Hand Osteoarthritis: Molecular Mechanisms, Randomized Controlled Trials, and the Future of Targeted Treatment
Abstract
1. Introduction
1.1. Epidemiology of Hand OA
1.2. Treatment of Hand OA
1.3. Mechanisms Underlying the Molecular Pathogenesis of Hand OA
2. Methods
3. Tumor Necrosis Factor Alpha (TNFα) and the Interleukins
3.1. Tumor Necrosis Factor Alpha (TNFα)
3.2. Interleukin-1 (IL-1)
3.3. Interleukin-4 and Its Receptor (IL-4/IL-4R)
3.4. Interleukin-6 (IL-6)
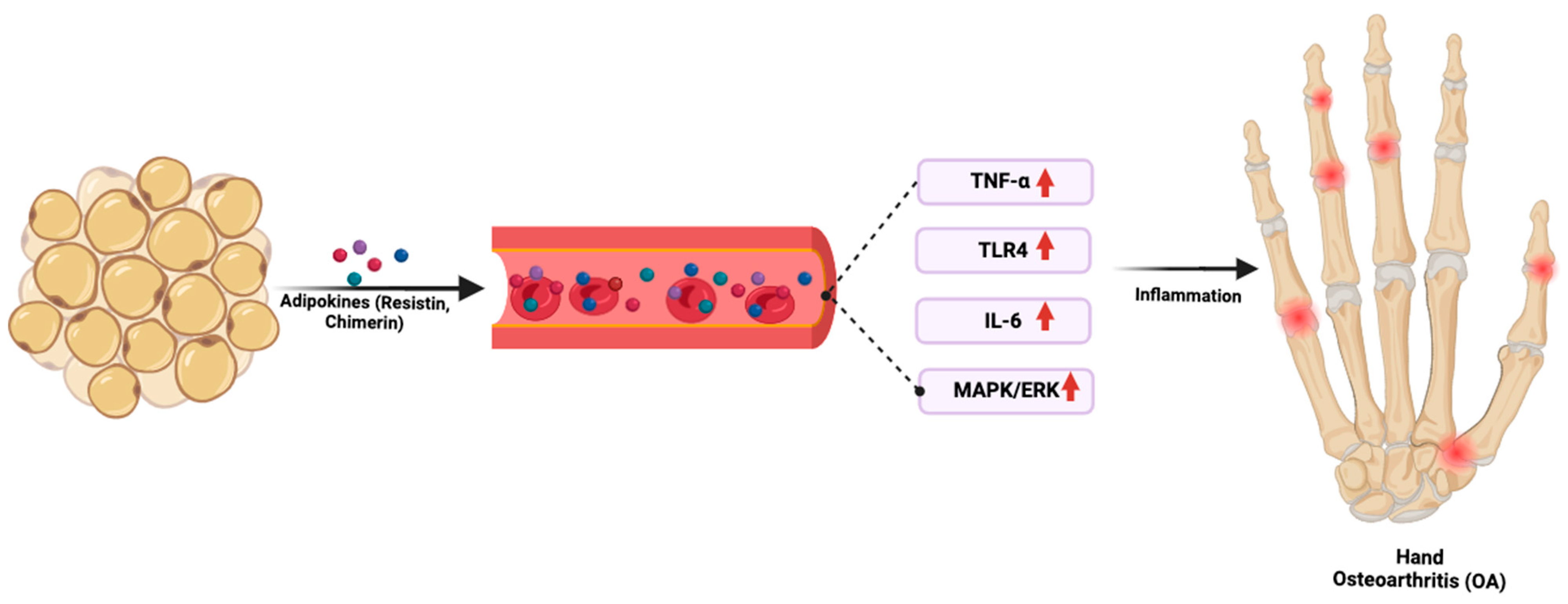
4. The Adipokines
5. Other Pathways and Genes Implicated in Hand OA
5.1. Transforming Growth Factor Alpha (TGFα)
5.2. Matrix Gla Protein (MGP)
5.3. Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1)
5.4. TEA Domain Transcription Factor 1 (TEAD1)
6. Thumb CMC OA: A Unique Subset of Hand OA
6.1. Interleukin-7 (IL-7)
6.2. WNT9A (Wnt Family Member 9A)
6.3. ALDH1A2 (Aldehyde Dehydrogenase 1 Family, Member A2) and Retinoic Acid Metabolism
6.4. MATN3 (Matrilin-3)
7. Beyond the Influence of Genetic and Inflammatory Factors in Hand OA
7.1. Epigenetic Regulation of Hand OA Pathophysiology
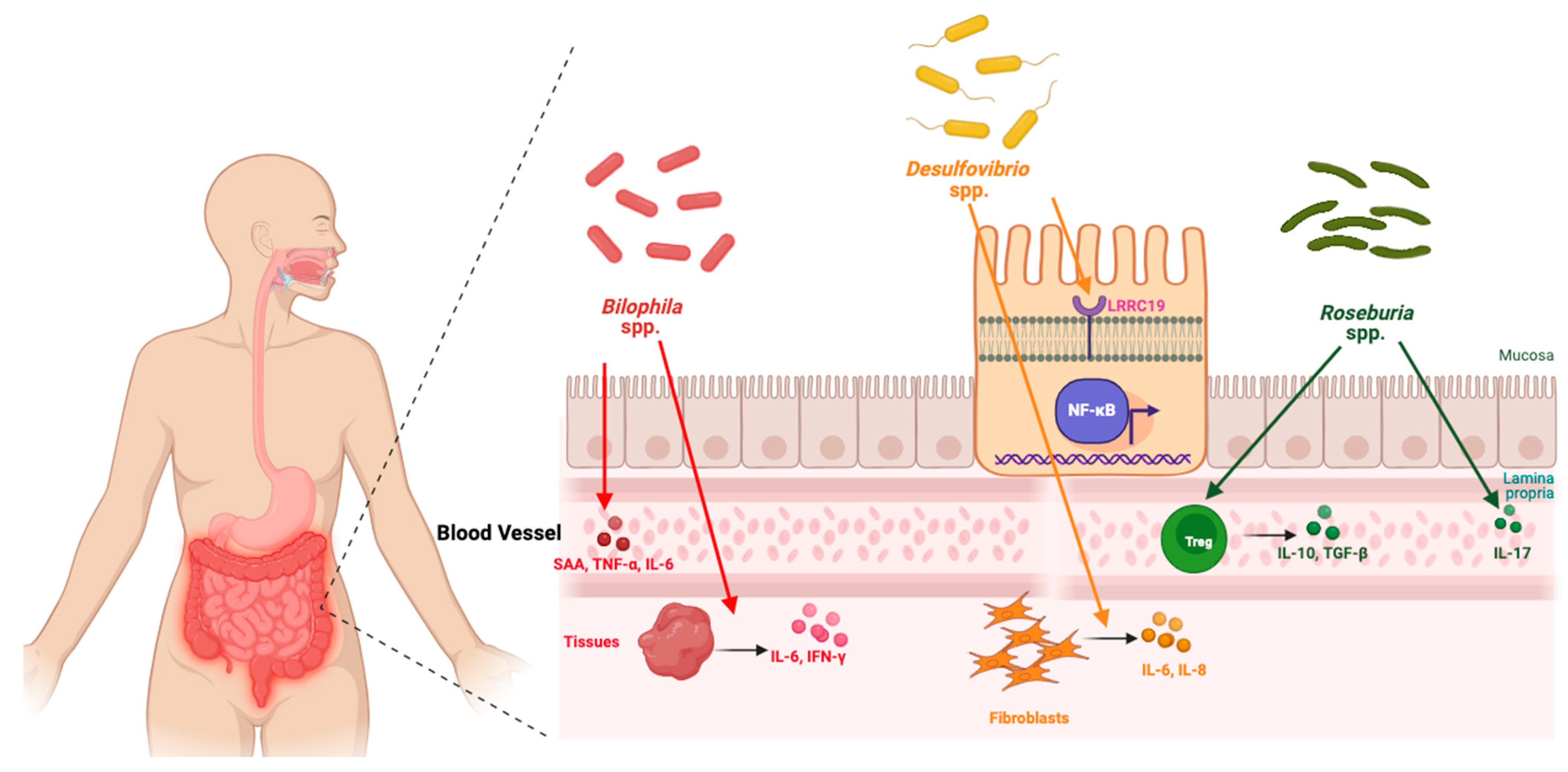
7.2. Emerging Gut Connection
8. Randomized Controlled Trials (RCTs) in Hand OA
8.1. Methods
8.2. Methotrexate (MTX)
8.3. Botulinum Toxin A (BoNT-A)
8.4. Topical Cetylated Fatty Acids (CFAs)
8.5. Tocilizumab: IL-6 Receptor (IL-6R) Blockade
8.6. Adalimumab: TNF-α Blockade
8.7. Colchicine
8.8. Corticosteroids
8.9. Platelet-Rich Plasma (PRP)
8.10. Hypertonic Dextrose Therapy (Prolotherapy)
8.11. Estrogen Replacement Therapy
8.12. Hydroxychloroquine (HCQ)
8.13. Hyaluronic Acid (HA)
8.14. Efficacy of Corticosteroids vs. Other Intra-Articular Therapies
8.15. Why Have Pharmacological Targeted Therapies Largely Failed to Deliver?
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martel-Pelletier, J. Pathophysiology of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The genesis of pain. Rheumatology 2018, 57 (Suppl. 4), iv43–iv50. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.-A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Eaton, C.B.; Schaefer, L.F.; Duryea, J.; Driban, J.B.; Lo, G.H.; Roberts, M.B.; Haugen, I.K.; Lu, B.; Nevitt, M.C.; Hochberg, M.C.; et al. Prevalence, Incidence, and Progression of Radiographic and Symptomatic Hand Osteoarthritis: The Osteoarthritis Initiative. Arthritis Rheumatol. 2022, 74, 992–1000. [Google Scholar] [CrossRef]
- Plotz, B.; Bomfim, F.; Sohail, M.A.; Samuels, J. Current Epidemiology and Risk Factors for the Development of Hand Osteoarthritis. Curr. Rheumatol. Rep. 2021, 23, 61. [Google Scholar] [CrossRef]
- Moran, S.L.; Berger, R.A. Biomechanics and hand trauma: What you need. Hand Clin. 2003, 19, 17–31. [Google Scholar] [CrossRef]
- Wan, J.; Qian, X.; He, Z.; Zhu, Z.; Cheng, P.; Chen, A. Epidemiological trends of hand osteoarthritis from 1990 to 2019: Estimates from the 2019 Global Burden of Disease study. Front. Med. 2022, 9, 922321. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, J.; Kelly-Hayes, M.; Chaisson, C.E.; Aliabadi, P.; Felson, D.T. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am. J. Epidemiol. 2002, 156, 1021–1027. [Google Scholar] [CrossRef]
- Haugen, I.K.; Englund, M.; Aliabadi, P.; Niu, J.; Clancy, M.; Kvien, T.K.; Felson, D.T. Prevalence, incidence and progression of hand osteoarthritis in the general population: The Framingham Osteoarthritis Study. Ann. Rheum. Dis. 2011, 70, 1581–1586. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Kroon, F.P.; Blanco, F.J.; Doherty, M.; Dziedzic, K.S.; Greibrokk, E.; Haugen, I.K.; Herrero-Beaumont, G.; Jonsson, H.; Kjeken, I.; et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann. Rheum. Dis. 2019, 78, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Osteoarthritis in over 16s: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK588843/ (accessed on 22 March 2025).
- Rannou, F.; Dimet, J.; Boutron, I.; Baron, G.; Fayad, F.; Macé, Y.; Beaudreuil, J.; Richette, P.; Ravaud, P.; Revel, M.; et al. Splint for Base-of-Thumb Osteoarthritis. Ann. Intern. Med. 2009, 150, 661–669. [Google Scholar] [CrossRef]
- Kjeken, I.; Smedslund, G.; Moe, R.H.; Slatkowsky-Christensen, B.; Uhlig, T.; Hagen, K.B. Systematic review of design and effects of splints and exercise programs in hand osteoarthritis. Arthritis Care Res. 2011, 63, 834–848. [Google Scholar] [CrossRef]
- Gomes Carreira, A.C.; Jones, A.; Natour, J. Assessment of the effectiveness of a functional splint for osteoarthritis of the trapeziometacarpal joint on the dominant hand: A randomized controlled study. J. Rehabil. Med. 2010, 42, 469–474. [Google Scholar] [CrossRef]
- Derry, S.; Conaghan, P.; Da Silva, J.A.P.; Wiffen, P.J.; Moore, R.A. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2016, 2016, CD007400. [Google Scholar] [CrossRef]
- Zacher, J.; Altman, R.; Bellamy, N.; Brühlmann, P.; Da Silva, J.; Huskisson, E.; Taylor, R.S. Topical diclofenac and its role in pain and inflammation: An evidence-based review. Curr. Med. Res. Opin. 2008, 24, 925–950. [Google Scholar] [CrossRef]
- Zeng, C.; Doherty, M.; Persson, M.S.M.; Yang, Z.; Sarmanova, A.; Zhang, Y.; Wei, J.; Kaur, J.; Li, X.; Lei, G.; et al. Comparative efficacy and safety of acetaminophen, topical and oral non-steroidal anti-inflammatory drugs for knee osteoarthritis: Evidence from a network meta-analysis of randomized controlled trials and real-world data. Osteoarthr. Cartil. 2021, 29, 1242–1251. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: A systematic review and network meta-analysis. Ann. Intern. Med. 2015, 162, 46–54. [Google Scholar] [CrossRef]
- Kroon, F.P.B.; Rubio, R.; Schoones, J.W.; Kloppenburg, M. Intra-Articular Therapies in the Treatment of Hand Osteoarthritis: A Systematic Literature Review. Drugs Aging 2016, 33, 119–133. [Google Scholar] [CrossRef] [PubMed]
- de Oliva Spolidoro Paschoal, N.; Natour, J.; Machado, F.S.; de Oliveira, H.A.V.; Furtado, R.N.V. Effectiveness of Triamcinolone Hexacetonide Intraarticular Injection in Interphalangeal Joints: A 12-week Randomized Controlled Trial in Patients with Hand Osteoarthritis. J. Rheumatol. 2015, 42, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.A.; Fruth, K.M.; Rizzo, M.; Moran, S.L.; Kakar, S. Prosthetic Arthroplasty Versus Arthrodesis for Osteoarthritis and Posttraumatic Arthritis of the Index Finger Proximal Interphalangeal Joint. J. Hand Surg. 2015, 40, 1937–1948. [Google Scholar] [CrossRef]
- Sweets, T.M.; Stern, P.J. Proximal Interphalangeal Joint Prosthetic Arthroplasty. J. Hand Surg. 2010, 35, 1190–1193. [Google Scholar] [CrossRef]
- Vermeulen, G.M.; Slijper, H.; Feitz, R.; Hovius, S.E.R.; Moojen, T.M.; Selles, R.W. Surgical Management of Primary Thumb Carpometacarpal Osteoarthritis: A Systematic Review. J. Hand Surg. 2011, 36, 157–169. [Google Scholar] [CrossRef]
- Rongières, M. Surgical treatment of degenerative osteoarthritis of the fingers. Chir. Main 2013, 32, 193–198. [Google Scholar] [CrossRef]
- Oo, W.M.; Hunter, D.J. Efficacy, Safety, and Accuracy of Intra-articular Therapies for Hand Osteoarthritis: Current Evidence. Drugs Aging 2023, 40, 1–20. [Google Scholar] [CrossRef]
- Kjeken, I.; Bordvik, D.H.; Osteras, N.; Haugen, I.K.; Aasness Fjeldstad, K.; Skaalvik, I.; Kloppenburg, M.; Kroon, F.P.B.; Tveter, A.T.; Smedslund, G. Efficacy and safety of non-pharmacological, pharmacological and surgical treatments for hand osteoarthritis in 2024: A systematic review. RMD Open 2025, 11, e004963. [Google Scholar] [CrossRef]
- Kroon, F.P.B.; Carmona, L.; Schoones, J.W.; Kloppenburg, M. Efficacy and safety of non-pharmacological, pharmacological and surgical treatment for hand osteoarthritis: A systematic literature review informing the 2018 update of the EULAR recommendations for the management of hand osteoarthritis. RMD Open 2018, 4, e000734. [Google Scholar] [CrossRef]
- Boer, C.G.; Hatzikotoulas, K.; Southam, L.; Stefánsdóttir, L.; Zhang, Y.; de Almeida, R.C.; Wu, T.T.; Zheng, J.; Hartley, A.; Teder-Laving, M. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 2021, 184, 4784–4818. [Google Scholar] [CrossRef]
- Hatzikotoulas, K.; Southam, L.; Boer, C.G. Large-scale genome-wide meta-analyses provide insights for the development of new disease modifying targets for osteoarthritis. Osteoarthr. Cartil. 2021, 29, S302. [Google Scholar] [CrossRef]
- Henkel, C.; Styrkársdóttir, U.; Thorleifsson, G.; Stefánsdóttir, L.; Björnsdóttir, G.; Banasik, K.; Brunak, S.; Erikstrup, C.; Dinh, K.M.; Hansen, T.F.; et al. Genome-wide association meta-analysis of knee and hip osteoarthritis uncovers genetic differences between patients treated with joint replacement and patients without joint replacement. Ann. Rheum. Dis. 2023, 82, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Armada, M.J.; Carames, B.; Lires-Dean, M.; Cillero-Pastor, B.; Ruiz-Romero, C.; Galdo, F.; Blanco, F.J. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthr. Cartil. 2006, 14, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Kumar, A.; Arshad, M.; Singh, A.; Khan, H.; Swaroop, S. Association of cytokine TNF-α in development of osteoarthritis: A comprehensive study. J. Ecophysiol. Occup. Health 2018, 18, 117–121. [Google Scholar]
- Hämäläinen, S.; Solovieva, S.; Vehmas, T.; Leino-Arjas, P.; Hirvonen, A. Variations in the TNFα gene and their interactions with the IL4R and IL10 genes in relation to hand osteoarthritis. BMC Musculoskelet. Disord. 2014, 15, 311. [Google Scholar] [CrossRef]
- Alexander, L.C.; McHorse, G.; Huebner, J.L.; Bay-Jensen, A.-C.; Karsdal, M.A.; Kraus, V.B. A matrix metalloproteinase-generated neoepitope of CRP can identify knee and multi-joint inflammation in osteoarthritis. Arthritis Res. Ther. 2021, 23, 226. [Google Scholar] [CrossRef]
- Na, H.S.; Park, J.-S.; Cho, K.-H.; Kwon, J.Y.; Choi, J.; Jhun, J.; Kim, S.J.; Park, S.-H.; Cho, M.-L. Interleukin-1-interleukin-17 signaling axis induces cartilage destruction and promotes experimental osteoarthritis. Front. Immunol. 2020, 11, 730. [Google Scholar]
- Yau, M.S.; Okoro, P.C.; Haugen, I.K.; Lynch, J.A.; Nevitt, M.C.; Lewis, C.E.; Torner, J.; Felson, D.T. Epigenome-Wide Analysis Of Osteoarthritis In The Multicenter Osteoarthritis Studyepigenome-Wide Analysis Of Osteoarthritis In The Multicenter Osteoarthritis Study. Osteoarthr. Cartil. 2023, 31, S362–S363. [Google Scholar] [CrossRef]
- Yau, M.S.; Joehanes, R.; Hsu, Y.-H.; Kiel, D.P.; Felson, D.T. Genome-wide DNA methylation study of hand osteoarthritis. Osteoarthr. Cartil. 2018, 26, S158. [Google Scholar] [CrossRef]
- Vidal, L.; Lopez-Golan, Y.; Rego-Perez, I.; Horvath, S.; Blanco, F.J.; Riancho, J.A.; Gomez-Reino, J.J.; Gonzalez, A. Specific increase of methylation age in osteoarthritis cartilage. Osteoarthr. Cartil. 2016, 24, S63. [Google Scholar] [CrossRef]
- Jin, L.; Ma, J.; Chen, Z.; Wang, F.; Li, Z.; Shang, Z.; Dong, J. Osteoarthritis related epigenetic variations in miRNA expression and DNA methylation. BMC Med. Genom. 2023, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Kreitmaier, P.; Swift, D.; Wilkinson, J.M.; Zeggini, E. Epigenomic differences between osteoarthritis grades in primary cartilage. Osteoarthr. Cartil. 2024, 32, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, X.; Xiao, Y.; Zhang, Y.; Zhang, W.; Doherty, M.; Nestor, J.; Li, C.; Ye, J.; Sha, T. Combining single-cell RNA sequencing and population-based studies reveals hand osteoarthritis-associated chondrocyte subpopulations and pathways. Bone Res. 2023, 11, 58. [Google Scholar] [CrossRef]
- Vincent, T.L. IL-1 in osteoarthritis: Time for a critical review of the literature. F1000Research 2019, 8, F1000. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6589928/ (accessed on 28 October 2024). [CrossRef]
- Moxley, G.; Han, J.; Stern, A.G.; Riley, B.P. Potential influence of IL1B haplotype and IL1A–IL1B–IL1RN extended haplotype on hand osteoarthritis risk. Osteoarthr. Cartil. 2007, 15, 1106–1112. [Google Scholar] [CrossRef]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Solovieva, S.; Kämäräinen, O.-P.; Hirvonen, A.; Hämäläinen, S.; Laitala, M.; Vehmas, T.; Luoma, K.; Näkki, A.; Riihimäki, H.; Ala-Kokko, L. Association between interleukin 1 gene cluster polymorphisms and bilateral distal interphalangeal osteoarthritis. J. Rheumatol. 2009, 36, 1977–1986. [Google Scholar] [CrossRef]
- von Kaeppler, E.P.; Wang, Q.; Raghu, H.; Bloom, M.S.; Wong, H.; Robinson, W.H. Interleukin 4 promotes anti-inflammatory macrophages that clear cartilage debris and inhibits osteoclast development to protect against osteoarthritis. Clin. Immunol. 2021, 229, 108784. [Google Scholar] [CrossRef]
- Song, S.Y.; Hong, J.; Go, S.; Lim, S.; Sohn, H.S.; Kang, M.; Jung, G.; Yoon, J.; Kang, M.L.; Im, G.; et al. Interleukin-4 Gene Transfection and Spheroid Formation Potentiate Therapeutic Efficacy of Mesenchymal Stem Cells for Osteoarthritis. Adv. Healthc. Mater. 2020, 9, 1901612. [Google Scholar] [CrossRef] [PubMed]
- Steen-Louws, C.; Popov-Celeketic, J.; Mastbergen, S.C.; Coeleveld, K.; Hack, C.E.; Eijkelkamp, N.; Tryfonidou, M.; Spruijt, S.; van Roon, J.A.G.; Lafeber, F. IL4-10 fusion protein has chondroprotective, anti-inflammatory and potentially analgesic effects in the treatment of osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Pulsatelli, L.; Dolzani, P.; Platano, D.; Olivotto, E.; Filardo, G.; Trisolino, G.; Facchini, A.; Borzì, R.M.; Meliconi, R. Human Osteoarthritic Cartilage Shows Reduced In Vivo Expression of IL-4, a Chondroprotective Cytokine that Differentially Modulates IL-1β-Stimulated Production of Chemokines and Matrix-Degrading Enzymes In Vitro. PLoS ONE 2014, 9, e96925. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef]
- Agere, S.A.; Akhtar, N.; Watson, J.M.; Ahmed, S. RANTES/CCL5 Induces Collagen Degradation by Activating MMP-1 and MMP-13 Expression in Human Rheumatoid Arthritis Synovial Fibroblasts. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Vargiolu, M.; Silvestri, T.; Bonora, E.; Dolzani, P.; Pulsatelli, L.; Addimanda, O.; Mancarella, L.; Punzi, L.; Fioravanti, A.; Facchini, A. Interleukin-4/interleukin-4 receptor gene polymorphisms in hand osteoarthritis. Osteoarthr. Cartil. 2010, 18, 810–816. [Google Scholar] [CrossRef]
- Liao, Y.; Ren, Y.; Luo, X.; Mirando, A.J.; Long, J.T.; Leinroth, A.; Ji, R.-R.; Hilton, M.J. Interleukin-6 signaling mediates cartilage degradation and pain in posttraumatic osteoarthritis in a sex-specific manner. Sci. Signal. 2022, 15, eabn7082. [Google Scholar] [CrossRef]
- Wiegertjes, R.; Thielen, N.G.M.; van Caam, A.P.M.; van Laar, M.; van Beuningen, H.M.; Koenders, M.I.; van Lent, P.; van der Kraan, P.M.; van de Loo, F.A.J.; Davidson, E.B. Increased IL-6 receptor expression and signaling in ageing cartilage can be explained by loss of TGF-β-mediated IL-6 receptor suppression. Osteoarthr. Cartil. 2021, 29, 773–782. [Google Scholar] [CrossRef]
- Blumenfeld, O.; Williams, F.M.; Valdes, A.; Hart, D.J.; Malkin, I.; Spector, T.D.; Livshits, G. Association of interleukin-6 gene polymorphisms with hand osteoarthritis and hand osteoporosis. Cytokine 2014, 69, 94–101. [Google Scholar] [CrossRef]
- Kämäräinen, O.-P.; Solovieva, S.; Vehmas, T.; Luoma, K.; Riihimäki, H.; Ala-Kokko, L.; Männikkö, M.; Leino-Arjas, P. Common interleukin-6 promoter variants associate with the more severe forms of distal interphalangeal osteoarthritis. Arthritis Res. Ther. 2008, 10, R21. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-J.; Lee, D.S.; Kim, W.K.; Han, B.S.; Lee, S.C.; Bae, K.-H. Metabolic adaptation in obesity and type II diabetes: Myokines, adipokines and hepatokines. Int. J. Mol. Sci. 2016, 18, 8. [Google Scholar] [CrossRef]
- Lee, S.S.; Choi, S.E.; Kang, J.H.; Park, D.J.; Shin, M.H. POS0383 Low Serum Adiponectin Levels Were Associated with Radiographic Progression of Hand Osteoarthritis in a 6-Year Follow-Up of the Dong-Gu Study. 2024. Available online: https://ard.bmj.com/content/83/Suppl_1/394.1.abstract (accessed on 28 October 2024).
- Yan, S.; Liu, H.; Nie, H.; Bu, G.; Yuan, W.; Wang, S. Common Variants of RARRES2 and Retn Contribute to Susceptibility to Hand Osteoarthritis and Related Pain. Biomark. Med. 2022, 16, 731–738. [Google Scholar] [CrossRef]
- Reilly, M.P.; Lehrke, M.; Wolfe, M.L.; Rohatgi, A.; Lazar, M.A.; Rader, D.J. Resistin Is an Inflammatory Marker of Atherosclerosis in Humans. Circulation 2005, 111, 932–939. [Google Scholar] [CrossRef]
- Filková, M.; Haluzík, M.; Gay, S.; Šenolt, L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009, 133, 157–170. [Google Scholar] [CrossRef]
- Choe, J.-Y.; Bae, J.; Jung, H.-Y.; Park, S.-H.; Lee, H.-J.; Kim, S.-K. Serum resistin level is associated with radiographic changes in hand osteoarthritis: Cross-sectional study. Jt. Bone Spine 2012, 79, 160–165. [Google Scholar] [CrossRef]
- Lee, J.H.; Ort, T.; Ma, K.; Picha, K.; Carton, J.; Marsters, P.A.; Lohmander, L.S.; Baribaud, F.; Song, X.-Y.; Blake, S. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthr. Cartil. 2009, 17, 613–620. [Google Scholar] [CrossRef]
- Eisinger, K.; Bauer, S.; Schäffler, A.; Walter, R.; Neumann, E.; Buechler, C.; Müller-Ladner, U.; Frommer, K.W. Chemerin induces CCL2 and TLR4 in synovial fibroblasts of patients with rheumatoid arthritis and osteoarthritis. Exp. Mol. Pathol. 2012, 92, 90–96. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Huang, L.; Liu, J.; Zhou, Q.; Du, G.; Lao, S. Chemerin promotes MAPK/ERK activation to induce inflammatory factor production in rat synoviocytes. Exp. Ther. Med. 2022, 24, 684. [Google Scholar] [CrossRef]
- Hämäläinen, S.; Solovieva, S.; Vehmas, T.; Hirvonen, A.; Leino-Arjas, P. Adipokine genes and radiographic hand osteoarthritis in Finnish women: A cross-sectional study. Scand. J. Rheumatol. 2018, 47, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.; Schimmack, S.; Lawrence, B.; Alaimo, D.; Modlin, I.M. EGFR/TGFα and TGFβ/CTGF Signaling in Neuroendocrine Neoplasia: Theoretical Therapeutic Targets. Neuroendocrinology 2012, 97, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Jia, H.; Ma, X.; Tong, W.; Doyran, B.; Sun, Z.; Wang, L.; Zhang, X.; Zhou, Y.; Badar, F.; Chandra, A.; et al. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc. Natl. Acad. Sci. USA 2016, 113, 14360–14365. [Google Scholar] [CrossRef]
- Hallbeck, A.L.; Walz, T.M.; Briheim, K.; Wasteson, Å. TGF-α and ErbB2 production in synovial joint tissue: Increased expression in arthritic joints. Scand. J. Rheumatol. 2005, 34, 204–211. [Google Scholar] [CrossRef]
- Appleton, C.T.G.; Usmani, S.E.; Bernier, S.M.; Aigner, T.; Beier, F. Transforming growth factor α suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis. Arthritis Rheum. 2007, 56, 3693–3705. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Liu, F.; Li, Y.; Chandra, A.; Levin, L.S.; Beier, F.; Enomoto-Iwamoto, M.; Qin, L. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res. 2014, 2, 14015. [Google Scholar] [CrossRef]
- Den Hollander, W.; Boer, C.G.; Hart, D.J.; Yau, M.S.; Ramos, Y.F.; Metrustry, S.; Broer, L.; Deelen, J.; Cupples, L.A.; Rivadeneira, F. Genome-wide association and functional studies identify a role for matrix Gla protein in osteoarthritis of the hand. Ann. Rheum. Dis. 2017, 76, 2046–2053. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Spronk, H.M.H.; Skepper, J.N.; Hackeng, T.M.; Shanahan, C.M.; Vermeer, C.; Weissberg, P.L.; Proudfoot, D. Post-translational modifications regulate matrix Gla protein function: Importance for inhibition of vascular smooth muscle cell calcification. J. Thromb. Haemost. JTH 2007, 5, 2503–2511. [Google Scholar] [CrossRef]
- Neogi, T.; Booth, S.L.; Zhang, Y.Q.; Jacques, P.F.; Terkeltaub, R.; Aliabadi, P.; Felson, D.T. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006, 54, 1255–1261. [Google Scholar] [CrossRef]
- Neogi, T.; Felson, D.T.; Sarno, R.; Booth, S.L. Vitamin K in hand osteoarthritis: Results from a randomised clinical trial. Ann. Rheum. Dis. 2008, 67, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Pac, M.; Krata, N.; Moszczuk, B.; Wyczałkowska-Tomasik, A.; Kaleta, B.; Foroncewicz, B.; Rudnicki, W.; Pączek, L.; Mucha, K. NR3C1 glucocorticoid receptor gene polymorphisms are associated with membranous and IgA nephropathies. Cells 2021, 10, 3186. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Dolhain, R.J.; Koper, J.W.; Van Rossum, E.F.; Emonts, M.; Han, K.H.; Wouters, J.M.; Hazes, J.M.; Lamberts, S.W.; Feelders, R.A. Polymorphisms in the glucocorticoid receptor gene that modulate glucocorticoid sensitivity are associated with rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R159. [Google Scholar] [CrossRef] [PubMed]
- Malaise, O.; Relic, B.; Charlier, E.; Zeddou, M.; Neuville, S.; Deroyer, C.; Gillet, P.; Louis, E.; Malaise, M.G.; De Seny, D. Glucocorticoid-induced leucine zipper (GILZ) is involved in glucocorticoid-induced and mineralocorticoid-induced leptin production by osteoarthritis synovial fibroblasts. Arthritis Res. Ther. 2016, 18, 219. [Google Scholar] [CrossRef]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop. J. Sports Med. 2015, 3, 2325967115581163. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.-Y.; Chinnaiyan, A.M. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, B.; Wang, P.; Chen, F.; Dong, Z.; Yang, H.; Guan, K.-L.; Xu, Y. Structural insights into the YAP and TEAD complex. Genes Dev. 2010, 24, 235–240. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, J.; Li, W.; Wu, A.; Zhang, X.; Tong, W.; Ho, K.K.; Qin, L.; Song, H.; Mak, K.K. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 2018, 9, 4564. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.-J.; Liu, R.; Zhan, J.-F.; Tan, C.; Fang, Y.-F.; Chen, Y.; Yu, B. Inhibition of YAP with siRNA prevents cartilage degradation and ameliorates osteoarthritis development. J. Mol. Med. 2019, 97, 103–114. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Zhang, Y.; Zhang, M.; Sun, Q.; Chen, H.; Dong, L.; Chu, Z.; Xue, B.; Hoff, W.D.; et al. Photo-tunable hydrogels reveal cellular sensing of rapid rigidity changes through the accumulation of mechanical signaling molecules. Cell Stem Cell 2025, 32, 121–136.e6. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Pan, X.; Wang, P.; Wei, Q. Synergistic Influence of Fibrous Pattern Orientation and Modulus on Cellular Mechanoresponse. Nano Lett. 2024, 24, 6376–6385. [Google Scholar] [CrossRef] [PubMed]
- Loening, A.M.; James, I.E.; Levenston, M.E.; Badger, A.M.; Frank, E.H.; Kurz, B.; Nuttall, M.E.; Hung, H.-H.; Blake, S.M.; Grodzinsky, A.J.; et al. Injurious Mechanical Compression of Bovine Articular Cartilage Induces Chondrocyte Apoptosis. Arch. Biochem. Biophys. 2000, 381, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Cook, M.N.; DiMicco, M.A.; Blake, S.M.; James, I.E.; Kumar, S.; Cole, A.A.; Lark, M.W.; Grodzinsky, A.J. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: Interaction with exogenous cytokines. Arthritis Rheum. 2003, 48, 1292–1301. [Google Scholar] [CrossRef]
- Patwari, P.; Cheng, D.M.; Cole, A.A.; Kuettner, K.E.; Grodzinsky, A.J. Analysis of the Relationship between Peak Stress and Proteoglycan Loss following Injurious Compression of Human Post-mortem Knee and Ankle Cartilage. Biomech. Model. Mechanobiol. 2007, 6, 83–89. [Google Scholar] [CrossRef]
- Mow, V.C.; Huiskes, R. Basic Orthopaedic Biomechanics & Mechano-Biology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Lawrence, J.S.; Bremner, J.M.; Bier, F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and X-ray changes. Ann. Rheum. Dis. 1966, 25, 1. [Google Scholar]
- Armstrong, A.L.; Hunter, J.B.; Davis, T.R.C. The Prevalence of Degenerative Arthritis of the Base of the Thumb in Post-Menopausal Women. J. Hand Surg. 1994, 19, 340–341. [Google Scholar] [CrossRef]
- Becker, S.J.E.; Briet, J.P.; Hageman, M.G.J.S.; Ring, D. Death, Taxes, and Trapeziometacarpal Arthrosis. Clin. Orthop. 2013, 471, 3738–3744. [Google Scholar] [CrossRef]
- Snyder, E.A.; Alvarez, C.; Golightly, Y.M.; Renner, J.B.; Jordan, J.M.; Nelson, A.E. Incidence and progression of hand osteoarthritis in a large community-based cohort: The Johnston County Osteoarthritis Project. Osteoarthr. Cartil. 2020, 28, 446–452. [Google Scholar] [CrossRef]
- Edmunds, J.O. Current concepts of the anatomy of the thumb trapeziometacarpal joint. J. Hand Surg. 2011, 36, 170–182. [Google Scholar] [CrossRef]
- Ladd, A.L.; Weiss, A.-P.C.; Crisco, J.J.; Hagert, E.; Wolf, J.M.; Glickel, S.Z.; Yao, J. The thumb carpometacarpal joint: Anatomy, hormones, and biomechanics. Instr. Course Lect. 2013, 62, 165. [Google Scholar] [PubMed]
- Long, D.; Blake, S.; Song, X.-Y.; Lark, M.; Loeser, R.F. Human articular chondrocytes produce IL-7 and respond to IL-7 with increased production of matrix metalloproteinase-13. Arthritis Res. Ther. 2008, 10, R23. [Google Scholar] [CrossRef] [PubMed]
- Hartgring, S.A.Y.; Bijlsma, J.W.J.; Lafeber, F.; Van Roon, J.A.G. Interleukin-7 induced immunopathology in arthritis. Ann. Rheum. Dis. 2006, 65 (Suppl. 3), iii69–iii74. [Google Scholar] [CrossRef]
- Van Roon, J.A.G.; Verweij, M.C.; Wijk, M.W.; Jacobs, K.M.G.; Bijlsma, J.W.J.; Lafeber, F.P.J.G. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact–dependent activation of CD4+ T cells and macrophages. Arthritis Rheum. 2005, 52, 1700–1710. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Cenci, S.; Rifas, L.; Brown, C.; Pacifici, R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood J. Am. Soc. Hematol. 2000, 96, 1873–1878. [Google Scholar]
- Ratneswaran, A.; Rockel, J.S.; Antflek, D.; Matelski, J.J.; Shestopaloff, K.; Kapoor, M.; Baltzer, H. Investigating molecular signatures underlying trapeziometacarpal osteoarthritis through the evaluation of systemic cytokine expression. Front. Immunol. 2022, 12, 794792. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Roberts, M.B.; Haugen, I.K.; Schaefer, L.F.; Duryea, J.; Driban, J.B.; Booth, S.L.; Petty, G.A.; Eaton, C.B. Cytokine/adipokine profile shows no evidence of an inflammatory or metabolic basis for incident erosive hand oa in the osteoarthritis initiative cohort. Osteoarthr. Cartil. 2020, 28, S66–S67. [Google Scholar] [CrossRef]
- Baloun, J.; Kropáčková, T.; Hulejova, H.; Tomčík, M.; Ruužičková, O.; Šléglová, O.; Gatterová, J.; Vencovskỳ, J.; Pavelka, K.; Šenolt, L. Chemokine and cytokine profiles in patients with hand osteoarthritis. Biomolecules 2020, 11, 4. [Google Scholar] [CrossRef]
- Rübenhagen, R.; Schüttrumpf, J.P.; Stürmer, K.M.; Frosch, K.-H. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop. 2012, 83, 59–64. [Google Scholar] [CrossRef]
- Boer, C.G.; Yau, M.S.; Rice, S.J.; Coutinho de Almeida, R.; Cheung, K.; Styrkarsdottir, U.; Southam, L.; Broer, L.; Wilkinson, J.M.; Uitterlinden, A.G.; et al. Genome-wide association of phenotypes based on clustering patterns of hand osteoarthritis identify WNT9A as novel osteoarthritis gene. Ann. Rheum. Dis. 2021, 80, 367–375. [Google Scholar] [CrossRef]
- Cheng, J.; Li, M.; Bai, R. The Wnt signaling cascade in the pathogenesis of osteoarthritis and related promising treatment strategies. Front. Physiol. 2022, 13, 954454. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jiang, H.; Cheng, Z.; Li, Y.; Yang, Z.; Cheng, C.; Tang, X. Genetic polymorphism of WNT9A is functionally associated with thumb osteoarthritis in the Chinese population. Adv. Rheumatol. 2024, 64, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, M.; Zuscik, M.; Wu, Q.; Wang, Y.; Rosier, R.N.; O’Keefe, R.J.; Chen, D. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008, 58, 2053–2064. [Google Scholar] [CrossRef]
- Nalesso, G.; Thomas, B.L.; Sherwood, J.C.; Yu, J.; Addimanda, O.; Eldridge, S.E.; Thorup, A.-S.; Dale, L.; Schett, G.; Zwerina, J. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 218–226. [Google Scholar] [CrossRef]
- Styrkarsdottir, U.; Thorleifsson, G.; Helgadottir, H.T.; Bomer, N.; Metrustry, S.; Bierma-Zeinstra, S.; Strijbosch, A.M.; Evangelou, E.; Hart, D.; Beekman, M. Severe osteoarthritis of the hand associates with common variants within the ALDH1A2 gene and with rare variants at 1p31. Nat. Genet. 2014, 46, 498–502. [Google Scholar] [CrossRef]
- Nallamshetty, S.; Wang, H.; Rhee, E.-J.; Kiefer, F.W.; Brown, J.D.; Lotinun, S.; Le, P.; Baron, R.; Rosen, C.J.; Plutzky, J. Deficiency of Retinaldehyde Dehydrogenase 1 Induces BMP2 and Increases Bone Mass In Vivo. PLoS ONE 2013, 8, e71307. [Google Scholar] [CrossRef]
- Nallamshetty, S.; Le, P.T.; Wang, H.; Issacsohn, M.J.; Reeder, D.J.; Rhee, E.-J.; Kiefer, F.W.; Brown, J.D.; Rosen, C.J.; Plutzky, J. Retinaldehyde dehydrogenase 1 deficiency inhibits PPARγ-mediated bone loss and marrow adiposity. Bone 2014, 67, 281–291. [Google Scholar] [CrossRef]
- Unguryte, A.; Bernotiene, E.; Bagdonas, E.; Garberyte, S.; Porvaneckas, N.; Jorgensen, C. Human articular chondrocytes with higher aldehyde dehydrogenase activity have stronger expression of COL2A1 and SOX9. Osteoarthr. Cartil. 2016, 24, 873–882. [Google Scholar] [CrossRef]
- Zhu, L.; Kamalathevan, P.; Koneva, L.A.; Zarebska, J.M.; Chanalaris, A.; Ismail, H.; Wiberg, A.; Ng, M.; Muhammad, H.; Walsby-Tickle, J.; et al. Variants in ALDH1A2 reveal an anti-inflammatory role for retinoic acid and a new class of disease-modifying drugs in osteoarthritis. Sci. Transl. Med. 2022, 14, eabm4054. [Google Scholar] [CrossRef]
- Min, J.L.; Meulenbelt, I.; Riyazi, N.; Kloppenburg, M.; Houwing-Duistermaat, J.J.; Seymour, A.B.; van Duijn, C.M.; Slagboom, P.E. Association of matrilin-3 polymorphisms with spinal disc degeneration and osteoarthritis of the first carpometacarpal joint of the hand. Ann. Rheum. Dis. 2006, 65, 1060–1066. [Google Scholar] [CrossRef]
- Pullig, O.; Tagariello, A.; Schweizer, A.; Swoboda, B.; Schaller, P.; Winterpacht, A. MATN3 (matrilin-3) sequence variation (pT303M) is a risk factor for osteoarthritis of the CMC1 joint of the hand, but not for knee osteoarthritis. Ann. Rheum. Dis. 2007, 66, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Stefánsson, S.E.; Jónsson, H.; Ingvarsson, T.; Manolescu, I.; Jónsson, H.H.; Ólafsdóttir, G.; Pálsdottir, E.; Stefánsdottir, G.; Sveinbjörnsdóttir, G.; Frigge, M.L. Genomewide scan for hand osteoarthritis: A novel mutation in matrilin-3. Am. J. Hum. Genet. 2003, 72, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.R.; Becker, A.-K.A.; Neacsu, C.D.; Paulsson, M.; Wagener, R. The matrilins: Modulators of extracellular matrix assembly. Int. J. Biochem. Cell Biol. 2011, 43, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fleischhauer, L.; Nicolae, C.; Prein, C.; Farkas, Z.; Saller, M.M.; Prall, W.C.; Wagener, R.; Heilig, J.; Niehoff, A. Mice lacking the matrilin family of extracellular matrix proteins develop mild skeletal abnormalities and are susceptible to age-associated osteoarthritis. Int. J. Mol. Sci. 2020, 21, 666. [Google Scholar] [CrossRef]
- Vincourt, J.-B.; Etienne, S.; Grossin, L.; Cottet, J.; Bantsimba-Malanda, C.; Netter, P.; Mainard, D.; Libante, V.; Gillet, P.; Magdalou, J. Matrilin-3 switches from anti-to pro-anabolic upon integration to the extracellular matrix. Matrix Biol. 2012, 31, 290–298. [Google Scholar] [CrossRef]
- Budde, B.; Blumbach, K.; Ylöstalo, J.; Zaucke, F.; Ehlen, H.W.A.; Wagener, R.; Ala-Kokko, L.; Paulsson, M.; Bruckner, P.; Grässel, S. Altered Integration of Matrilin-3 into Cartilage Extracellular Matrix in the Absence of Collagen IX. Mol. Cell. Biol. 2005, 25, 10465–10478. [Google Scholar] [CrossRef]
- Nicolae, C.; Ko, Y.-P.; Miosge, N.; Niehoff, A.; Studer, D.; Enggist, L.; Hunziker, E.B.; Paulsson, M.; Wagener, R.; Aszodi, A. Abnormal collagen fibrils in cartilage of matrilin-1/matrilin-3-deficient mice. J. Biol. Chem. 2007, 282, 22163–22175. [Google Scholar] [CrossRef]
- Jayasuriya, C.T.; Goldring, M.B.; Terek, R.; Chen, Q. Matrilin-3 Induction of IL-1 receptor antagonist Is required for up-regulating collagen II and aggrecan and down-regulating ADAMTS-5 gene expression. Arthritis Res. Ther. 2012, 14, R197. [Google Scholar] [CrossRef]
- Muttigi, M.S.; Han, I.; Park, H.-K.; Park, H.; Lee, S.-H. Matrilin-3 role in cartilage development and osteoarthritis. Int. J. Mol. Sci. 2016, 17, 590. [Google Scholar] [CrossRef]
- Hämäläinen, S.; Solovieva, S.; Vehmas, T.; Hirvonen, A.; Leino-Arjas, P. THU0017 DNA Methylation of Regulatory Sites of Hand Osteoarthritis Susceptibility Genes in Finnish Women. 2018. Available online: https://ard.bmj.com/content/77/Suppl_2/237.2.abstract (accessed on 28 October 2024).
- Bertoncelj, M.F.; Masterson, T.; Karouzakis, E.; Kolling, C.; Filer, A.; Buckley, C.D.; Gay, S.; Distler, O.; Ospelt, C. SAT0066 the Long Noncoding RNA (LNCRNA) Hottip Is a Master Regulator of Cell Cycle in Hand Synovial Fibroblasts in Arthritis. 2018. Available online: https://ard.bmj.com/content/77/Suppl_2/896.3.abstract (accessed on 28 October 2024).
- Kuroiwa, T.; Tsuboi, Y.; Michikawa, T.; Tajima, K.; Uraya, Y.; Maeda, A.; Shizu, K.; Suzuki, K.; Suzuki, K.; Kawano, Y.; et al. DNA methylation of bone morphogenetic protein 7 in leukocytes as a possible biomarker for hand osteoarthritis: A pilot study. J. Orthop. Res. 2024, 43, 84–93. [Google Scholar] [CrossRef]
- Boehler, C.; Gauthier, L.R.; Mortusewicz, O.; Biard, D.S.; Saliou, J.-M.; Bresson, A.; Sanglier-Cianferani, S.; Smith, S.; Schreiber, V.; Boussin, F.; et al. Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc. Natl. Acad. Sci. USA 2011, 108, 2783. [Google Scholar] [CrossRef] [PubMed]
- Belousova, E.A.; Ishchenko, A.A.; Lavrik, O.I. DNA is a New Target of Parp3. Sci. Rep. 2018, 8, 4176. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.; Liu, Z.; Kraus, W.L. PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological outcomes. Genes Dev. 2017, 31, 101–126. [Google Scholar] [CrossRef]
- Hassa, P.O.; Hottiger, M.O. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol. Chem. 1999, 380, 953–959. [Google Scholar] [CrossRef]
- Rodriguez-Vargas, J.-M.; Martin-Hernandez, K.; Wang, W.; Kunath, N.; Suganthan, R.; Amé, J.-C.; Oliver, F.J.; Ye, J.; Bjørås, M.; Dantzer, F. Parp3 promotes astrocytic differentiation through a tight regulation of Nox4-induced ROS and mTorc2 activation. Cell Death Dis. 2020, 11, 954. [Google Scholar] [CrossRef]
- Karicheva, O.; Rodriguez-Vargas, J.M.; Wadier, N.; Martin-Hernandez, K.; Vauchelles, R.; Magroun, N.; Tissier, A.; Schreiber, V.; Dantzer, F. PARP3 controls TGFβ and ROS driven epithelial-to-mesenchymal transition and stemness by stimulating a TG2-Snail-E-cadherin axis. Oncotarget 2016, 7, 64109. [Google Scholar] [CrossRef]
- Sorkin, A.; Goh, L.K. Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 2009, 315, 683–696. [Google Scholar] [CrossRef]
- Guan, Z.; Jia, J.; Zhang, C.; Sun, T.; Zhang, W.; Yuan, W.; Leng, H.; Song, C. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clin. Sci. 2020, 134, 3159–3174. [Google Scholar] [CrossRef]
- Sane, F.; Piva, F.; Romond, M.-B. Free lipoproteins from Bifidobacterium longum alleviate osteoarthritis through modulation of the gut microbiome. Microbiome Res. Rep. 2023, 2, 18. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10688786/ (accessed on 28 October 2024). [CrossRef]
- Schlupp, L.; Prinz, E.; Dyson, G.; Barrett, M.; Izda, V.; Dunn, C.M.; Jeffries, M.A. Sex-Linked Discrepancies in C57BL6/J Mouse Osteoarthritis are Associated With the Gut Microbiome and are Transferrable by Microbiome Transplantation. Arthritis Rheumatol. 2024, 76, 231–237. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Z.; Li, J.; Zhang, Y.; Zhang, W.; Doherty, M.; Yang, T.; Yang, Y.; Li, H.; Wang, Y. Association between gut microbiome-related metabolites and symptomatic hand osteoarthritis in two independent cohorts. EBioMedicine 2023, 98, 104892. Available online: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(23)00458-9/fulltext (accessed on 28 October 2024). [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Long, W.; Hao, B.; Ding, D.; Ma, X.; Zhao, L.; Pang, X. A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog. 2017, 9, 59. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; Da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef]
- Xie, R.; Gu, Y.; Li, M.; Li, L.; Yang, Y.; Sun, Y.; Zhou, B.; Liu, T.; Wang, S.; Liu, W.; et al. Desulfovibrio vulgaris interacts with novel gut epithelial immune receptor LRRC19 and exacerbates colitis. Microbiome 2024, 12, 4. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Yu, Y.; Wang, Q.; Yang, C.; Yan, Y.; Wang, F.; Mao, Y. Desulfovibrio desulfuricans and its derived metabolites confer resistance to FOLFOX through METTL3. EBioMedicine 2024, 102, 105041. Available online: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(24)00076-8/fulltext (accessed on 28 October 2024). [CrossRef]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef]
- Dzierzewicz, Z.; Szczerba, J.; Lodowska, J.; Wolny, D.; Gruchlik, A.; Orchel, A.; Weglarz, L. The role of Desulfovibrio desulfuricans lipopolysaccharides in modulation of periodontal inflammation through stimulation of human gingival fibroblasts. Arch. Oral Biol. 2010, 55, 515–522. [Google Scholar] [CrossRef]
- Bisson-Boutelliez, C.; Massin, F.; Dumas, D.; Miller, N.; Lozniewski, A. Desulfovibrio spp. survive within KB cells and modulate inflammatory responses. Mol. Oral Microbiol. 2010, 25, 226–235. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Hillman, E.T.; Kozik, A.J.; Hooker, C.A.; Burnett, J.L.; Heo, Y.; Kiesel, V.A.; Nevins, C.J.; Oshiro, J.M.K.I.; Robins, M.M.; Thakkar, R.D.; et al. Comparative genomics of the genus Roseburia reveals divergent biosynthetic pathways that may influence colonic competition among species. Microb. Genom. 2020, 6, mgen000399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Song, K.; Shen, Z.; Quan, Y.; Tan, B.; Luo, W.; Wu, S.; Tang, K.; Yang, Z.; Wang, X. Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T cells differentiation in colitis. Mol. Med. Rep. 2018, 17, 7567–7574. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Shen, Z.; Deng, M.; Li, X.; Tan, B.; Xiao, M.; Wu, S.; Yang, Z.; Zhu, C.; Tian, L.; et al. Roseburia intestinalis supernatant ameliorates colitis induced in mice by regulating the immune response. Mol. Med. Rep. 2019, 20, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, C.; Quan, Y.; Yang, J.; Yuan, W.; Yang, Z.; Wu, S.; Luo, W.; Tan, B.; Wang, X. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol. 2018, 33, 1751–1760. [Google Scholar] [CrossRef]
- Binvignat, M.; Emond, P.; Mifsud, F.; Miao, B.; Courties, A.; Lefèvre, A.; Maheu, E.; Crema, M.D.; Klatzmann, D.; Kloppenburg, M. Serum tryptophan metabolites are associated with erosive hand osteoarthritis and pain: Results from the DIGICOD cohort. Osteoarthr. Cartil. 2023, 31, 1132–1143. [Google Scholar] [CrossRef]
- Meng, D.I.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef]
- Hong, S.-H.; Hong, Y.; Lee, M.; Keum, B.-R.; Kim, G.-H. Natural product skatole ameliorates lipotoxicity-induced multiple hepatic damage under hyperlipidemic conditions in hepatocytes. Nutrients 2023, 15, 1490. [Google Scholar] [CrossRef]
- Kurata, K.; Kawahara, H.; Nishimura, K.; Jisaka, M.; Yokota, K.; Shimizu, H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem. Biophys. Res. Commun. 2019, 510, 649–655. [Google Scholar] [CrossRef]
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr. Polym. 2022, 276, 118699. [Google Scholar] [CrossRef]
- Wang, A.; Guan, C.; Wang, T.; Mu, G.; Tuo, Y. Lactiplantibacillus plantarum -Derived Indole-3-lactic Acid Ameliorates Intestinal Barrier Integrity through the AhR/Nrf2/NF-κB Axis. J. Agric. Food Chem. 2024, 72, 9236–9246. [Google Scholar] [CrossRef]
- Jolivet, J.; Cowan, K.H.; Curt, G.A.; Clendeninn, N.J.; Chabner, B.A. The Pharmacology and Clinical Use of Methotrexate. N. Engl. J. Med. 1983, 309, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.J.; Jacobs, J.W.G. Methotrexate: Still the anchor drug in RA treatment. Jt. Bone Spine 2009, 76, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Wittoek, R.; Allado, E.; Cruzel, C.; Fontas, E.; Breuil, V.; Ziegler, L.; Kremer, J.; Loeuille, D.; Roux, C.H. Methotrexate treatment in hand osteoarthritis refractory to usual treatments: A randomised, double-blind, placebo-controlled trial. Semin. Arthritis Rheum. 2021, 51, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Harman, G.; McMillan, R.B.; Stoy, N.; Stone, T.W.; Darlington, L.G. Modulation of cytokine release by purine receptors in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, 89–92. [Google Scholar]
- Varani, K.; Padovan, M.; Vincenzi, F.; Targa, M.; Trotta, F.; Govoni, M.; Borea, P.A. A2A and A3 adenosine receptor expression in rheumatoid arthritis: Upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res. Ther. 2011, 13, R197. [Google Scholar] [CrossRef]
- Wang, Y.; Jones, G.; Keen, H.I.; Hill, C.L.; Wluka, A.E.; Kasza, J.; Teichtahl, A.J.; Antony, B.; O’Sullivan, R.; Cicuttini, F.M. Methotrexate to treat hand osteoarthritis with synovitis (METHODS): An Australian, multisite, parallel-group, double-blind, randomised, placebo-controlled trial. Lancet 2023, 402, 1764–1772. [Google Scholar] [CrossRef]
- Simpson, L.L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol. Rev. 1981, 33, 155–188. [Google Scholar] [CrossRef]
- Matak, I.; Bölcskei, K.; Bach-Rojecky, L.; Helyes, Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef]
- Schäffer, M.; Beiter, T.; Becker, H.D.; Hunt, T.K. Neuropeptides: Mediators of Inflammation and Tissue Repair? Arch. Surg. 1998, 133, 1107–1116. [Google Scholar] [CrossRef]
- Scott, D.T.; Lam, F.Y.; Ferrell, W.R. Acute joint inflammation--mechanisms and mediators. Gen. Pharmacol. 1994, 25, 1285–1296. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Chu, X.; Li, T.; Shen, N.; Fan, C.; Niu, Z.; Zhang, X.; Hu, L. Intra-articular injection of Botulinum toxin A reduces neurogenic inflammation in CFA-induced arthritic rat model. Toxicon 2017, 126, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Abdoul, H.; Campagna, R.; Guerini, H.; Jilet, L.; Bedin, C.; Chagny, F.; Couraud, G.; Daste, C.; Drapé, J.-L.; et al. Intra-articular botulinum toxin A injection for painful base-of-thumb osteoarthritis: A double-blind, randomised, controlled, phase 3 trial (RHIBOT). Lancet Rheumatol. 2022, 4, e480–e489. [Google Scholar] [CrossRef] [PubMed]
- Suwannaphisit, S.; Sinnathakorn, N.; Suwanno, P.; Fongsri, W.; Tangtrakulwanich, B. Impact of topical cetylated fatty acid cream on hand osteoarthritis: A randomized, double-blind clinical trial. Sci. Rep. 2025, 15, 4587. [Google Scholar] [CrossRef] [PubMed]
- Hesslink, R.; Armstrong, D.; Nagendran, M.V.; Sreevatsan, S.; Barathur, R. Cetylated fatty acids improve knee function in patients with osteoarthritis. J. Rheumatol. 2002, 29, 1708–1712. [Google Scholar]
- Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Maresh, C.M.; Tiberio, D.P.; Joyce, M.E.; Messinger, B.N.; French, D.N.; Rubin, M.R.; Gómez, A.L.; et al. Effect of a cetylated fatty acid topical cream on functional mobility and quality of life of patients with osteoarthritis. J. Rheumatol. 2004, 31, 767–774. [Google Scholar]
- Hudita, A.; Galateanu, B.; Dinescu, S.; Costache, M.; Dinischiotu, A.; Negrei, C.; Stan, M.; Tsatsakis, A.; Nikitovic, D.; Lupuliasa, D.; et al. In Vitro Effects of Cetylated Fatty Acids Mixture from Celadrin on Chondrogenesis and Inflammation with Impact on Osteoarthritis. Cartilage 2020, 11, 88–97. [Google Scholar] [CrossRef]
- Richette, P.; Latourte, A.; Sellam, J.; Wendling, D.; Piperno, M.; Goupille, P.; Pers, Y.-M.; Eymard, F.; Ottaviani, S.; Ornetti, P.; et al. Efficacy of tocilizumab in patients with hand osteoarthritis: Double blind, randomised, placebo-controlled, multicentre trial. Ann. Rheum. Dis. 2021, 80, 349–355. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Yang, S.; Shin, Y.; Rhee, J.; Chun, C.-H.; Chun, J.-S. Interleukin-6 plays an essential role in hypoxia-inducible factor 2α-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2011, 63, 2732–2743. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; Jiang, H.; Zhou, J.; Tang, Y. Inhibition of interleukin-6 function attenuates the central sensitization and pain behavior induced by osteoarthritis. Eur. J. Pharmacol. 2017, 811, 260–267. [Google Scholar] [CrossRef]
- Eitner, A.; Hofmann, G.O.; Schaible, H.-G. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Front. Mol. Neurosci. 2017, 10, 349. [Google Scholar] [CrossRef]
- Steen Pettersen, P.; Neogi, T.; Magnusson, K.; Berner Hammer, H.; Uhlig, T.; Kvien, T.K.; Haugen, I.K. Peripheral and Central Sensitization of Pain in Individuals With Hand Osteoarthritis and Associations With Self-Reported Pain Severity. Arthritis Rheumatol. 2019, 71, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Malfait, A.-M.; Miller, R.E. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 2020, 28, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Ravaud, P.; Maheu, E.; Baron, G.; Rialland, A.; Vergnaud, P.; Roux, C.; Maugars, Y.; Mulleman, D.; Lukas, C.; et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2015, 74, 1697–1705. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Restrepo-Escobar, M.; de Jesús Carmona-Franceschi, M.; Donado Gómez, J.H. Colchicine treatment in adult patients with knee osteoarthritis: Systematic review of the literature. Rev. Colomb. Reumatol. 2017, 24, 102–111. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Haaland, B.; Huebner, J.L.; Wong, S.B.S.; Tjai, M.; Wang, C.; Chowbay, B.; Thumboo, J.; Chakraborty, B.; Tan, M.H.; et al. Colchicine lack of effectiveness in symptom and inflammation modification in knee osteoarthritis (COLKOA): A randomized controlled trial. Osteoarthr. Cartil. 2018, 26, 631–640. [Google Scholar] [CrossRef]
- Amirpour, A.; Mousavi, M.A.; Abolghasemi, R.; Taziki, O.; Khoddami Vishteh, H.R. The Effect of Colchicine in Improving the Symptoms of Patients with Knee Osteoarthritis. J. Babol Univ. Med. Sci. 2016, 18, 7–13. [Google Scholar] [CrossRef]
- Davis, C.R.; Ruediger, C.D.; Dyer, K.A.; Lester, S.; Graf, S.W.; Kroon, F.P.B.; Whittle, S.L.; Hill, C.L. Colchicine is not effective for reducing osteoarthritic hand pain compared to placebo: A randomised, placebo-controlled trial (COLAH). Osteoarthr. Cartil. 2021, 29, 208–214. [Google Scholar] [CrossRef]
- Døssing, A.; Henriksen, M.; Ellegaard, K.; Nielsen, S.M.; Stamp, L.K.; Müller, F.C.; Kloppenburg, M.; Haugen, I.K.; McCarthy, G.M.; Conaghan, P.G.; et al. Colchicine twice a day for hand osteoarthritis (COLOR): A double-blind, randomised, placebo-controlled trial. Lancet Rheumatol. 2023, 5, e254–e262. [Google Scholar] [CrossRef]
- Brophy, R.H.; Fillingham, Y.A. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J. Am. Acad. Orthop. Surg. 2022, 30, e721–e729. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Saadé, N.E.; Safieh-Garabedian, B. Interleukin-10 and the regulation of mitogen-activated protein kinases: Are these signalling modules targets for the anti-inflammatory action of this cytokine? Cell. Signal. 2003, 15, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Schäcke, H.; Cato, A.C.B. Dichotomy of glucocorticoid action in the immune system. Trends Immunol. 2002, 23, 120–122. [Google Scholar] [CrossRef]
- Berrebi, D.; Bruscoli, S.; Cohen, N.; Foussat, A.; Migliorati, G.; Bouchet-Delbos, L.; Maillot, M.-C.; Portier, A.; Couderc, J.; Galanaud, P.; et al. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: An anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood 2003, 101, 729–738. [Google Scholar] [CrossRef]
- Mocellin, S.; Panelli, M.C.; Wang, E.; Nagorsen, D.; Marincola, F.M. The dual role of IL-10. Trends Immunol. 2003, 24, 36–43. [Google Scholar] [CrossRef]
- Kroon, F.P.B.; Kortekaas, M.C.; Boonen, A.; Böhringer, S.; Reijnierse, M.; Rosendaal, F.R.; Riyazi, N.; Starmans, M.; Turkstra, F.; van Zeben, J.; et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): A double-blind, randomised, placebo-controlled trial. Lancet Lond. Engl. 2019, 394, 1993–2001. [Google Scholar] [CrossRef]
- Wenham, C.Y.J.; Hensor, E.M.A.; Grainger, A.J.; Hodgson, R.; Balamoody, S.; Doré, C.J.; Emery, P.; Conaghan, P.G. A randomized, double-blind, placebo-controlled trial of low-dose oral prednisolone for treating painful hand osteoarthritis. Rheumatol. Oxf. Engl. 2012, 51, 2286–2294. [Google Scholar] [CrossRef]
- Meenagh, G.K.; Patton, J.; Kynes, C.; Wright, G.D. A randomised controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann. Rheum. Dis. 2004, 63, 1260–1263. [Google Scholar] [CrossRef]
- Wang, Y.; Estee, M.M.; Gan, D.; Lim, Y.Z.; Heritier, S.; Wluka, A.E.; Hussain, S.M.; Trevaskis, N.L.; Cicuttini, F.M. Effect of 6-week treatment with topical betamethasone dipropionate in patients with symptomatic hand osteoarthritis: A randomized double-blind, placebo-controlled trial. Osteoarthr. Cartil. Open 2023, 5, 100382. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef]
- Smyth, N.A.; Murawski, C.D.; Fortier, L.A.; Cole, B.J.; Kennedy, J.G. Platelet-rich plasma in the pathologic processes of cartilage: Review of basic science evidence. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2013, 29, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O’Shaughnessey, K.; Hoeppner, J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.; Tuca, A.-C.; Justich, I.; Tschauner, S.; Friedl, H.; Girsch, W.; Lebo, P.; Zrim, R.; Lumenta, D.B.; Kamolz, L.-P. Minimally Invasive Treatment of Trapeziometacarpal Osteoarthritis: Results of a Blinded Randomized Controlled Trial. Plast. Reconstr. Surg. 2023, 152, 1277–1285. [Google Scholar] [CrossRef]
- Pumberger, P.; Wechselberger, G.; Schwaiger, K.; Zimmermann, V. The Use of Stromal Vascular Fraction, Platelet-rich Plasma, and Stem Cells in the Treatment of Thumb Carpometacarpal Osteoarthritis. Plast. Reconstr. Surg. Glob. Open 2025, 13, e6481. [Google Scholar] [CrossRef]
- Malahias, M.-A.; Roumeliotis, L.; Nikolaou, V.S.; Chronopoulos, E.; Sourlas, I.; Babis, G.C. Platelet-Rich Plasma versus Corticosteroid Intra-Articular Injections for the Treatment of Trapeziometacarpal Arthritis: A Prospective Randomized Controlled Clinical Trial. Cartilage 2021, 12, 51–61. [Google Scholar] [CrossRef]
- Oh, J.H.; Ha, H.; Yu, M.R.; Lee, H.B. Sequential effects of high glucose on mesangial cell transforming growth factor-beta 1 and fibronectin synthesis. Kidney Int. 1998, 54, 1872–1878. [Google Scholar] [CrossRef]
- Fukuda, K.; Kawata, S.; Inui, Y.; Higashiyama, S.; Matsuda, Y.; Igura, T.; Tamura, S.; Taniguchi, N.; Matsuzawa, Y. High concentration of glucose increases mitogenic responsiveness to heparin-binding epidermal growth factor-like growth factor in rat vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1962–1968. [Google Scholar] [CrossRef]
- Pugliese, G.; Pricci, F.; Locuratolo, N.; Romeo, G.; Romano, G.; Giannini, S.; Cresci, B.; Galli, G.; Rotella, C.M.; Di Mario, U. Increased activity of the insulin-like growth factor system in mesangial cells cultured in high glucose conditions. Relation to glucose-enhanced extracellular matrix production. Diabetologia 1996, 39, 775–784. [Google Scholar] [CrossRef]
- Topol, G.A.; Reeves, K.D.; Hassanein, K.M. Efficacy of dextrose prolotherapy in elite male kicking-sport athletes with chronic groin pain. Arch. Phys. Med. Rehabil. 2005, 86, 697–702. [Google Scholar] [CrossRef]
- Ustun, I.; Çağlar, S. Comparison of the effect of prolotherapy and paraffin wax for hand osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9510–9520. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.; Tracey, I. Hormones and their Interaction with the Pain Experience. Rev. Pain 2008, 2, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.E.; Chester-Jones, M.; Minns Lowe, C.; Goff, M.V.; Francis, A.; Brewer, G.; Marian, I.; Morris, S.L.; Warwick, D.; Eldridge, L.; et al. Hormone replacement therapy (conjugated oestrogens plus bazedoxifene) for post-menopausal women with symptomatic hand osteoarthritis: Primary report from the HOPE-e randomised, placebo-controlled, feasibility study. Lancet Rheumatol. 2022, 4, e725–e737. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.-H.; Choi, J.-H.; Byun, M.-S.; Jue, D.-M. Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology 2006, 45, 703–710. [Google Scholar] [CrossRef]
- van den Borne, B.E.; Dijkmans, B.A.; de Rooij, H.H.; le Cessie, S.; Verweij, C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997, 24, 55–60. [Google Scholar]
- Lee, W.; Ruijgrok, L.; Boxma-de Klerk, B.; Kok, M.R.; Kloppenburg, M.; Gerards, A.; Huisman, M.; Hazes, M.; de Sonnaville, P.; Grillet, B.; et al. Efficacy of Hydroxychloroquine in Hand Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care Res. 2018, 70, 1320–1325. [Google Scholar] [CrossRef]
- Kingsbury, S.R.; Tharmanathan, P.; Keding, A.; Ronaldson, S.J.; Grainger, A.; Wakefield, R.J.; Arundel, C.; Birrell, F.; Doherty, M.; Vincent, T.; et al. Hydroxychloroquine Effectiveness in Reducing Symptoms of Hand Osteoarthritis: A Randomized Trial. Ann. Intern. Med. 2018, 168, 385–395. [Google Scholar] [CrossRef]
- Kogan, G.; Soltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 1996, 74, A1–A7. [Google Scholar] [CrossRef]
- Fam, H.; Bryant, J.T.; Kontopoulou, M. Rheological properties of synovial fluids. Biorheology 2007, 44, 59–74. [Google Scholar] [CrossRef]
- Band, P.A.; Heeter, J.; Wisniewski, H.-G.; Liublinska, V.; Pattanayak, C.W.; Karia, R.J.; Stabler, T.; Balazs, E.A.; Kraus, V.B. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthr. Cartil. 2015, 23, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Percival, S.S.; Conrad, B.P.; Seay, A.N.; Montero, C.; Vincent, K.R. Hyaluronic Acid (HA) Viscosupplementation on Synovial Fluid Inflammation in Knee Osteoarthritis: A Pilot Study. Open Orthop. J. 2013, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Lin, Y.-T.; Chiang, B.-L.; Lin, Y.-H.; Hou, S.-M. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr. Cartil. 2006, 14, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chiang, C.-F.; Kuo, F.-C.; Su, S.-C.; Huang, C.-L.; Liu, J.-S.; Lu, C.-H.; Hsieh, C.-H.; Wang, C.-C.; Lee, C.-H.; et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11917. [Google Scholar] [CrossRef]
- Mandl, L.A.; Wolfe, S.; Daluiski, A.; Hotchkiss, R.N.; Lyman, S.L.; Katz, J.N. A Randomized Controlled Trial of Hylan GF 20 for the Treatment of Carpometacarpal Osteoarthritis. In Arthritis and Rheumatism; Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 64, pp. S475–S476. [Google Scholar]
- Ioppolo, F.; Saracino, F.; Rizzo, R.S.; Monacelli, G.; Lanni, D.; Di Sante, L.; Cacchio, A.; Santilli, V.; Venditto, T. Comparison Between Extracorporeal Shock Wave Therapy and Intra-articular Hyaluronic Acid Injections in the Treatment of First Carpometacarpal Joint Osteoarthritis. Ann. Rehabil. Med. 2018, 42, 92–100. [Google Scholar] [CrossRef]
- Sabaah, H.M.A.; Fattah, R.A.E.; Al Zifzaf, D.; Saad, H. A comparative study for different types of thumb base osteoarthritis injections: A randomized controlled interventional study. Ortop. Traumatol. Rehabil. 2020, 22, 447–454. [Google Scholar]
- Monfort, J.; Rotés-Sala, D.; Segalés, N.; Montañes, F.-J.; Orellana, C.; Llorente-Onaindia, J.; Mojal, S.; Padró, I.; Benito, P. Comparative efficacy of intra-articular hyaluronic acid and corticoid injections in osteoarthritis of the first carpometacarpal joint: Results of a 6-month single-masked randomized study. Jt. Bone Spine 2015, 82, 116–121. [Google Scholar] [CrossRef]
- Jahangiri, A.; Moghaddam, F.R.; Najafi, S. Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: A double-blind randomized clinical trial. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2014, 19, 737–743. [Google Scholar] [CrossRef]
- Thakker, A.; Sharma, S.C.; Johnson, N.; Dias, J.J. Comparison between intra-articular injections of corticosteroids, hyaluronic acid, PRP and placebo for thumb base osteoarthritis: A frequentist network meta-analysis. J. Orthop. 2023, 45, 78–86. [Google Scholar] [CrossRef]
- Joseph, Y. Created with BioRender. Figure 1. TNF-α-Driven Inflammatory Pathways in Joint Degradation. 2025. Available online: https://BioRender.com/dka0yn2 (accessed on 28 February 2025).
- Joseph, Y. Created with BioRender. Figure 2. Adipokine-Driven Inflammation Linking Obesity to Hand OA. 2025. Available online: https://BioRender.com/al6q05j (accessed on 28 February 2025).
- Joseph, Y. Created with BioRender. Figure 3. Dual Roles of YAP/TAZ in Fibroblast-like Synoviocytes (FLSs) and Mesenchymal Stem Cells (MSCs) in Cartilage Regulation. 2025. Available online: https://BioRender.com/h7fyi8q (accessed on 28 February 2025).
- Joseph, Y. Created with BioRender. Figure 4. The Gut–Joint Axis in Hand OA. 2025. Available online: https://BioRender.com/8wosytf (accessed on 28 February 2025).


| Mechanism Category | Molecular Factor | Role in Hand OA | Joint Involvement | Key References |
|---|---|---|---|---|
| Cytokines | TNFα | Pro-inflammatory; linked with cartilage breakdown and synovial inflammation | Hand OA | [37,45] |
| IL-1β | Induces cartilage degradation; enhances inflammation via MMPs and ADAMTS | DIP OA, Hand OA | [47,50] | |
| IL-4 | Inhibits IL-1β-induced protein expression of MMP-13; prevents ECM breakdown | Hand OA | [58] | |
| IL-6 | Synovial inflammation; cartilage damage | Hand OA | [61,62] | |
| IL-7 | Activates inflammatory T-cell responses; biomarker potential | Thumb CMC OA | [109] | |
| Other Signalling Pathways | WNT9A | Regulates chondrogenesis; influences thumb CMC OA severity | Thumb CMC OA | [115] |
| TGFα | Responds to mechanical stress; dual roles in cartilage catabolism/anabolism | Hand OA | [31] | |
| YAP/TAZ-TEAD | Mediates cartilage homeostasis; cellular response to mechanical cues | Hand OA | [31] | |
| ALDH1A2 | Retinoic acid metabolism; impacts cartilage repair and homeostasis | Hand OA | [118,122] | |
| Epigenetic Factors | DNA methylation of PARP3 | DNA methylation affects DNA repair; modulates NF-κB signaling; influences inflammation and cartilage homeostasis | Hand OA | [41] |
| DNA methylation of EPS15 | Affects EGFR endocytosis and bone mineralization pathways; influences inflammation, immune response, and cartilage metabolism | Hand OA | [41] | |
| DNA methylation of COL2A1 | Alters collagen type II synthesis, cartilage structural integrity; methylation associated with radiographic severity | Hand OA | [73] | |
| DNA methylation of BMP7 | Impairs cartilage repair; elevated methylation linked to OA severity; potential systemic biomarker for hand OA diagnosis and monitoring | Hand OA | [135] | |
| Adiponectin | Lower levels linked with radiographic hand OA progression; potential protective role in OA through anti-inflammatory effects | Hand OA | [65] | |
| Resistin | SNPs associated with OA risk and severity; interacts with TNFα and IL-6 to exacerbate inflammation and OA progression | Hand OA | [66] | |
| Chemerin | SNPs associated with OA risk and pain severity; promotes inflammation via TLR4 and MAPK/ERK pathways | Hand OA | [66] | |
| Gut-Joint Axis | Bilophila | Elevated in symptomatic hand OA; induces pro-inflammatory cytokines IFN-γ, IL-6, TNF-α, and systemic inflammatory markers; linked to inflammation in other inflammatory diseases | Hand OA | [146] |
| Desulfovibrio | Elevated in symptomatic hand OA; stimulates inflammatory genes, activates NF-κB pathway, and produces IL-6 and IL-8; contributes to inflammation | Hand OA | [146] | |
| Roseburia | Reduced abundance in hand OA; typically anti-inflammatory; inhibits IL-17 secretion; promotes Treg cells and IL-10 production | Hand OA | [146] | |
| Extracellular Matrix Proteins | MATN3 | Chondrocyte metabolism; regulates the synthesis of ECM proteins | Thumb CMC OA | [123,124,125] |
| MGP | Vitamin K-dependent; prevents cartilage calcification | Hand OA | [80,82,83] | |
| Glucocorticoid Receptor | NR3C1 | Modulates inflammation; regulates glucocorticoid response; influences cartilage and synovial homeostasis | Hand OA | [31] |
| Therapy | Mechanistic Target/Action | Joints Studied | Key Outcomes | References |
|---|---|---|---|---|
| Methotrexate | Reduction of inflammatory cytokines (TNFα, IL-1β, IL-6) | Thumb CMC, IP OA | Reduced pain at 6 months; no significant functional improvement | [171] |
| Botulinum Toxin A | Inhibition of neurogenic inflammation via neuropeptides; reduction of inflammatory cytokines (TNFα, IL-1β) | Thumb CMC OA | Short-term pain relief (3 months); transient motor weakness | [177] |
| Topical Cetylated Fatty Acids (CFAs) | Reduction of inflammatory cytokines (TNFα, IL-6) | Thumb CMC, IP OA | Modest pain relief; improved patient symptom perception | [178] |
| Tocilizumab (IL-6 blockade) | IL-6 receptor blockade; inflammation modulation | DIP, PIP OA | No significant clinical benefit compared to placebo | [182] |
| Adalimumab (TNF-α blockade) | TNF-α inhibition; inflammation modulation | Hand OA | No significant clinical benefit compared to placebo | [188] |
| Colchicine | Inflammasome inhibition; reduction of inflammatory cytokines (IL-1β, IL-6) | Thumb CMC, IP OA | No significant improvement over placebo | [193,194] |
| Oral Corticosteroids | Anti-inflammatory effects (IL-1β, IL-6, TNF-α inhibition) | Thumb CMC, IP OA | Short-term pain relief; mixed evidence on hand function | [202,203] |
| Intra-articular Corticosteroids | Anti-inflammatory effects (IL-1β, IL-6, TNF-α inhibition) | Thumb CMC, IP OA | Effective in pain relief for IP OA; limited effectiveness in thumb CMC OA | [23,204] |
| Topical Corticosteroids | Anti-inflammatory effects (IL-1β, IL-6, TNF-α inhibition) | Hand OA | No significant pain reduction compared to placebo | [205] |
| Platelet-rich Plasma (PRP) | Regenerative; reduction of inflammatory cytokines (IL-1β, TNFα); promotion of anti-inflammatory cytokines (IL-4, IL-10) | Thumb CMC OA | Improved pain and function, sustained in most trials | [209,210,211] |
| Hypertonic Dextrose (Prolotherapy) | Tissue regeneration via induced growth factors (TGF-β, IGF-1, PDGF) | Thumb CMC, IP, DIP, PIP OA | Improved function and pain vs. placebo | [216] |
| Estrogen Replacement Therapy | Anti-inflammatory effects (IL-6, TNF-α inhibition); promotion of anti-inflammatory cytokines (TGF-β, IL-10) | Hand OA | No significant improvement in hand pain vs. placebo | [219] |
| Hydroxychloroquine (HCQ) | Anti-inflammatory effects (TLR, IL-1β, IL-6, TNF-α inhibition) | Hand OA | No clinical benefit compared to placebo | [222,223] |
| Hyaluronic Acid (HA) | Joint lubrication; anti-inflammatory effects (IL-1β, TNF-α inhibition) | Thumb CMC OA | Comparable effectiveness to corticosteroids; moderate effects in hand pain and function | [231,232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, Y.D.; Ladd, A.L.; Bhutani, N. Hand Osteoarthritis: Molecular Mechanisms, Randomized Controlled Trials, and the Future of Targeted Treatment. Int. J. Mol. Sci. 2025, 26, 4537. https://doi.org/10.3390/ijms26104537
Joseph YD, Ladd AL, Bhutani N. Hand Osteoarthritis: Molecular Mechanisms, Randomized Controlled Trials, and the Future of Targeted Treatment. International Journal of Molecular Sciences. 2025; 26(10):4537. https://doi.org/10.3390/ijms26104537
Chicago/Turabian StyleJoseph, Yemisi D., Amy L. Ladd, and Nidhi Bhutani. 2025. "Hand Osteoarthritis: Molecular Mechanisms, Randomized Controlled Trials, and the Future of Targeted Treatment" International Journal of Molecular Sciences 26, no. 10: 4537. https://doi.org/10.3390/ijms26104537
APA StyleJoseph, Y. D., Ladd, A. L., & Bhutani, N. (2025). Hand Osteoarthritis: Molecular Mechanisms, Randomized Controlled Trials, and the Future of Targeted Treatment. International Journal of Molecular Sciences, 26(10), 4537. https://doi.org/10.3390/ijms26104537






