Metabolic Alterations in Colombian Women with Rheumatoid Arthritis and Systemic Lupus Erythematosus Reveal Potential Lipid Biomarkers Associated with Inflammation and Cardiovascular Risk
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Participants
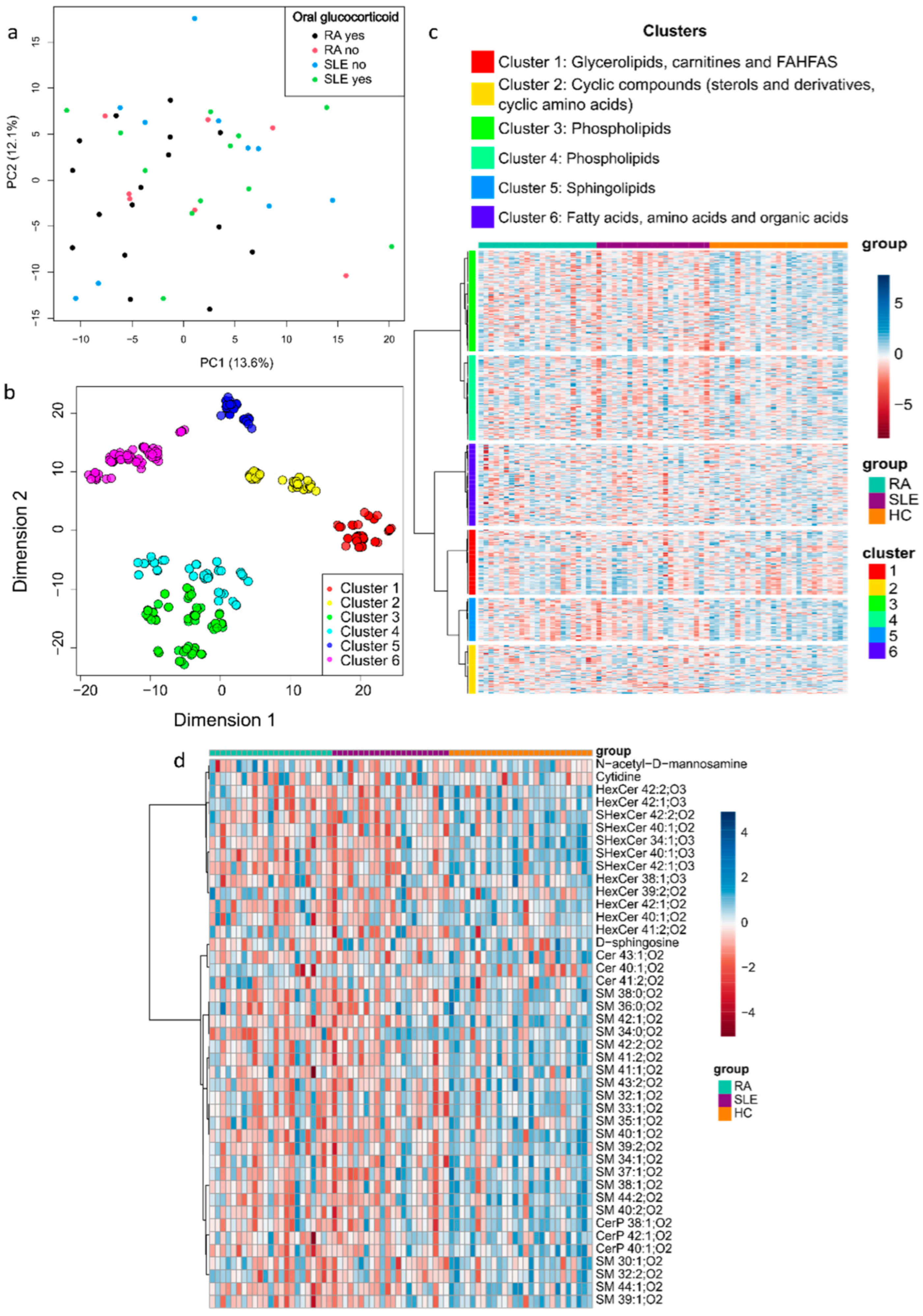
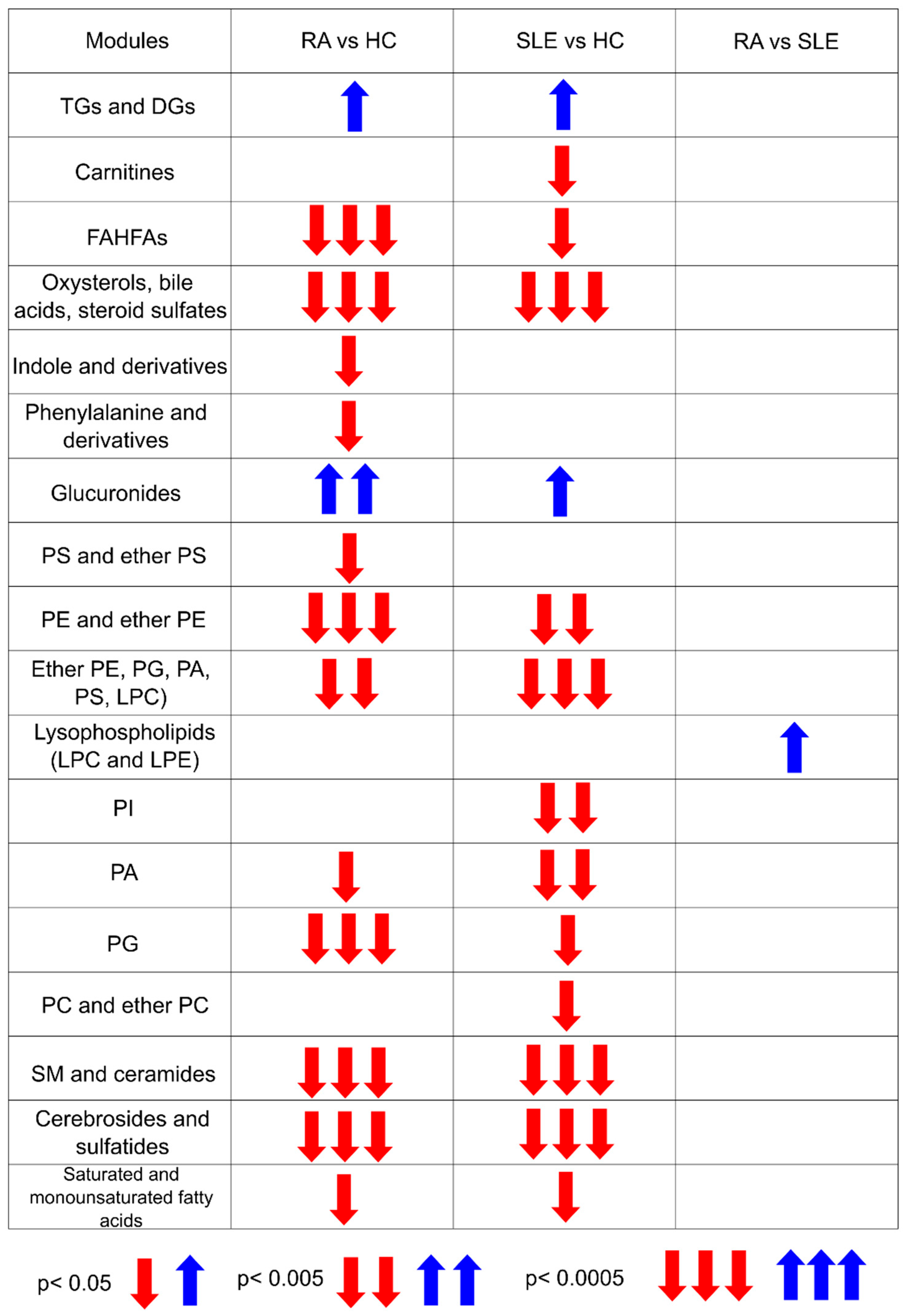
2.2. Metabolite Mapping of RA and SLE Patients
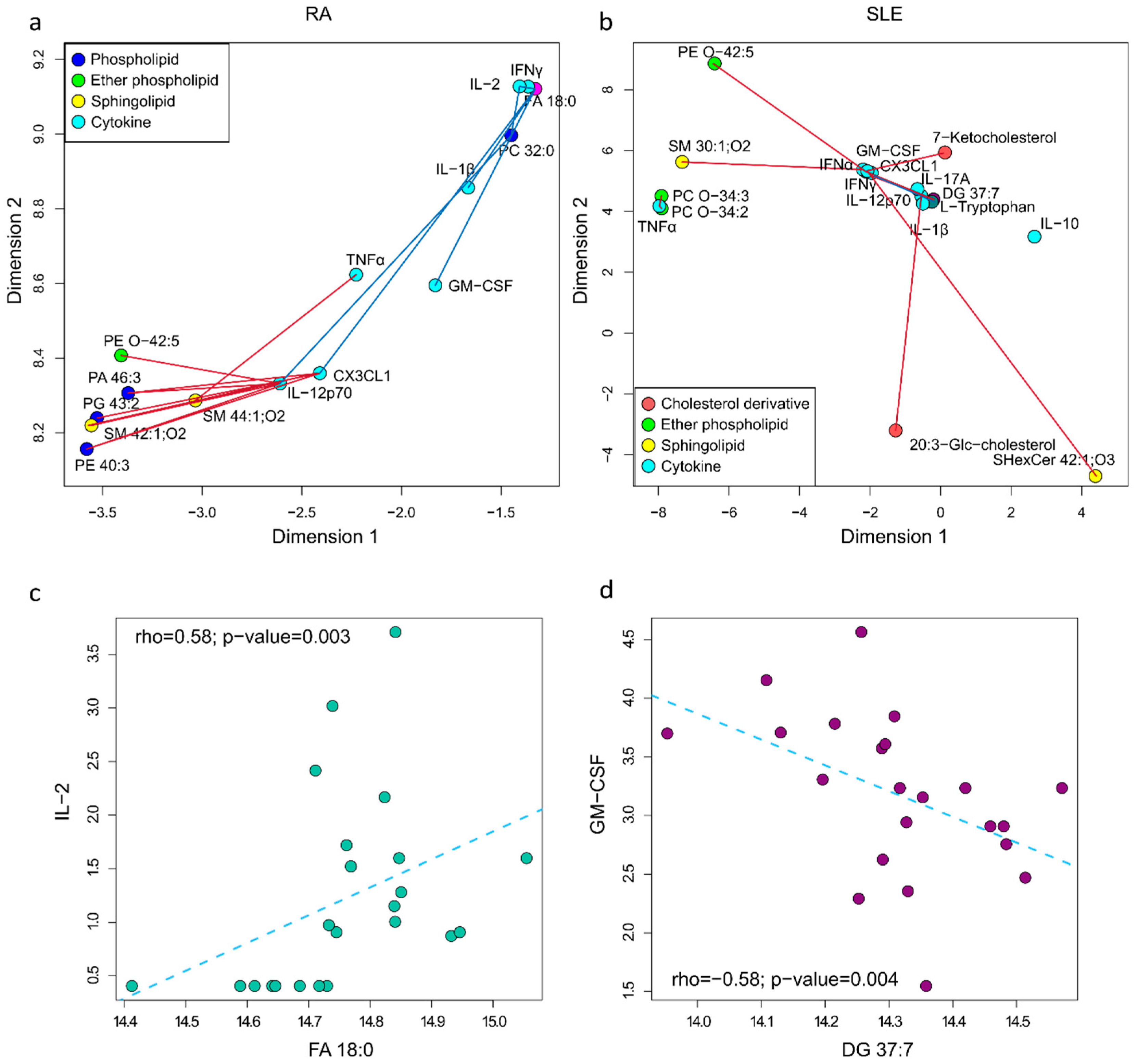
2.3. Metabolite and Cytokine Correlation Network in RA and SLE
2.4. Predictive Performance of Combined Metabolites in RA and SLE
2.5. Metabolite and HDL Correlation Network
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Metabolite Extraction
4.3. Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF-MS)
4.4. Gas Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry (GC-QTOF-MS)
4.5. Quality Control Samples
4.6. Metabolomics and Lipidomics Data Analysis
4.7. Statistical Analysis
4.8. Metabolite Mapping
4.9. Correlation Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theofilopoulos, A.N.; Kono, D.H.; Baccala, R. The Multiple Pathways to Autoimmunity. Nat. Immunol. 2018, 18, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Canizares, N.; Wahezi, D.; Putterman, C.; Einstein, A. Diagnostic and Prognostic Tests in Systemic Lupus Erythematosus. Best. Pract. Res. Clin. Rheumatol. 2018, 31, 351–363. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Li, J.; Tang, H.; Shang, Z.; Chen, R.; Meng, X.; Cheng, X.; Song, Z.; Li, S.; Zhang, R.; Lv, W. Identifying functional subtypes and common mechanisms of rheumatoid arthritis and systemic lupus erythematosus. Genes Dis. 2025, 101527. [Google Scholar] [CrossRef]
- Miller, F.W. The increasing prevalence of autoimmunity and autoimmune diseases: An urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 2023, 80, 102266. [Google Scholar] [CrossRef]
- Anaya, J.-M.; Beltrán, S. The autoimmune tautology revisited. J. Transl. Autoimmun. 2023, 7, 100204. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Kemp, A.S.; Ponsonby, A.L. Gene-environment interaction in autoimmune disease. Expert Rev. Mol. Med. 2014, 16, e4. [Google Scholar] [CrossRef]
- Arriens, C.; Mohan, C. Systemic lupus erythematosus diagnostics in the ‘omics’ era. Int. J. Clin. Rheumatol. 2013, 8, 671–687. [Google Scholar] [CrossRef]
- Anaya, J.M.; Restrepo-Jiménez, P.; Ramírez-Santana, C. The autoimmune ecology: An update. Curr. Opin. Rheumatol. 2018, 30, 350–360. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Priori, R.; Scrivo, R.; Brandt, J.; Valerio, M.; Casadei, L.; Valesini, G.; Manetti, C. Metabolomics in rheumatic diseases: The potential of an emerging methodology for improved patient diagnosis, prognosis, and treatment efficacy. Autoimmun. Rev. 2013, 12, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Luan, H.H.; Medzhitov, R. An evolutionary perspective on immunometabolism. Science 2019, 363, 3932. [Google Scholar] [CrossRef]
- Huang, N.; Perl, A. Metabolism as a Target for Modulation in Autoimmune Diseases. Trends Immunol. 2018, 39, 562–576. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Morel, L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2017, 13, 280–290. [Google Scholar] [CrossRef]
- Fernández-Ávila, D.G.; Rincón-Riaño, D.N.; Bernal-Macías, S.; Gutiérrez Dávila, J.M.; Rosselli, D. Prevalencia de la artritis reumatoide en Colombia según información del Sistema Integral de Información de la Protección Social. Rev. Colomb. Reumatol. 2019, 26, 83–87. [Google Scholar] [CrossRef]
- Fernández-Ávila, D.G.; Bernal-Macías, S.; Rincón-Riaño, D.N.; Gutiérrez Dávila, J.M.; Rosselli, D. Prevalence of systemic lupus erythematosus in Colombia: Data from the national health registry 2012–2016. Lupus 2019, 28, 1273–1278. [Google Scholar] [CrossRef]
- Londoño, J.; Ballestas, I.P.; Cuervo, F.; Angarita, I.; Giraldo, R.; Rueda, J.C.; Ballesteros, J.G.; Baquero, R.; Forero, E.; Cardiel, M.; et al. Prevalencia de la enfermedad reumática en Colombia, según estrategia COPCORD-Asociación Colombiana de Reumatología. Estudio de prevalencia de enfermedad reumática en población colombiana mayor de 18 años. Rev. Colomb. De Reumatol. 2018, 5, 245–256. [Google Scholar] [CrossRef]
- Sahin, D.; Di Matteo, A.; Emery, P. Biomarkers in the diagnosis, prognosis and management of rheumatoid arthritis: A comprehensive review. Ann. Clin. Biochem. Int. J. Lab. Med. 2024. [Google Scholar] [CrossRef]
- Sandhu, V.; Quan, M. SLE and Serum Complement: Causative, Concomitant or Coincidental? Open Rheumatol. J. 2017, 11, 113–122. [Google Scholar] [CrossRef]
- Arriens, C.; Wren, J.D.; Munroe, M.E.; Mohan, C. Systemic lupus erythematosus biomarkers: The challenging quest. Rheumatology 2017, 56, i32–i45. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Tiziani, S.; Firestein, G.S. Metabolomics in rheumatic diseases: Desperately seeking biomarkers. Nat. Rev. Rheumatol. 2016, 12, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Delgado, N.P.; Cala, M.P.; Barreto, A.; Rodríguez, C.L.S. Metabolites and metabolic pathways associated with rheumatoid arthritis and systemic lupus erythematosus. J. Transl. Autoimmun. 2022, 5, 100150. [Google Scholar] [CrossRef]
- Li, S.; Todor, A.; Luo, R. Blood transcriptomics and metabolomics for personalized medicine. Comput. Struct. Biotechnol. J. 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Szabó, M.Z.; Szodoray, P.; Kiss, E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 2017, 65, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, S.; Han, L.; Ba, X.; Shen, P.; Lin, W.; Li, T.; Zhang, R.; Huang, Y.; Huang, Y.; et al. Dyslipidemia in rheumatoid arthritis: The possible mechanisms. Front. Immunol. 2023, 14, 1254753. [Google Scholar] [CrossRef]
- Cui, W.; Liu, D.; Gu, W.; Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef]
- Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and sphingolipid enriched lipid rafts as therapeutic targets in cancer. Int. J. Mol. Sci. 2021, 22, 726. [Google Scholar] [CrossRef]
- Harden, O.C.; Hammad, S.M. Sphingolipids and Diagnosis, Prognosis, and Organ Damage in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 586737. [Google Scholar] [CrossRef]
- Zhang, B.H.; Yin, F.; Qiao, Y.N.; Guo, S.D. Triglyceride and Triglyceride-Rich Lipoproteins in Atherosclerosis. Front. Mol. Biosci. 2022, 9, 909151. [Google Scholar] [CrossRef]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: A review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Duarte-Delgado, N.P.; Segura, K.; Gómez, O.; Pulido, S.; Tovar-Sánchez, C.; Bello-Gualtero, J.M.; Fernández-Ávila, D.G.; Amado-Garzón, S.B.; Romero-Sanchez, C.; Cacciatore, S.; et al. Cytokine profiles and their correlation with clinical and blood parameters in rheumatoid arthritis and systemic lupus erythematosus. Sci. Rep. 2024, 14, 23475. [Google Scholar] [CrossRef]
- Zhou, B.; Xia, Y.; She, J. Dysregulated serum lipid profile and its correlation to disease activity in young female adults diagnosed with systemic lupus erythematosus: A cross-sectional study. Lipids Health Dis. 2020, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; López-Mejías, R.; Alonso-Castro, S.; Abal, F.; Ballina-García, F.J.; González-Gay, M.Á.; Suárez, A. High triglycerides and low high-density lipoprotein cholesterol lipid profile in rheumatoid arthritis: A potential link among inflammation, oxidative status, and dysfunctional high-density lipoprotein. J. Clin. Lipidol. 2017, 11, 1043–1054.e2. [Google Scholar] [CrossRef] [PubMed]
- Cas, M.D.; Roda, G.; Li, F.; Secundo, F. Functional lipids in autoimmune inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 3074. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Fang, L.; Mundra, P.A.; Fan, F.; Galvin, A.; Weir, J.M.; Wong, G.; Chin-Dusting, J.; Cicuttini, F.; Meikle, P.; Dart, A.M. Plasma lipidomic profiling in patients with rheumatoid arthritis. Metabolomics 2016, 12, 136. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, J.; Yang, S.; Li, H.; Wang, C.; Fang, X.; Fan, Y.; Zhang, J.; Han, X.; Wen, C. Oxidative stress leads to reduction of plasmalogen serving as a novel biomarker for systemic lupus erythematosus. Free Radic. Biol. Med. 2016, 101, 475–481. [Google Scholar] [CrossRef]
- Robinson, G.; Pineda-Torra, I.; Ciurtin, C.; Jury, E.C. Lipid metabolism in autoimmune rheumatic disease: Implications for modern and conventional therapies. J. Clin. Investig. 2022, 132, e148552. [Google Scholar] [CrossRef]
- Lei, Q.; Yang, J.; Li, L.; Zhao, N.; Lu, C.; Lu, A.; He, X. Lipid metabolism and rheumatoid arthritis. Front. Immunol. 2023, 14, 1190607. [Google Scholar] [CrossRef]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative stress and lipid mediators modulate immune cell functions in autoimmune diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef] [PubMed]
- Frommer, K.W.; Schäffler, A.; Rehart, S.; Lehr, A.; Müller-Ladner, U.; Neumann, E. Free fatty acids: Potential proinflammatory mediators in rheumatic diseases. Ann. Rheum. Dis. 2015, 74, 303–310. [Google Scholar] [CrossRef]
- Masuoka, S.; Nishio, J.; Yamada, S.; Saito, K.; Kaneko, K.; Kaburaki, M.; Tanaka, N.; Sato, H.; Muraoka, S.; Kawazoe, M.; et al. Relationship Between the Lipidome Profile and Disease Activity in Patients with Rheumatoid Arthritis. Inflammation 2024, 47, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Varshney, S.; Gandha, S.; Maurya, A.; Mittal, P.; Jangra, S.; Garg, R.; Saraf, A. Association of cardiovascular risks in rheumatoid arthritis patients: Management, treatment and future perspectives. Health Sci. Rev. 2023, 8, 100108. [Google Scholar] [CrossRef]
- Ferreira, H.B.; Melo, T.; Paiva, A.; Domingues, M.D.R. Insights in the role of lipids, oxidative stress and inflammation in rheumatoid arthritis unveiled by new trends in lipidomic investigations. Antioxidants 2021, 10, 45. [Google Scholar] [CrossRef]
- Sutter, I.; Velagapudi, S.; Othman, A.; Riwanto, M.; Manz, J.; Rohrer, L.; Rentsch, K.; Hornemann, T.; Landmesser, U.; von Eckardstein, A. Plasmalogens of high-density lipoproteins (HDL) are associated with coronary artery disease and anti-apoptotic activity of HDL. Atherosclerosis 2015, 241, 539–546. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, 5–9. [Google Scholar] [CrossRef]
- Prevoo, M.L.L.; Van’T Hof, M.A.; Kuper, H.H.; Van Leeuwen, M.A.; Van De Putte, L.B.A.; Van Riel, P.L.C.M. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and Validation of Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. Comm. Progn. Stud. SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Kind, T.; Cajka, T.; Hazen, S.L.; Tang, W.W.; Kaddurah-Daouk, R.; Irvin, M.R.; Arnett, D.K.; Barupal, D.K.; Fiehn, O. Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data. Anal. Chem. 2019, 91, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Ogle, D.; Doll, J.; Wheeler, A.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods, R package version 0.9.5, 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 14 April 2025).
- Cacciatore, S.; Tenori, L.; Luchinat, C.; Bennett, P.R.; MacIntyre, D.A. KODAMA: An R package for knowledge discovery and data mining. Bioinformatics 2017, 33, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Abdel-Shafy, E.A.; Melak, T.; MacIntyre, D.A.; Zadra, G.; Zerbini, L.F.; Piazza, S.; Cacciatore, S. MetChem: A new pipeline to explore structural similarity across metabolite modules. Bioinform. Adv. 2023, 3, vbad053. [Google Scholar] [CrossRef]
- Cacciatore, S.; Luchinat, C.; Tenori, L. Knowledge discovery by accuracy maximization. Proc. Natl. Acad. Sci. USA 2014, 111, 5117–5122. [Google Scholar] [CrossRef]


| Feature | HC | RA | SLE | p-Value |
|---|---|---|---|---|
| age, median [IQR] (c) | 40 [31–49.5] | 43 [39.5–49] | 35 [25–40.75] | 0.042 |
| BMI, median [IQR] | 24.5 [22.3–26.5] | 25.1 [23.1–26.7] | 25.1 [23.4–26.7] | 0.771 |
| familiar antecedent of autoimmunity | 0.000546 | |||
| no, n (%) | 27 (100.0) | 14 (60.9) | 17 (77.3) | |
| yes, n (%) | 0 (0.0) | 9 (39.1) | 5 (22.7) | |
| diagnostic time, median [IQR] | - | 4.5 [3.0–9.0] | 5.8 [3.8–11.3] | 0.22 |
| SLEDAI score, median [IQR] | 6 [4–6] | |||
| DAS28-ESR score, median [IQR] | 3.6 [3.4–3.8] | |||
| renal involvement in SLE | 1 | |||
| no, n (%) | - | - | 12 (54.5) | |
| yes, n (%) | - | - | 10 (45.5) | |
| HDL, median [IQR] (b) | 52.5 [45–60.2] | 48 [37.5–56.5] | 45.5 [37.5–49.5] | 0.017 |
| LDL, median [IQR] (b) | 123.6 [100.9–145.9] | 109 [86.1–120.1] | 93.5 [72.9–116.9] | 0.022 |
| total cholesterol, median [IQR] (b) | 202.4 [179.5–237.5] | 172.9 [156.5–209.1] | 164.4 [144.6–189.3] | 0.009 |
| triglycerides, median [IQR] | 106.1 [88.6–144.7] | 129.4 [89.2–166.8] | 122.7 [86.8–171.2] | 0.773 |
| glucose, median [IQR] | 86 [83.2–91] | 83 [76.5–92] | 82.5 [76–92.7] | 0.273 |
| HbA1c, median [IQR] | 5.15 [5–5.4] | 5.2 [5–5.315] | 5.2 [5–5.4] | 0.982 |
| hematocrit, median [IQR] (a) | 43.7 [41.7–44.7] | 40.6 [38.9–43.2] | 41.7 [38.4–43.7] | 0.027 |
| hemoglobin, median [IQR] (a) | 14.6 [14.1–15.1] | 13.6 [12.8–14.5] | 13.85 [12.7–14.6] | 0.008 |
| ESR, median [IQR] (a) | 7 [5.3–10] | 13 [6.5–19.5] | 9.5 [6–16.2] | 0.040 |
| platelets, median [IQR] | 273,500 [233,400–314,175] | 289,500 [255,150–381,550] | 312,950 [272,250–326,025] | 0.259 |
| leukocytes, median [IQR] | 5900 [5025–6450] | 6800 [5450–7850] | 5600 [4675–6275] | 0.152 |
| lymphocytes, median [IQR] (b) | 1900 [1800–2175] | 1700 [1250–2000] | 1450 [1125–1775] | 0.010 |
| monocytes, median [IQR] | 400 [300–500] | 500 [400–550] | 400 [300–500] | 0.164 |
| neutrophils, median [IQR] (a) | 3300 [2650–3800] | 4400 [3300–5000] | 3700 [3100–4075] | 0.016 |
| CRP, median [IQR] | 0.26 [0.1–0.4] | 0.34 [0.12–1.17] | 0.31 [0.16–0.7] | 0.346 |
| RF, median [IQR] | 102 [32–138.75] | |||
| ACPA, median [IQR] | 327 [173.75–579] | |||
| anti-dsDNA, n(%) | 6 (28.6) | |||
| C3, median [IQR] | 97.1 [81–109] | |||
| C4, median [IQR] | 20.9 [11.8–24.1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte-Delgado, N.P.; Bello-Gualtero, J.M.; Fernández-Ávila, D.G.; Romero-Sánchez, C.; Cacciatore, S.; Cala, M.P.; Rodríguez Camacho, L.-S. Metabolic Alterations in Colombian Women with Rheumatoid Arthritis and Systemic Lupus Erythematosus Reveal Potential Lipid Biomarkers Associated with Inflammation and Cardiovascular Risk. Int. J. Mol. Sci. 2025, 26, 4527. https://doi.org/10.3390/ijms26104527
Duarte-Delgado NP, Bello-Gualtero JM, Fernández-Ávila DG, Romero-Sánchez C, Cacciatore S, Cala MP, Rodríguez Camacho L-S. Metabolic Alterations in Colombian Women with Rheumatoid Arthritis and Systemic Lupus Erythematosus Reveal Potential Lipid Biomarkers Associated with Inflammation and Cardiovascular Risk. International Journal of Molecular Sciences. 2025; 26(10):4527. https://doi.org/10.3390/ijms26104527
Chicago/Turabian StyleDuarte-Delgado, Nancy Paola, Juan Manuel Bello-Gualtero, Daniel G. Fernández-Ávila, Consuelo Romero-Sánchez, Stefano Cacciatore, Mónica P. Cala, and Luz-Stella Rodríguez Camacho. 2025. "Metabolic Alterations in Colombian Women with Rheumatoid Arthritis and Systemic Lupus Erythematosus Reveal Potential Lipid Biomarkers Associated with Inflammation and Cardiovascular Risk" International Journal of Molecular Sciences 26, no. 10: 4527. https://doi.org/10.3390/ijms26104527
APA StyleDuarte-Delgado, N. P., Bello-Gualtero, J. M., Fernández-Ávila, D. G., Romero-Sánchez, C., Cacciatore, S., Cala, M. P., & Rodríguez Camacho, L.-S. (2025). Metabolic Alterations in Colombian Women with Rheumatoid Arthritis and Systemic Lupus Erythematosus Reveal Potential Lipid Biomarkers Associated with Inflammation and Cardiovascular Risk. International Journal of Molecular Sciences, 26(10), 4527. https://doi.org/10.3390/ijms26104527






