LncRNA-Mediated Tissue-Specific Plastic Responses to Salinity Changes in Oysters
Abstract
:1. Introduction
2. Results
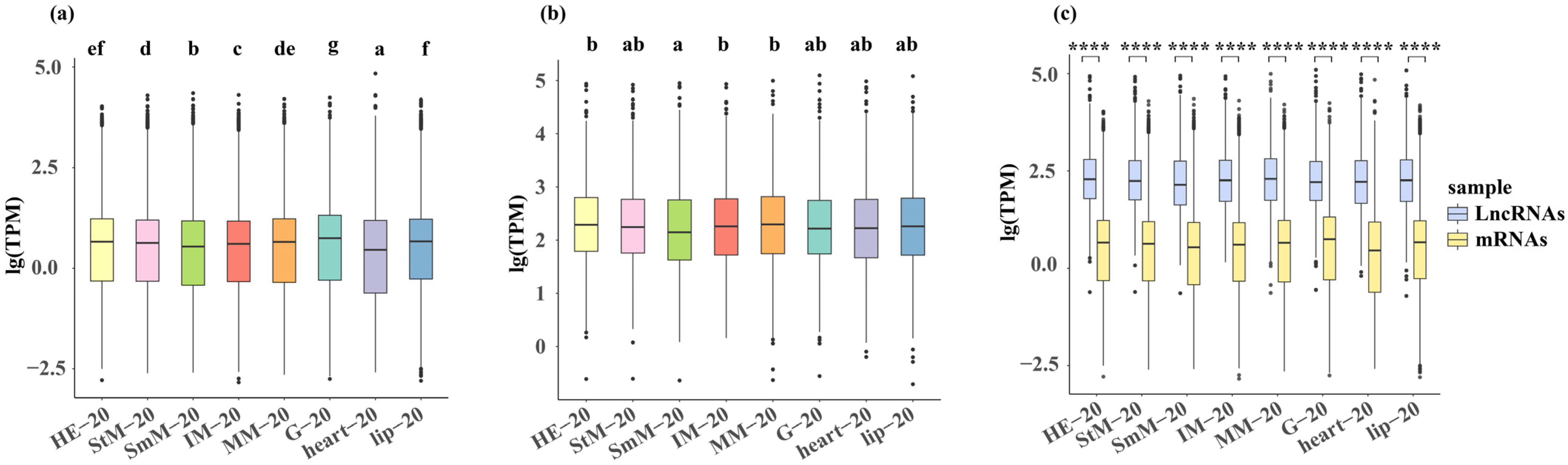
2.1. Differences in the Response to Salinity Among the Eight Tissues
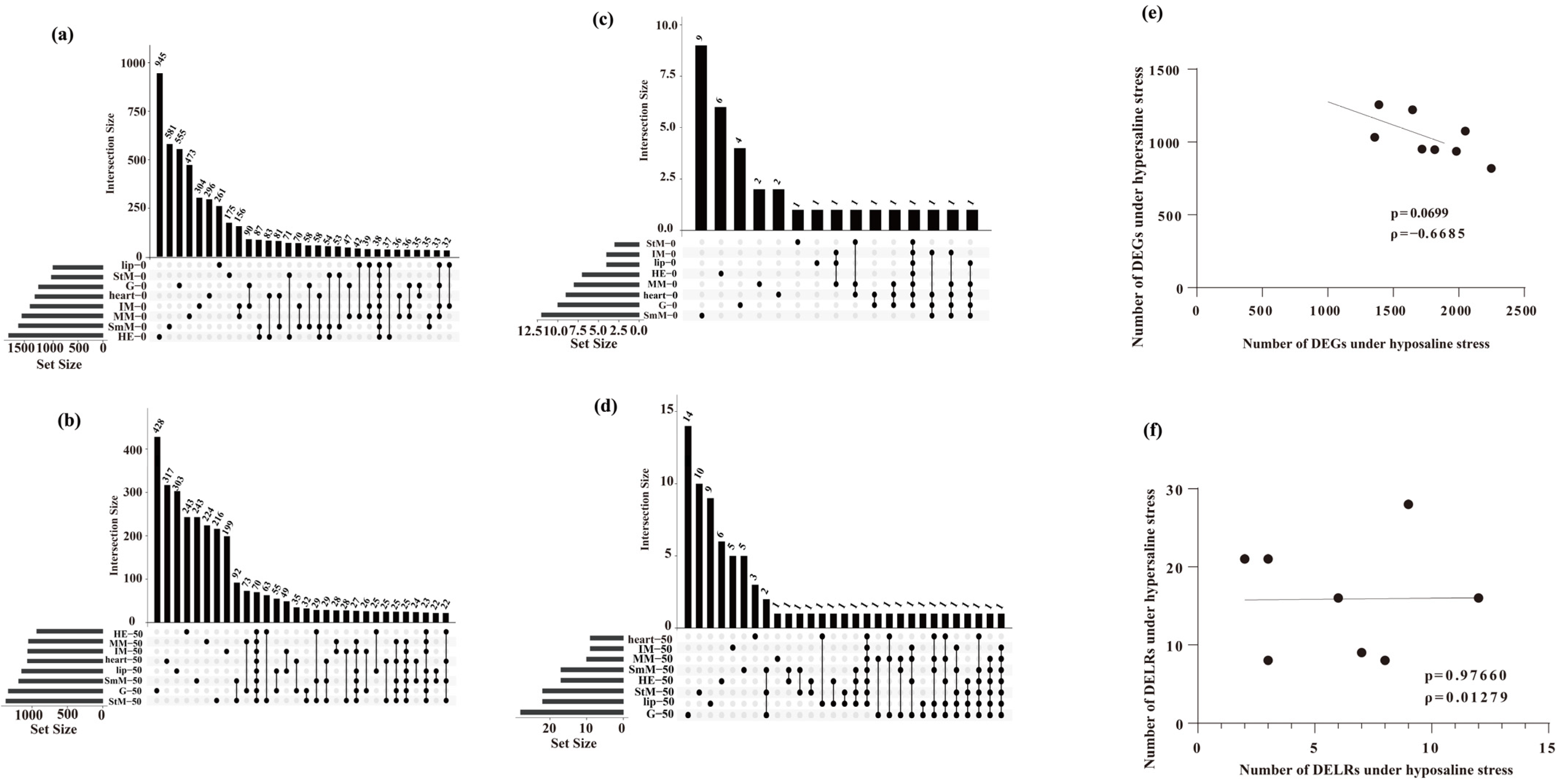
2.2. DEGs and DELRs in Response to Hyper- and Hypo-Saline Stresses
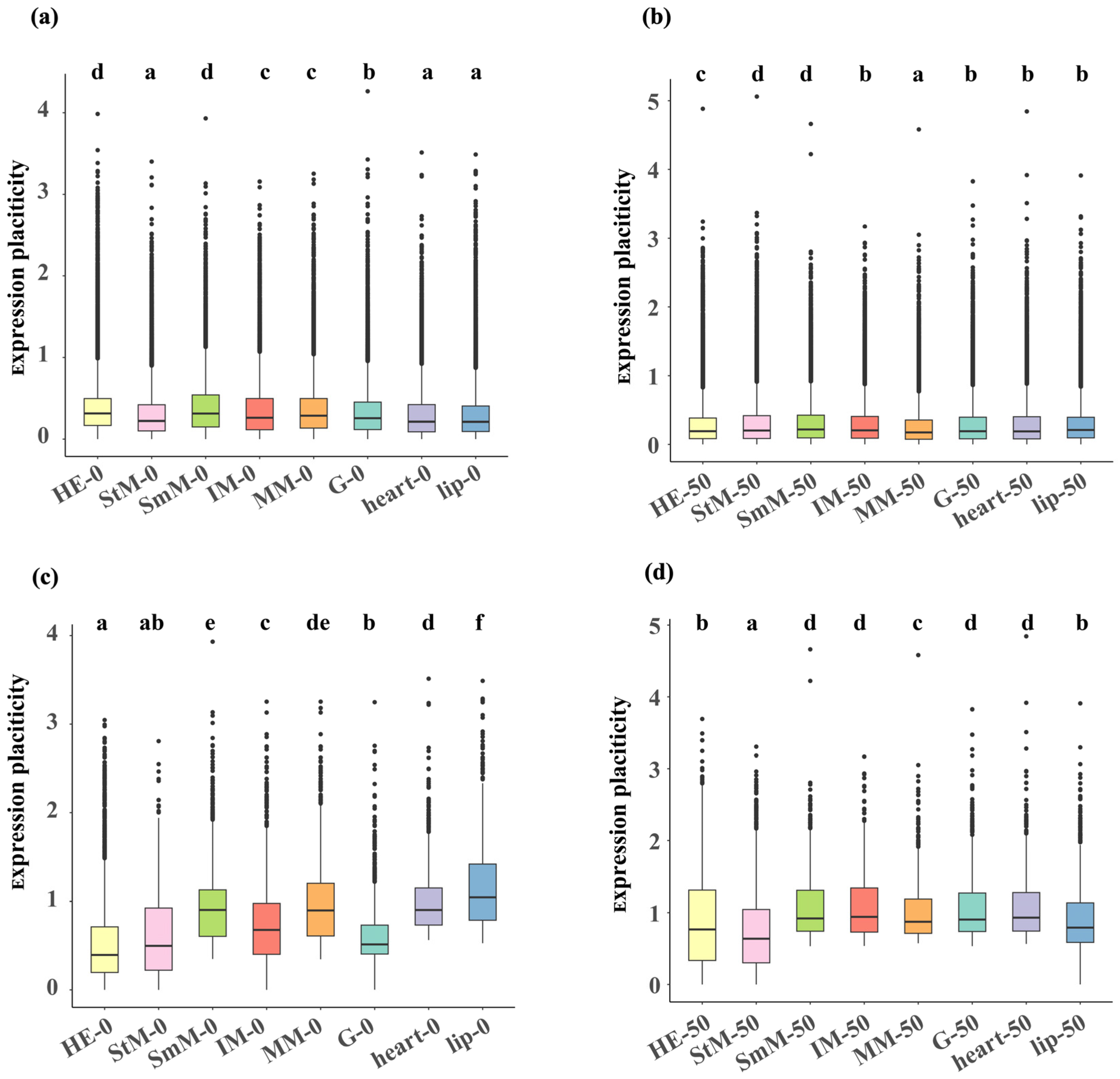
2.3. Transcriptional Changes in Genome-Wide Genes and DEGs
2.4. Expression Patterns of mRNAs and lncRNAs in HE and StM
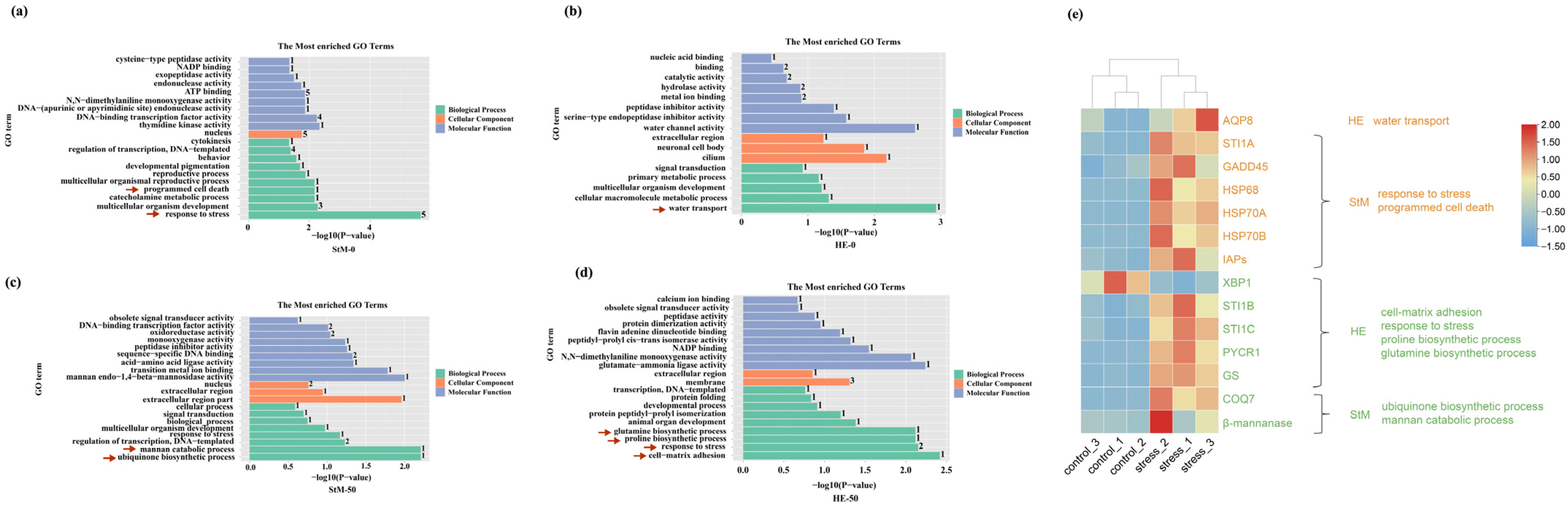
2.5. Biological Processes of HE and StM upon Hypo- and Hyper-Saline Conditions
3. Discussion
4. Materials and Methods
4.1. Oyster Samples
4.2. RNA-Sequencing
4.3. Identification of lncRNAs
4.4. Differential Expression Analyses and Cluster Analysis
4.5. Transcriptional Changes in Genes upon Salinity Stresses
4.6. Construction of lncRNA-mRNA Co-Expression Network and Functional Enrichment Analyses
4.7. High-Throughput Chromosome Conformation Capture (Hi-C) and Assay for Transposase-Accessible Chromatin Sequencing (ATAC-Seq)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLusky, D.S.; Hagerman, L.; Mitchell, P. Effect of salinity acclimation on osmoregulation in Crangon crangon and Praunus flexuosus. Ophelia 1982, 21, 89–100. [Google Scholar] [CrossRef]
- Gong, J.; Li, Q.; Yu, H.; Liu, S.; Kong, L. Effects of low salinity on hemolymph osmolality and transcriptome of the Iwagaki oyster, Crassostrea nippona. Fish Shellfish Immunol. 2022, 126, 211–216. [Google Scholar] [CrossRef]
- Johnson, C.R.; Banks, S.C.; Barrett, N.S.; Cazassus, F.; Dunstan, P.K.; Edgar, G.J.; Frusher, S.D.; Gardner, C.; Haddon, M.; Helidoniotis, F.; et al. Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J. Exp. Mar. Biol. Ecol. 2011, 400, 17–32. [Google Scholar] [CrossRef]
- Philippart, C.J.M.; Anadón, R.; Danovaro, R.; Dippner, J.W.; Drinkwater, K.F.; Hawkins, S.J.; Oguz, T.; O’Sullivan, G.; Reid, P.C. Impacts of climate change on European marine ecosystems: Observations, expectations and indicators. J. Exp. Mar. Biol. Ecol. 2011, 400, 52–69. [Google Scholar] [CrossRef]
- Gienapp, P.; Teplitsky, C.; Alho, J.S.; Mills, J.A.; Merilä, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef]
- Kenkel, C.D.; Matz, M.V. Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat. Ecol. Evol. 2017, 1, 14. [Google Scholar] [CrossRef]
- Li, L.; Li, A.; Song, K.; Meng, J.; Guo, X.; Li, S.; Li, C.; De Wit, P.; Que, H.; Wu, F.; et al. Divergence and plasticity shape adaptive potential of the Pacific oyster. Nat. Ecol. Evol. 2018, 2, 1751–1760. [Google Scholar] [CrossRef]
- Chevin, L.-M.; Lande, R.; Mace, G.M. Adaptation, Plasticity, and Extinction in a Changing Environment: Towards a Predictive Theory. PLoS Biol. 2010, 8, e1000357. [Google Scholar] [CrossRef]
- Li, A.; Zhao, M.; Zhang, Z.; Wang, C.; Zhang, K.; Zhang, X.; De Wit, P.R.; Wang, W.; Gao, J.; Guo, X.; et al. Genome architecture and selective signals compensatorily shape plastic response to a new environment. Innovation 2023, 4, 100464. [Google Scholar] [CrossRef]
- Li, A.; Li, L.; Zhang, Z.; Li, S.; Wang, W.; Guo, X.; Zhang, G.; Saitou, N. Noncoding variation and transcriptional plasticity promote thermal adaptation in oysters by altering energy metabolism. Mol. Biol. Evol. 2021, 38, 5144–5155. [Google Scholar] [CrossRef]
- Li, A.; Dai, H.; Guo, X.; Zhang, Z.; Zhang, K.; Wang, C.; Wang, X.; Wang, W.; Chen, H.; Li, X.; et al. Genome of the estuarine oyster provides insights into climate impact and adaptive plasticity. Commun. Biol. 2021, 4, 1287. [Google Scholar] [CrossRef]
- Fischer, E.K.; Ghalambor, C.K.; Hoke, K.L. Can a network approach resolve how adaptive vs nonadaptive plasticity impacts evolutionary trajectories? Integr. Comp. Biol. 2016, 56, 877–888. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Wund, M.A.; Snell-Rood, E.C.; Cruickshank, T.; Schlichting, C.D.; Moczek, A.P. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 2010, 25, 459–467. [Google Scholar] [CrossRef]
- Wheaton, F.; Hall, S. Research needs for automated oyster shucking. Aquac. Eng. 2007, 37, 67–72. [Google Scholar] [CrossRef]
- Zhang, K.; Li, A.; Qi, H.; Yang, Q.; Du, M.; Wang, X.; Zhang, Z.; Wang, C.; Wang, W.; Zhang, G.; et al. The development of a 30 K SNP genotyping tool targeting genomic regions of temperature and salinity adaptation in estuarine oyster. Aquaculture 2023, 566, 739168. [Google Scholar] [CrossRef]
- She, Z.; Li, L.; Meng, J.; Jia, Z.; Que, H.; Zhang, G. Population resequencing reveals candidate genes associated with salinity adaptation of the Pacific oyster Crassostrea gigas. Sci. Rep. 2018, 8, 8683. [Google Scholar] [CrossRef] [PubMed]
- Dineshram, R.; Chandramouli, K.; Ko, G.W.; Zhang, H.; Qian, P.Y.; Ravasi, T.; Thiyagarajan, V. Quantitative analysis of oyster larval proteome provides new insights into the effects of multiple climate change stressors. Glob. Change Biol. 2016, 22, 2054–2068. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Q.; Du, M.; Zhang, Z.; Wang, W.; Zhang, G.; Li, A.; Li, L. Genome-wide mapping of regulatory variants for temperature- and salinity-adaptive genes reveals genetic basis of genotype-by-environment interaction in Crassostrea ariakensis. Environ. Res. 2023, 236, 116614. [Google Scholar] [CrossRef]
- Pérez–Velasco, R.; Manzano–Sarabia, M.; Hurtado–Oliva, M.Á. Effect of hypo–and hypersaline stress conditions on physiological, metabolic, and immune responses in the oyster Crassostrea corteziensis (Bivalvia: Ostreidae). Fish Shellfish Immunol. 2022, 120, 252–260. [Google Scholar] [CrossRef]
- Zhao, M.; Li, A.; Zhang, K.; Wang, W.; Zhang, G.; Li, L. The role of the balance between energy production and ammonia detoxification mediated by key amino acids in divergent hypersaline adaptation among Crassostrea oysters. Environ. Res. 2024, 248, 118213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, A.; She, Z.; Wang, X.; Jia, Z.; Wang, W.; Zhang, G.; Li, L. Adaptive divergence and underlying mechanisms in response to salinity gradients between two Crassostrea oysters revealed by phenotypic and transcriptomic analyses. Evol. Appl. 2023, 16, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, L.; Li, A.; Li, Y.; Wang, W.; Zhang, G. Transcriptome and gene coexpression network analyses of two wild populations provides insight into the high-salinity adaptation mechanisms of Crassostrea ariakensis. Mar. Biotechnol. 2019, 21, 596–612. [Google Scholar] [CrossRef]
- Meng, J.; Zhu, Q.; Zhang, L.; Li, C.; Li, L.; She, Z.; Huang, B.; Zhang, G. Genome and transcriptome analyses provide insight into the euryhaline adaptation mechanism of Crassostrea gigas. PLoS ONE 2013, 8, e58563. [Google Scholar] [CrossRef] [PubMed]
- Eierman, L.E.; Hare, M.P. Reef-specific patterns of gene expression plasticity in eastern oysters (Crassostrea virginica). J. Hered. 2016, 107, 90–100. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, H.; Kong, L.; Liu, S.; Li, Q. Comparative transcriptome analysis of two oysters, Crassostrea gigas and Crassostrea hongkongensis provides insights into adaptation to hypo-osmotic conditions. PLoS ONE 2014, 9, e111915. [Google Scholar] [CrossRef]
- Pourmozaffar, S.; Tamadoni Jahromi, S.; Rameshi, H.; Sadeghi, A.; Bagheri, T.; Behzadi, S.; Gozari, M.; Zahedi, M.R.; Abrari Lazarjani, S. The role of salinity in physiological responses of bivalves. Rev. Aquac. 2019, 12, 1548–1566. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Zhang, G.; Zhang, F. Classification of jinjiang oysters Crassostrea rivularis (Gould, 1861) from China, based on morphology and phylogenetic analysis. Aquaculture 2004, 242, 137–155. [Google Scholar] [CrossRef]
- Zhou, M.F.; Standish, K., Jr. A review of published work on Crassostrea ariakensis. J. Shellfish Res. 2003, 22, 1–20. [Google Scholar]
- Jiang, W.; Chen, L. Tissue specificity of gene expression evolves across mammal species. J. Comput. Biol. 2022, 29, 880–891. [Google Scholar] [CrossRef]
- Pan, B.; Wang, Y.; Li, D.; Wang, T.; Du, L. Tissue-specific distribution and bioaccumulation pattern of trace metals in fish species from the heavily sediment-laden Yellow River, China. J. Hazard. Mater. 2022, 425, 128050. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Zhang, Z.; Zhao, X.; Geng, H.; Xue, L.; Liu, D. Spatial distribution and enrichment dynamics of foodborne norovirus in oyster tissues. Foods 2023, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Barshir, R.; Shwartz, O.; Smoly, I.Y.; Yeger-Lotem, E. Comparative analysis of human tissue interactomes reveals factors leading to tissue-specific manifestation of hereditary diseases. PLoS Comput. Biol. 2014, 10, e1003632. [Google Scholar] [CrossRef]
- Gibcus, J.H.; Dekker, J. The hierarchy of the 3D genome. Mol. Cell 2013, 49, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Hamba, Y.; Kamatani, T.; Miya, F.; Boroevich, K.A.; Tsunoda, T. Topologically associating domain underlies tissue specific expression of long intergenic non-coding RNAs. iScience 2023, 26, 106640. [Google Scholar] [CrossRef]
- Marques, A.C.; Hughes, J.; Graham, B.; Kowalczyk, M.S.; Higgs, D.R.; Ponting, C.P. Chromatin signatures at transcriptional start sites separate two equally populated yet distinct classes of intergenic long noncoding RNAs. Genome Biol. 2013, 14, R131. [Google Scholar] [CrossRef]
- Mantica, F.; Iniguez, L.P.; Marquez, Y.; Permanyer, J.; Torres-Mendez, A.; Cruz, J.; Franch-Marro, X.; Tulenko, F.; Burguera, D.; Bertrand, S.; et al. Evolution of tissue-specific expression of ancestral genes across vertebrates and insects. Nat. Ecol. Evol. 2024, 8, 1140–1153. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Zou, C.; Yang, C.; Pan, G.; Ma, L.; Shen, Y. Integrated analysis of long non-coding RNAs and mRNAs reveals the regulatory network of maize seedling root responding to salt stress. BMC Genom. 2022, 23, 50. [Google Scholar] [CrossRef]
- Baruah, P.M.; Krishnatreya, D.B.; Bordoloi, K.S.; Gill, S.S.; Agarwala, N. Genome wide identification and characterization of abiotic stress responsive lncRNAs in Capsicum annuum. Plant Physiol. Biochem. 2021, 162, 221–236. [Google Scholar] [CrossRef]
- de Goede, O.M.; Nachun, D.C.; Ferraro, N.M.; Gloudemans, M.J.; Rao, A.S.; Smail, C.; Eulalio, T.Y.; Aguet, F.; Ng, B.; Xu, J.; et al. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell 2021, 184, 2633–2648.e19. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Author correction: Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, J.A.-O.; Terai, G.; Hamada, M. Computational prediction of lncRNA-mRNA interactionsby integrating tissue specificity in human transcriptome. Biol. Direct 2017, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Flower, C.T.; Chen, L.; Jung, H.J.; Raghuram, V.; Knepper, M.A.; Yang, C.R. An integrative proteogenomics approach reveals peptides encoded by annotated lincRNA in the mouse kidney inner medulla. Physiol. Genom. 2020, 52, 485–491. [Google Scholar] [CrossRef]
- Much, C.; Lasda, E.L.; Pereira, I.T.; Vallery, T.K.; Ramirez, D.; Lewandowski, J.P.; Dowell, R.D.; Smallegan, M.J.; Rinn, J.L. The temporal dynamics of lncRNA Firre-mediated epigenetic and transcriptional regulation. Nat. Commun. 2024, 15, 6821. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, F.; Wang, H.; Teschendorff, A.E.; Xie, F.; He, Y. Pan-cancer characterization of long non-coding RNA and DNA methylation mediated transcriptional dysregulation. eBioMedicine 2021, 68, 103399. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jin, S.; Lin, L.; Yang, Q.; Jiang, H. Changes in the long noncoding RNA expression profile in the development of the embryonic external ear after BMP5 gene mutation. J. Craniofac. Surg. 2023, 34, 1605–1609. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Zhong, Y.; Cai, M.; Gao, J.; Tan, C.; Han, X.; Guo, R.; Han, L. LncRNA H19 over-expression inhibited Th17 cell differentiation to relieve endometriosis through miR-342-3p/IER3 pathway. Cell Biosci. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, Q.; Liu, Y.L.; Xiang, Z.; Wang, Q.Q.; Yin, L.; Liu, S.L. LncRNA POU3F3 contributes to dacarbazine resistance of human melanoma through the MiR-650/MGMT axis. Front. Oncol. 2021, 11, 643613. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, Q.; Bing, J.; Songbuerbatu; Zheng, L.; Dorjee, T.; Liu, Q.; Zhou, Y.; Gao, F. Combined lncRNA and mRNA expression profiles identified the lncRNA-miRNA-mRNA modules regulating the cold stress response in Ammopiptanthus nanus. Int. J. Mol. Sci. 2023, 24, 6502. [Google Scholar] [CrossRef]
- Mirdar Mansuri, R.; Azizi, A.H.; Sadri, A.H.; Shobbar, Z.S. Long non-coding RNAs as the regulatory hubs in rice response to salt stress. Sci. Rep. 2022, 12, 21696. [Google Scholar] [CrossRef]
- Chen, L.; Shi, S.; Jiang, N.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Peng, X.; Xu, J.; Chen, Y.; Yu, Q.; et al. Genome-wide analysis of long non-coding RNAs affecting roots development at an early stage in the rice response to cadmium stress. BMC Genom. 2018, 19, 460. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Long, X.; Zhang, X.; Dong, X.; Wang, Z.; Jiang, H.; An, M.; Chen, J.; Gan, L. Identification and characterization of long non-coding RNAs in intestinal immune regulation of largemouth bass, Micropterus salmoides, under acute heat stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101132. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Qi, G. LncRNA-IRAR-mediated regulation of insulin receptor transcripts in Drosophila melanogaster during nutritional stress. Insect Mol. Biol. 2022, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, H.; Li, Q.; Liu, S. Transcription analysis for core networks of lncRNAs-mRNAs: Implication for potential role in sterility of Crassostrea gigas. Biology 2022, 11, 378. [Google Scholar] [CrossRef]
- Feng, D.; Li, Q.; Yu, H.; Kong, L.; Du, S. Transcriptional profiling of long non-coding RNAs in mantle of Crassostrea gigas and their association with shell pigmentation. Sci. Rep. 2018, 8, 1436. [Google Scholar] [CrossRef]
- Song, K. Genome-wide identification of long non-coding RNAs in Crassostrea gigas and their association with heat stress. Mar. Biotechnol. 2022, 24, 744–752. [Google Scholar] [CrossRef]
- Peng, M.; Cardoso, J.C.R.; Pearson, G.; Vm Canário, A.; Power, D.M. Core genes of biomineralization and cis-regulatory long non-coding RNA regulate shell growth in bivalves. J. Adv. Res. 2024, 64, 117–129. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, B.; Cai, W.; Yang, C.; Du, X. Identification of a long non-coding RNA (LncMSEN2) from pearl oyster and its potential roles in exoskeleton formation and LPS stimulation. Fish Shellfish Immunol. 2020, 103, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; He, Q.; Xie, B.; Xu, Z.; Wang, C.; Yang, C.; Liao, Y.; Zheng, Z. Long non-coding RNA LncMPEG1 responds to multiple environmental stressors by affecting biomineralization in pearl oyster Pinctada fucata martensii. Front. Mar. Sci. 2022, 9, 1014810. [Google Scholar] [CrossRef]
- Kurihara, M.; Otsuka, K.; Matsubara, S.; Shiraishi, A.; Satake, H.; Kimura, A.P. A Testis-Specific Long Non-Coding RNA, lncRNA-Tcam1, Regulates Immune-Related Genes in Mouse Male Germ Cells. Front. Endocrinol. 2017, 8, 229. [Google Scholar] [CrossRef]
- Gong, J.; Li, Q. Comparative transcriptome and WGCNA analysis reveal molecular responses to salinity change in larvae of the iwagaki oyster Crassostrea Nippona. Mar. Biotechnol. 2023, 25, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, C.; Wang, W.; Zhang, Z.; Liu, M.; She, Z.; Jia, Z.; Zhang, G.; Li, L. Molecular and fitness data reveal local adaptation of southern and northern estuarine oysters (Crassostrea ariakensis). Front. Mar. Sci. 2020, 7, 589099. [Google Scholar] [CrossRef]
- List, E.O.; Berryman, D.E.; Jensen, E.A.; Kulkarni, P.; McKenna, S.; Kopchick, J.J. New insights of growth hormone (GH) actions from tissue-specific GH receptor knockouts in mice. Arch. Endocrinol. Metab. 2019, 63, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Widman, N.; Feng, S.; Jacobsen, S.E.; Pellegrini, M. Epigenetic differences between shoots and roots in Arabidopsis reveals tissue-specific regulation. Epigenetics 2014, 9, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Modrek, B.; Lee, C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res. 2002, 30, 3754–3766. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nong, Q.; Solanki, M.K.; Liang, Q.; Xie, J.; Liu, X.; Li, Y.; Wang, W.; Yang, L.; Li, Y. Differential expression profiles and pathways of genes in sugarcane leaf at elongation stage in response to drought stress. Sci. Rep. 2016, 6, 25698. [Google Scholar] [CrossRef]
- Yao, H.; Liang, Z.; Wang, W.; Niu, C. Integrative analyses of transcriptomes and metabolomes provide insight into salinity adaption in Bangia (Rhodaphyta). Int. J. Biol. Macromol. 2023, 253, 127466. [Google Scholar] [CrossRef]
- Dupont-Prinet, A.; Chatain, B.; Grima, L.; Vandeputte, M.; Claireaux, G.; McKenzie, D.J. Physiological mechanisms underlying a trade-off between growth rate and tolerance of feed deprivation in the European sea bass (Dicentrarchus labrax). J. Exp. Biol. 2010, 213, 1143–1152. [Google Scholar] [CrossRef]
- Wang, C.; Li, A.; Wang, W.; Cong, R.; Wang, L.; Zhang, G.; Li, L. Integrated application of transcriptomics and metabolomics reveals the energy allocation-mediated mechanisms of growth-defense trade-offs in Crassostrea gigas and Crassostrea angulata. Front. Mar. Sci. 2021, 8, 744626. [Google Scholar] [CrossRef]
- Li, A.; Li, L.; Wang, W.; Song, K.; Zhang, G.F. Transcriptomics and fitness data reveal adaptive plasticity of thermal tolerance in oysters inhabiting different tidal zones. Front. Physiol. 2018, 9, 825. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, L.; Song, K.; Wang, W.; Zhang, G. Temperature, energy metabolism, and adaptive divergence in two oyster subspecies. Ecol. Evol. 2017, 7, 6151–6162. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, L.; Wang, W.; Zhang, G. Evolutionary trade-offs between baseline and plastic gene expression in two congeneric oyster species. Biol. Lett. 2019, 15, 20190202. [Google Scholar] [CrossRef] [PubMed]
- Murren, C.J.; Auld, J.R.; Callahan, H.; Ghalambor, C.K.; Handelsman, C.A.; Heskel, M.A.; Kingsolver, J.G.; Maclean, H.J.; Masel, J.; Maughan, H.; et al. Constraints on the evolution of phenotypic plasticity: Limits and costs of phenotype and plasticity. Heredity 2015, 115, 293–301. [Google Scholar] [CrossRef]
- DeWitt, T.J.; Sih, A.; Wilson, D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81. [Google Scholar] [CrossRef]
- Siljestam, M.; Östman, Ö. The combined effects of temporal autocorrelation and the costs of plasticity on the evolution of plasticity. J. Evol. Biol. 2017, 30, 1361–1371. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Li, Q. Physiological and gene expression responses of diploid and triploid Pacific oyster (Crassostrea gigas) to heat acclimation. Aquac. Res. 2022, 53, 6641–6650. [Google Scholar] [CrossRef]
- Ballantyne, J.S.; Berges, J.A. Enzyme activities of gill, hepatopancreas, mantle, and adductor muscle of the oyster (Crassostrea virginica) after changes in diet and salinity. Can. J. Fish. Aquat. Sci. 1991, 48, 1117–1123. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kawakami, K.; Morimoto, S. Changes in the free amino acid content of the shucked oyster Crassostrea gigas stored in salt water at 3 °C. Fish. Aquat. Sci. 2013, 16, 63–69. [Google Scholar] [CrossRef]
- Mendela, T.S.; Isaac, S.R.; Enzor, L.A. Impacts of elevated temperature, decreased salinity and microfibers on the bioenergetics and oxidative stress in eastern oyster, Crassostrea virginica. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 274, 111002. [Google Scholar] [CrossRef]
- Bresnahan, S.T.; Doke, M.A.; Giray, T.; Grozinger, C.M. Tissue-specific transcriptional patterns underlie seasonal phenotypes in honey bees (Apis mellifera). Mol. Ecol. 2022, 31, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Munro, T.; Mattick, J.S. The potential of long noncoding RNA therapies. Trends Pharmacol. Sci. 2022, 43, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Huanca-Mamani, W.; Arias-Carrasco, R.; Cardenas-Ninasivincha, S.; Rojas-Herrera, M.; Sepulveda-Hermosilla, G.; Caris-Maldonado, J.C.; Bastias, E.; Maracaja-Coutinho, V. Long non-coding RNAs responsive to salt and boron stress in the hyper-arid lluteno maize from Atacama Desert. Genes 2018, 9, 170. [Google Scholar] [CrossRef]
- Hu, W.; Wang, G.; Wang, S.; Nie, X.; Wang, C.; Wang, Y.; Zhang, H.; Ji, W. Co-regulation of long non-coding RNAs with allele-specific genes in wheat responding to powdery mildew infection. Agronomy 2020, 10, 896. [Google Scholar] [CrossRef]
- Long, Y.; Wang, X.; Youmans, D.T.; Cech, T.R. How do lncRNAs regulate transcription? Sci. Adv. 2017, 3, eaao2110. [Google Scholar] [CrossRef]
- Groff, A.F.; Barutcu, A.R.; Lewandowski, J.P.; Rinn, J.L. Enhancers in the peril lincRNA locus regulate distant but not local genes. Genome Biol. 2018, 19, 219. [Google Scholar] [CrossRef]
- Wang, C.; Li, A.; Cong, R.; Qi, H.; Wang, W.; Zhang, G.; Li, L.; Wittkopp, P. Cis- and trans-variations of stearoyl-CoA desaturase provide new insights into the mechanisms of diverged pattern of phenotypic plasticity for temperature adaptation in two congeneric oyster species. Mol. Biol. Evol. 2023, 40, msad015. [Google Scholar] [CrossRef]
- Sanford, E.; Kelly, M.W. Local adaptation in marine invertebrates. Annu. Rev. Mar. Sci. 2011, 3, 509–535. [Google Scholar] [CrossRef]
- Somero, G. The physiology of global change: Linking patterns to mechanisms. Annu. Rev. Mar. Sci. 2012, 4, 39–61. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Meng, J.; Qi, H.; Qu, T.; Xu, F.; Zhang, L. Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu. Rev. Anim. Biosci. 2016, 4, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, L. Proteomics to study adaptations in marine organisms to environmental stress. J. Proteom. 2014, 105, 92–106. [Google Scholar] [CrossRef]
- Burford, M.O.; Scarpa, J.; Cook, B.J.; Hare, M.P. Local adaptation of a marine invertebrate with a high dispersal potential: Evidence from a reciprocal transplant experiment of the eastern oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 2014, 505, 161–175. [Google Scholar] [CrossRef]
- Berger, V.J.; Kharazova, A.D. Mechanisms of salinity adaptations in marine molluscs. Hydrobiologia 1997, 355, 115–126. [Google Scholar] [CrossRef]
- Richardson, C.A.; Collis, S.A.; Ekaratne, K.; Dare, P.; Key, D. The age determination and growth rate of the European fat oyster, Ostrea edulis, in British waters determined from acetate peels ofumbo growth lines. ICES J. Mar. Sci. 1993, 50, 493–500. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth Gk Fau–Shi, W.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.A.-O. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Pertea, G.; Pertea, M.A.-O. GFF Utilities: GffRead and GffCompare. F1000 Res. 2024, 9, 304. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, J.; Zhou, Z. PLEK: A tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 2014, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, H.J.; Dasari, S.; Wang, S.; Kocher, J.P.; Li, W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef]
- Bateman, A.; Birney, E.; Durbin, R.; Eddy, S.R.; Howe, K.L.; Sonnhammer, E.L.L. The Pfam Protein Families Database. Nucleic Acids Res. 2000, 28, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenführer, J.; Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 2006, 22, 1600–1607. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]


| DELRs | DEGs | |
|---|---|---|
| HE-0 | 6 | 767 |
| HE-50 | 16 | 628 |
| StM-0 | 2 | 336 |
| StM-50 | 21 | 987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhao, J.; Li, A.; Zhao, M.; Huo, M.; Deng, J.; Wang, L.; Wang, W.; Zhang, G.; Li, L. LncRNA-Mediated Tissue-Specific Plastic Responses to Salinity Changes in Oysters. Int. J. Mol. Sci. 2025, 26, 4523. https://doi.org/10.3390/ijms26104523
Zhang M, Zhao J, Li A, Zhao M, Huo M, Deng J, Wang L, Wang W, Zhang G, Li L. LncRNA-Mediated Tissue-Specific Plastic Responses to Salinity Changes in Oysters. International Journal of Molecular Sciences. 2025; 26(10):4523. https://doi.org/10.3390/ijms26104523
Chicago/Turabian StyleZhang, Mengshi, Jinlong Zhao, Ao Li, Mingjie Zhao, Meitong Huo, Jinhe Deng, Luping Wang, Wei Wang, Guofan Zhang, and Li Li. 2025. "LncRNA-Mediated Tissue-Specific Plastic Responses to Salinity Changes in Oysters" International Journal of Molecular Sciences 26, no. 10: 4523. https://doi.org/10.3390/ijms26104523
APA StyleZhang, M., Zhao, J., Li, A., Zhao, M., Huo, M., Deng, J., Wang, L., Wang, W., Zhang, G., & Li, L. (2025). LncRNA-Mediated Tissue-Specific Plastic Responses to Salinity Changes in Oysters. International Journal of Molecular Sciences, 26(10), 4523. https://doi.org/10.3390/ijms26104523





