Glutathione Peroxidase and Selenoprotein P Evaluation in Well-Being Assessment After Total Thyroidectomy
Abstract
1. Introduction
- The antioxidant level, analyzing the relationship between SelP and GPx3 (primary endpoint).
- Quality of life (QoL), evaluated using a thyroid-specific QoL questionnaire (secondary endpoint).
- Thyroid hormones (secondary endpoint).
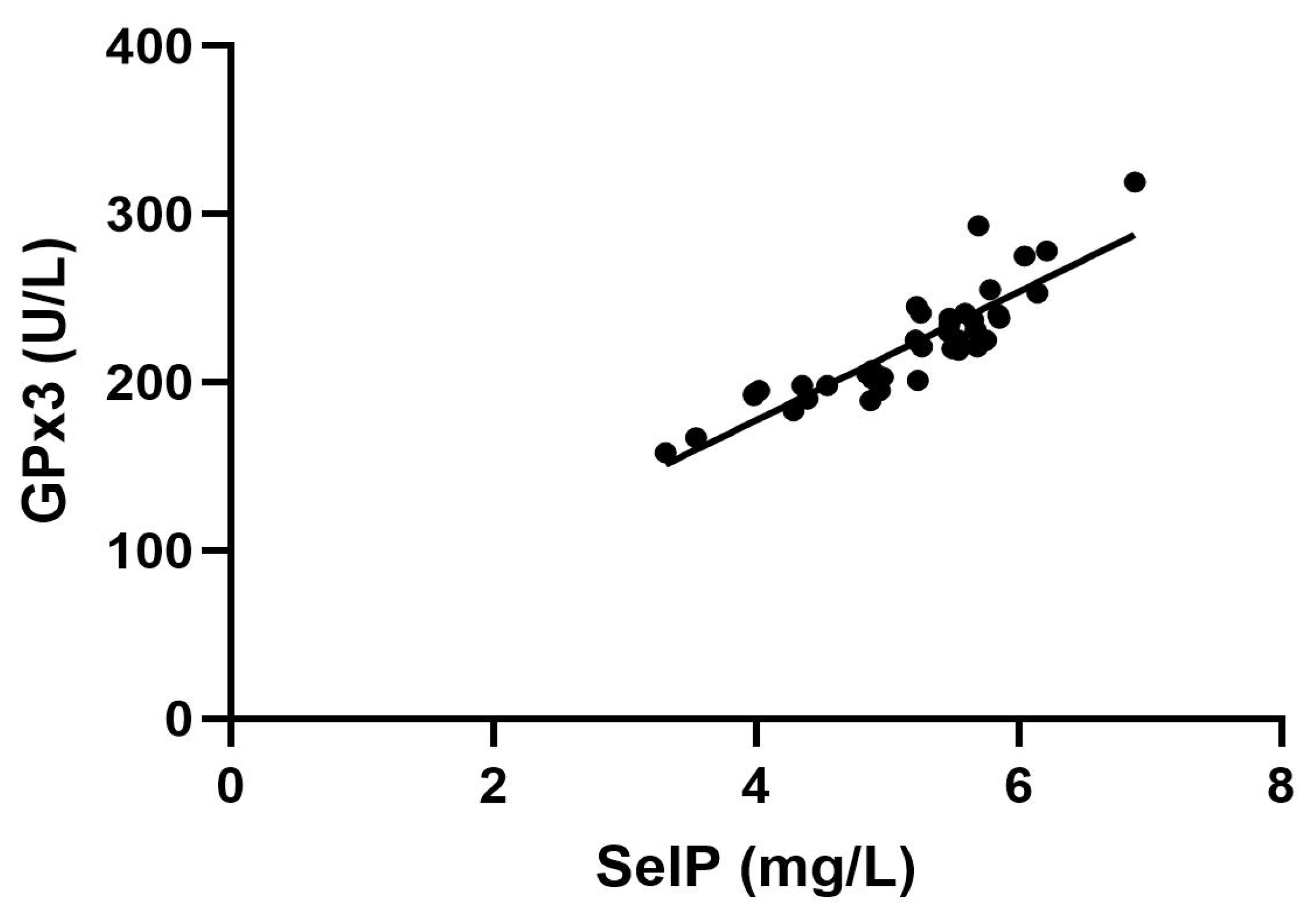
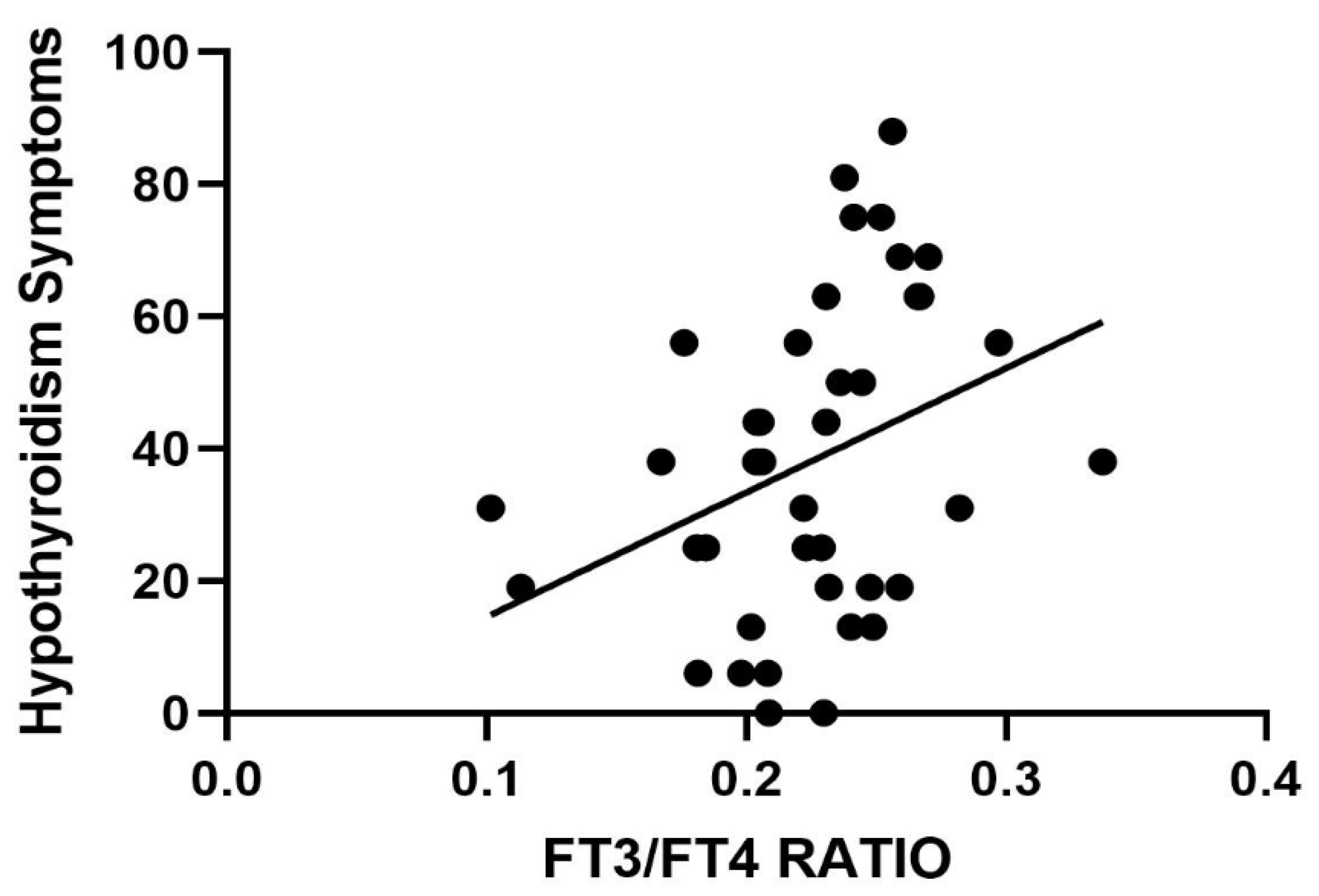
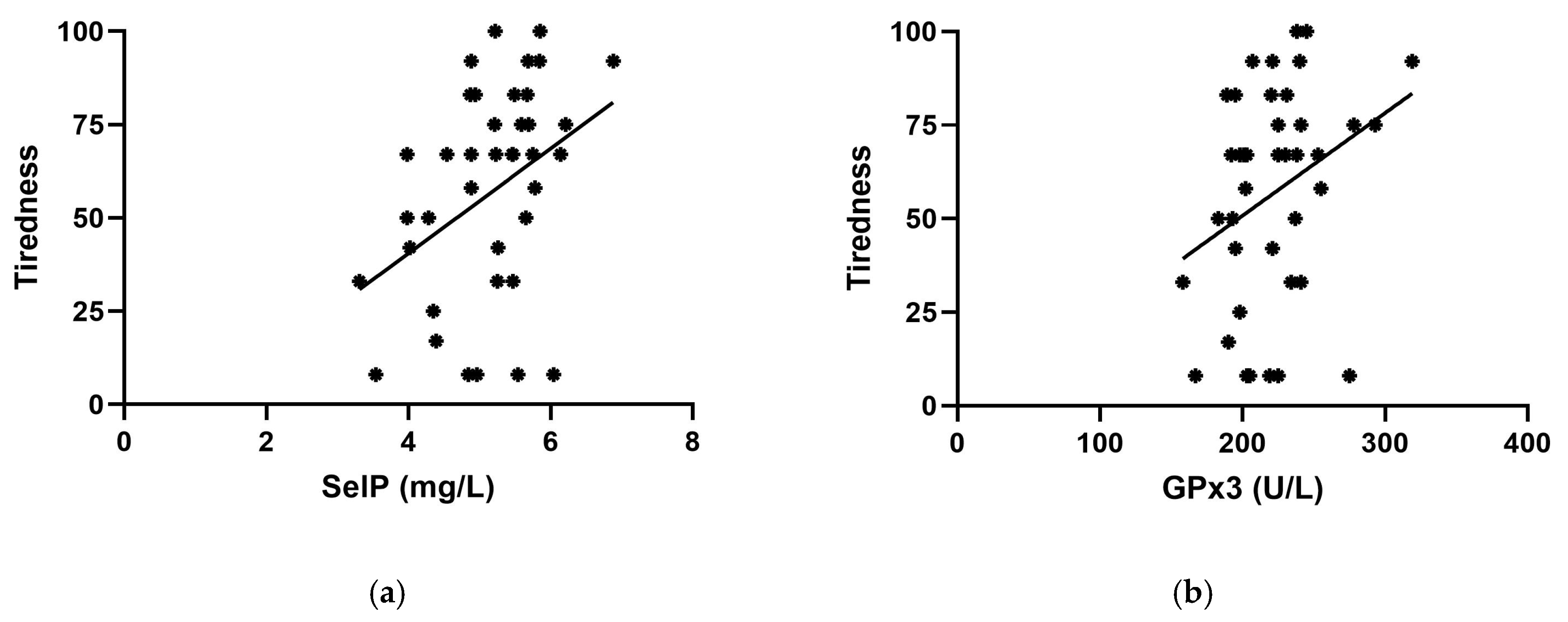
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Protocol and Population
4.2. Biochemical and Hormone Parameter Assays
4.3. Questionnaire Administration
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | catalase |
| fT4 | free thyroxine |
| fT3 | free triiodothyronine |
| GPx3 | glutathione peroxidase 3 |
| L-T4 | levothyroxine |
| OS | oxidative stress |
| QoL | quality of life |
| SelP | selenoprotein P |
| SOD | superoxide dismutase |
| TSH | thyroid stimulating hormone |
References
- Kim, B.W.; Bianco, A.C. For some, L-thyroxine replacement might not be enough: A genetic rationale. J. Clin. Endocrinol. Metab. 2009, 94, 1521–1523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peterson, S.J.; Cappola, A.R.; Castro, M.R.; Dayan, C.M.; Farwell, A.P.; Hennessey, J.V.; Kopp, P.A.; Ross, D.S.; Samuels, M.H.; Sawka, A.M.; et al. An Online Survey of Hypothyroid Patients Demonstrates Prominent Dissatisfaction. Thyroid 2018, 28, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.G.; Dentice, M.; Cantara, S.; Ambrosio, R.; Maino, F.; Porcelli, T.; Marzocchi, C.; Garbi, C.; Pacini, F.; Salvatore, D. DIO2 Thr92Ala Reduces Deiodinase-2 Activity and Serum-T3 Levels in Thyroid-Deficient Patients. J. Clin. Endocrinol. Metab. 2017, 102, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Balázs, C.; Rácz, K. The role of selenium in endocrine system diseases. Orvosi Hetilap 2013, 154, 1628–1635. [Google Scholar] [CrossRef]
- Silvestrini, A.; Mordente, A.; Martino, G.; Bruno, C.; Vergani, E.; Meucci, E.; Mancini, A. The Role of Selenium in Oxidative Stress and in Nonthyroidal Illness Syndrome (NTIS): An Overview. Curr. Med. Chem. 2020, 27, 423–449. [Google Scholar] [CrossRef]
- Nirgude, S.; Choudhary, B. Insights into the role of GPX3, a highly efficient plasma antioxidant, in cancer. Biochem. Pharmacol. 2021, 184, 114365. [Google Scholar] [CrossRef]
- Schomburg, L. Selenoprotein P—Selenium transport protein, enzyme and biomarker of selenium status. Free Radic. Biol. Med. 2022, 191, 150–163. [Google Scholar] [CrossRef]
- Watt, T.; Bjorner, J.B.; Groenvold, M.; Cramon, P.; Winther, K.H.; Hegedüs, L.; Bonnema, S.J.; Rasmussen, Å.K.; Ware, J.E., Jr.; Feldt-Rasmussen, U. Development of a Short Version of the Thyroid-Related Patient-Reported Outcome ThyPRO. Thyroid 2015, 25, 1069–1079. [Google Scholar] [CrossRef]
- Watt, T.; Bjorner, J.B.; Groenvold, M.; Rasmussen, A.K.; Bonnema, S.J.; Hegedus, L.; Feldt-Rasmussen, U. Establishing construct validity for the thyroid-specific patient-reported outcome measure (ThyPRO): An initial examination. Qual. Life Res. 2009, 18, 483–496. [Google Scholar] [CrossRef]
- Bottomley, A. The Cancer Patient and Quality of Life. Oncologist 2002, 7, 120–125. [Google Scholar] [CrossRef]
- Larsen, C.; Winther, K.H.; Cramon, P.K.; Rasmussen, Å.K.; Feldt-Rasmusssen, U.; Knudsen, N.J.; Bjorner, J.B.; Schomburg, L.; Demircan, K.; Chillon, T.S.; et al. Selenium supplementation and placebo are equally effective in improving quality of life in patients with hypothyroidism. Eur. Thyroid J. 2024, 13, e230175. [Google Scholar] [CrossRef]
- Pilli, T.; Cantara, S.; Schomburg, L.; Cenci, V.; Cardinale, S.; Heid, E.C.; Kühn, E.C.; Cevenini, G.; Sestini, F.; Fioravanti, C.; et al. IFNγ-Inducible Chemokines Decrease upon Selenomethionine Supplementation in Women with Euthyroid Autoimmune Thyroiditis: Comparison between Two Doses of Selenomethionine (80 or 160 μg) versus Placebo. Eur. Thyroid J. 2015, 4, 226–233. [Google Scholar] [CrossRef]
- Eskes, S.A.; Endert, E.; Fliers, E.; Birnie, E.; Hollenbach, B.; Schomburg, L.; Köhrle, J.; Wiersinga, W.M. Selenite supplementation in euthyroid subjects with thyroid peroxidase antibodies. Clin. Endocrinol. 2014, 80, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, G.; Schuetz, M.; Kontur, S.; Duan, H.; Kommata, S.; Schoen, R.; Antoni, A.; Kletter, K.; Dudczak, R.; Willheim, M. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid 2008, 18, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gartner, R.; Gasnier, B.C.; Dietrich, J.W.; Krebs, B.; Angstwurm, M.W. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J. Clin. Endocrinol. Met. 2002, 87, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.S.L.; Campos, R.O.; Braga, J.S.; Filho, J.D.S.; Ramos, H.E.; Anunciação, S.M.; Cassemiro, J.F.; Rende, P.R.F.; Hecht, F. Selenium nutritional status and thyroid dysfunction. Arch Endocrinol Metab. 2025, 69, e230348. [Google Scholar] [CrossRef]
- Nordio, M.; Basciani, S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto’s patients with subclinical hypothyroidism. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 51–59. [Google Scholar]
- Morón-Díaz, M.; Saavedra, P.; Alberiche-Ruano, M.P.; Rodríguez-Pérez, C.A.; López-Plasencia, Y.; Marrero-Arencibia, D.; González-Lleó, A.M.; Boronat, M. Correlation between TSH levels and quality of life among subjects with well-controlled primary hypothyroidism. Endocrine 2021, 72, 190–197. [Google Scholar] [CrossRef]
- Larsen, C.B.; Winther, K.H.; Cramon, P.K.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Groenvold, M.; Bjorner, J.B.; Hegedüs, L.; Watt, T.; Bonnema, S.J. Severity of hypothyroidism is inversely associated with impaired quality of life in patients referred to an endocrine clinic. Thyroid Res. 2023, 16, 37. [Google Scholar] [CrossRef]
- Winther, K.H.; Bonnema, S.J.; Cold, F.; Debrabant, B.; Nybo, M.; Cold, S.; Hegedüs, L. Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. Eur. J. Endocrinol. 2015, 172, 657–667. [Google Scholar] [CrossRef]
- Resch, U.; Helsel, G.; Tatzber, F.; Sinzinger, H. Antioxidant status in thyroid dysfunction. Clin. Chem. Lab. Med. 2002, 40, 1132–1134. [Google Scholar] [CrossRef]
- Asayama, K.; Kato, K. Oxidative muscular injury and its relevance to hyperthyroidism. Free Radic. Biol. Med. 1990, 8, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell Mol. Life. Sci. 2006, 63, 414–434. [Google Scholar] [CrossRef]
- Hoang, T.; Kuljanin, M.; Smith, M.D.; Jelokhani-Niaraki, M. A biophysical study on molecular physiology of the uncoupling proteins of the central nervous system. Biosci. Rep. 2015, 35, e00226. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Daniele, M.C.; Masullo, P.; Di Meo, S. Antioxidant-sensitive triiodothyronine effects on characteristics of rat liver mitochondrial population. Cell Physiol. Biochem. 1999, 9, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.; Kwon, T.H.; Kim, J.S.; Park, J.M. Role of the hepatic xanthine oxidase in thyroid dysfunction: Effect of thyroid hormones in oxidative stress in rat liver. Arch. Pharm. Res. 1998, 21, 236–240. [Google Scholar] [CrossRef]
- Choudhury, S.; Chainy, G.B.N.; Mishro, M.M. Experimentally induced hypo- and hyper-thyroidism influence on the antioxidant defense system in adult rat testis. Andrologia 2003, 35, 131–140. [Google Scholar] [CrossRef]
- Baskol, G.; Atmaca, H.; Tanriverdi, F.; Baskol, M.; Kocer, D.; Bayram, F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp. Clin. Endocrinol. Diabetes 2007, 115, 522–526. [Google Scholar] [CrossRef]
- Torun, A.N.; Kulaksizoglu, S.; Kulaksizoglu, M.; Pamuk, B.O.; Isbilen, E.; Tutuncu, N.B. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. 2009, 70, 469–474. [Google Scholar] [CrossRef]
- Santi, A.; Duarte, M.M.; de Menezes, C.C.; Loro, V.L. Association of lipids with oxidative stress biomarkers in subclinical hypothyroidism. Int. J. Endocrinol. 2012, 2012, 856359. [Google Scholar] [CrossRef]
- Öztürk, Ü.; Vural, P.; Özderya, A.; Karadağ, B.; Doğru-Abbasoğlu, S.; Uysal, M. Oxidative stress parameters in serum and low-density lipoproteins of Hashimoto’s thyroiditis patients with subclinical and overt hypothyroidism. Int. Immunopharmacol. 2012, 14, 349–352. [Google Scholar] [CrossRef]
- Estévez-Carmona, M.M.; Meléndez-Camargo, E.; Ortiz-Butron, R.; Pineda-Reynoso, M.; Franco-Colin, M.; Cano-Europa, E. Hypothyroidism maintained reactive oxygen species-steady state in the kidney of rats intoxicated with ethylene glycol: Effect related to an increase in the glutathione that maintains the redox environment. Toxicol. Ind. Health 2013, 29, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Cano-Europa, E.; Pérez-Severiano, F.; Vergara, P.; Ortiz-Butrón, R.; Ríos, C.; Segovia, J.; Pacheco-Rosado, J. Hypothyroidism induces selective oxidative stress in amygdala and hippocampus of rat. Metabol. Brain. Dis. 2008, 23, 275–287. [Google Scholar] [CrossRef]
- Ortiz-Butron, R.; Blas-Valdivia, V.; Franco-Colin, M.; Pineda-Reynoso, M.; Cano-Europa, E. An increase of oxidative stress markers and the alteration of the antioxidant enzymatic system are associated with spleen damage caused by methimazole-induced hypothyroidism. Drug Chem. Toxicol. 2011, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Danzi, S. Thyroid disease and the heart. Circulation 2007, 116, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Elnakish, M.T.; Ahmed, A.A.E.; Mohler, P.J.; Janssen, P.M. Role of oxidative stress in thyroid hormone-induced cardiomyocyte hypertrophy and associated cardiac dysfunction: An undisclosed story. Oxid. Med. Cell. Longev. 2015, 16, 854265. [Google Scholar] [CrossRef]
- Silvestrini, A.; Mancini, A. The Double-Edged Sword of Total Antioxidant Capacity: Clinical Significance and Personal Experience. Antioxidants 2024, 13, 933. [Google Scholar] [CrossRef]
- Peng, J.; Dai, X.; Zhang, T.; Hu, G.; Cao, H.; Guo, X.; Fan, H.; Chen, J.; Tang, W.; Yang, F. Copper as the driver of the lncRNA-TCONS-6251/miR-novel-100/TC2N axis: Unraveling ferroptosis in duck kidney. Int. J. Biol. Macromol. 2024, 282, 136797. [Google Scholar] [CrossRef]
- Fedala, A.; Adjroud, O.; Abid-Essefi, S.; Timoumi, R. Protective effects of selenium and zinc against potassium dichromate–induced thyroid disruption, oxidative stress, and DNA damage in pregnant Wistar rats. Environ. Sci. Pollut. Res. 2021, 28, 22563. [Google Scholar] [CrossRef]
- Wang, H.; Yang, F.; Ye, J.; Dai, X.; Liao, H.; Xing, C.; Jiang, Z.; Peng, C.; Gao, F.; Cao, H. Ginkgo biloba extract alleviates deltamethrin-induced testicular injury by upregulating SKP2 and inhibiting Beclin1-independent autophagy. Phytomedicine 2024, 135, 156245. [Google Scholar] [CrossRef]
- Chen, J.; Dai, X.; Xing, C.; Zhang, Y.; Cao, H.; Hu, G.; Guo, X.; Gao, X.; Liu, P.; Yang, F. Cooperative application of transcriptomics and ceRNA hypothesis: lncRNA-00742/miR-116 targets CD74 to mediate vanadium-induced mitochondrial apoptosis in duck liver. J. Hazard. Mater. 2024, 5, 135904. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Dai, X.; Chen, J.; Cao, H.; Hu, G.; Guo, X.; Liu, P.; Xing, C.; Yang, F. Single nucleus RNA sequencing reveals cellular and molecular responses to vanadium exposure in duck kidneys. J. Hazard. Mater. 2024, 5, 136492. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Zahan, A.E.; Watt, T.; Pascanu, I.; Rasmussen, A.K.; Hegedüs, L.; Bonnema, S.J.; Feldt-Rasmussen, U.; Bjorner, J.B.; Nadasan, V.; Boila, A.; et al. The Romanian version of the thyroid-related patient-reported outcomes THYPRO and THYPRO-39. Translation and assessment of reliability and cross-cultural validity. Acta Endocrinol. 2018, 14, 192–200. [Google Scholar] [CrossRef]
| Parameter | Mean ± SD |
|---|---|
| Age (years) | 56.01 ± 9.54 |
| BMI (kg/m2) | 27.46 ± 4.96 |
| SelP (mg/L) | 5.16 ± 0.76 |
| GPx3 (U/L) | 222.08 ± 32.92 |
| TSH (μU/mL) | 1.32 ± 1.28 |
| fT4 (ng/dL) | 1.40 ± 0.22 |
| fT3 (pg/mL) | 3.12 ± 0.61 |
| fT3/fT4 ratio | 0.22 ± 0.04 |
| Item | Mean % and Range |
|---|---|
| Hypothyroidism symptoms (4 questions) | 38 (0–88) |
| Hyperthyroidism symptoms (4 questions) | 29 (0–100) |
| Tiredness (3 questions) | 57 (8–100) |
| Cognitive (3 questions) | 41 (0–100) |
| Anxiety (3 questions) | 39 (0–100) |
| Depression (3 questions) | 36 (0–100) |
| Emotional susceptibility (2 questions) | 60 (0–100) |
| Impaired social life (3 questions) | 15 (0–83) |
| Impaired daily life (3 questions) | 27 (0–83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traini, E.; Ianni, F.; Vergani, E.; Carnassale, G.; Daloiso, G.; Mancini, A.; Silvestrini, A. Glutathione Peroxidase and Selenoprotein P Evaluation in Well-Being Assessment After Total Thyroidectomy. Int. J. Mol. Sci. 2025, 26, 4521. https://doi.org/10.3390/ijms26104521
Traini E, Ianni F, Vergani E, Carnassale G, Daloiso G, Mancini A, Silvestrini A. Glutathione Peroxidase and Selenoprotein P Evaluation in Well-Being Assessment After Total Thyroidectomy. International Journal of Molecular Sciences. 2025; 26(10):4521. https://doi.org/10.3390/ijms26104521
Chicago/Turabian StyleTraini, Emanuela, Francesca Ianni, Edoardo Vergani, Giulia Carnassale, Giuseppe Daloiso, Antonio Mancini, and Andrea Silvestrini. 2025. "Glutathione Peroxidase and Selenoprotein P Evaluation in Well-Being Assessment After Total Thyroidectomy" International Journal of Molecular Sciences 26, no. 10: 4521. https://doi.org/10.3390/ijms26104521
APA StyleTraini, E., Ianni, F., Vergani, E., Carnassale, G., Daloiso, G., Mancini, A., & Silvestrini, A. (2025). Glutathione Peroxidase and Selenoprotein P Evaluation in Well-Being Assessment After Total Thyroidectomy. International Journal of Molecular Sciences, 26(10), 4521. https://doi.org/10.3390/ijms26104521








