Progressive Elevation of Store-Operated Calcium Entry-Associated Regulatory Factor (SARAF) and Calcium Pathway Dysregulation in Multiple Sclerosis
Abstract
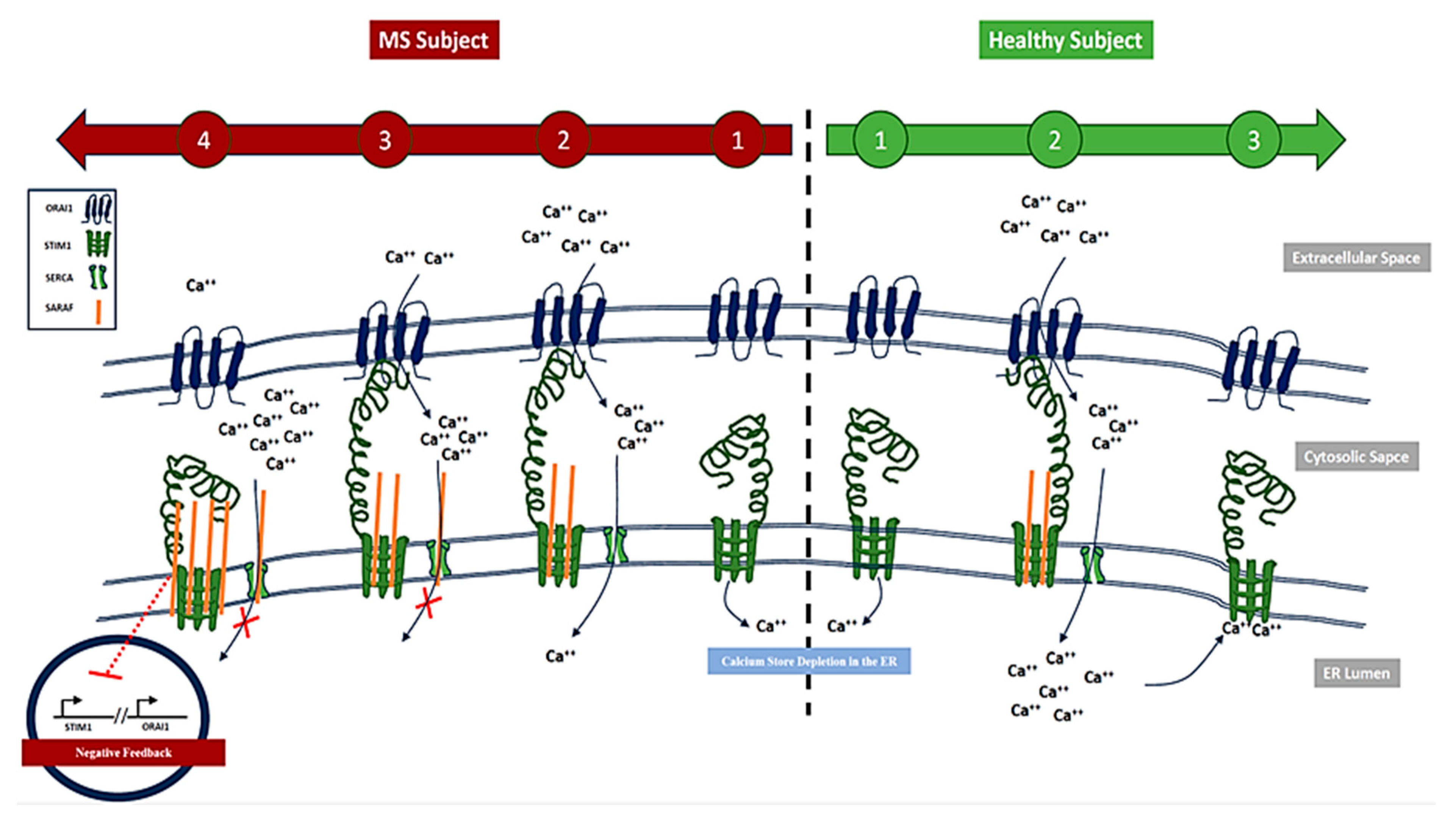
1. Introduction
2. Results
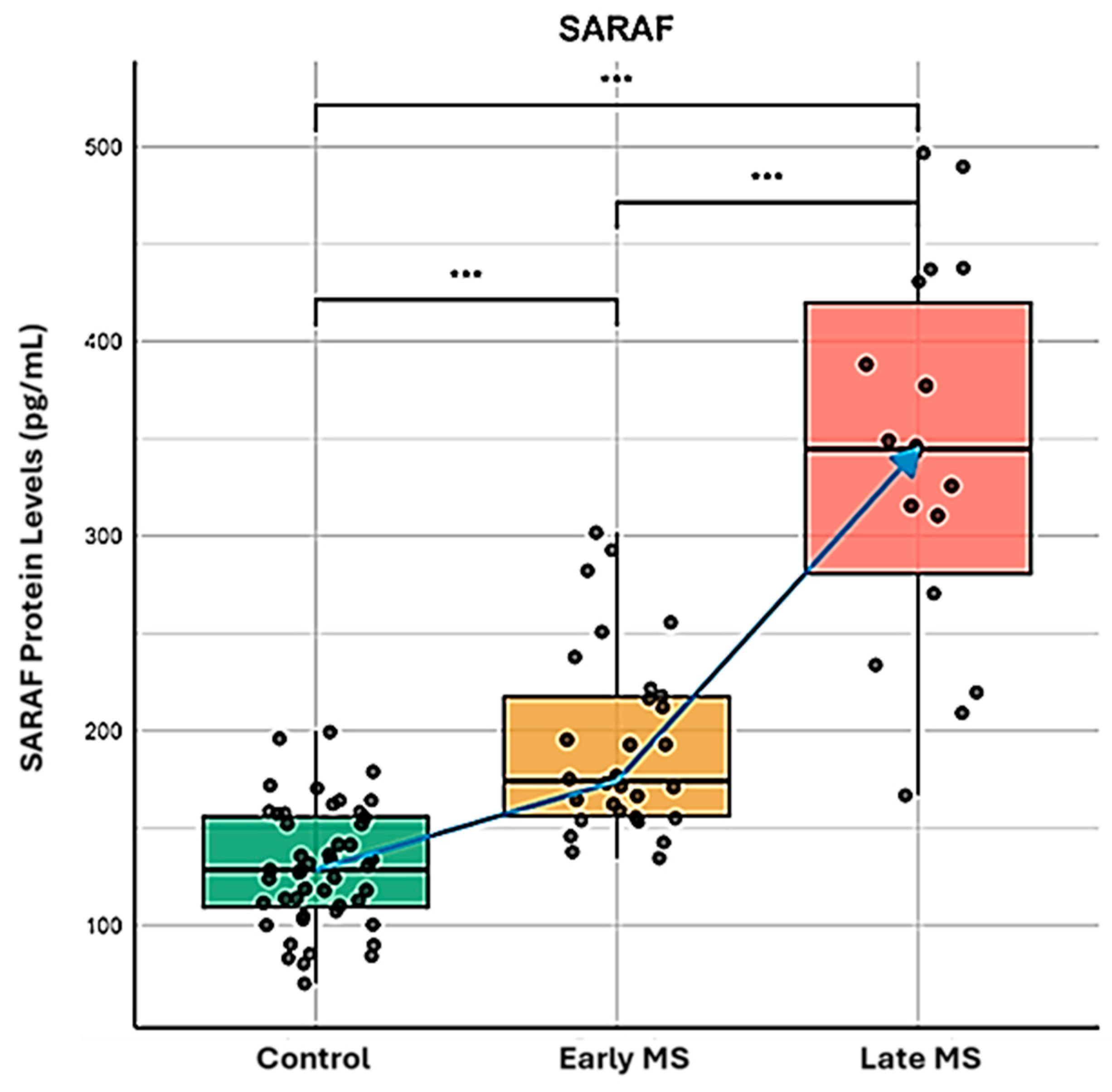
2.1. SARAF Protein in MS Patients Compared to Healthy Controls Using ELISA
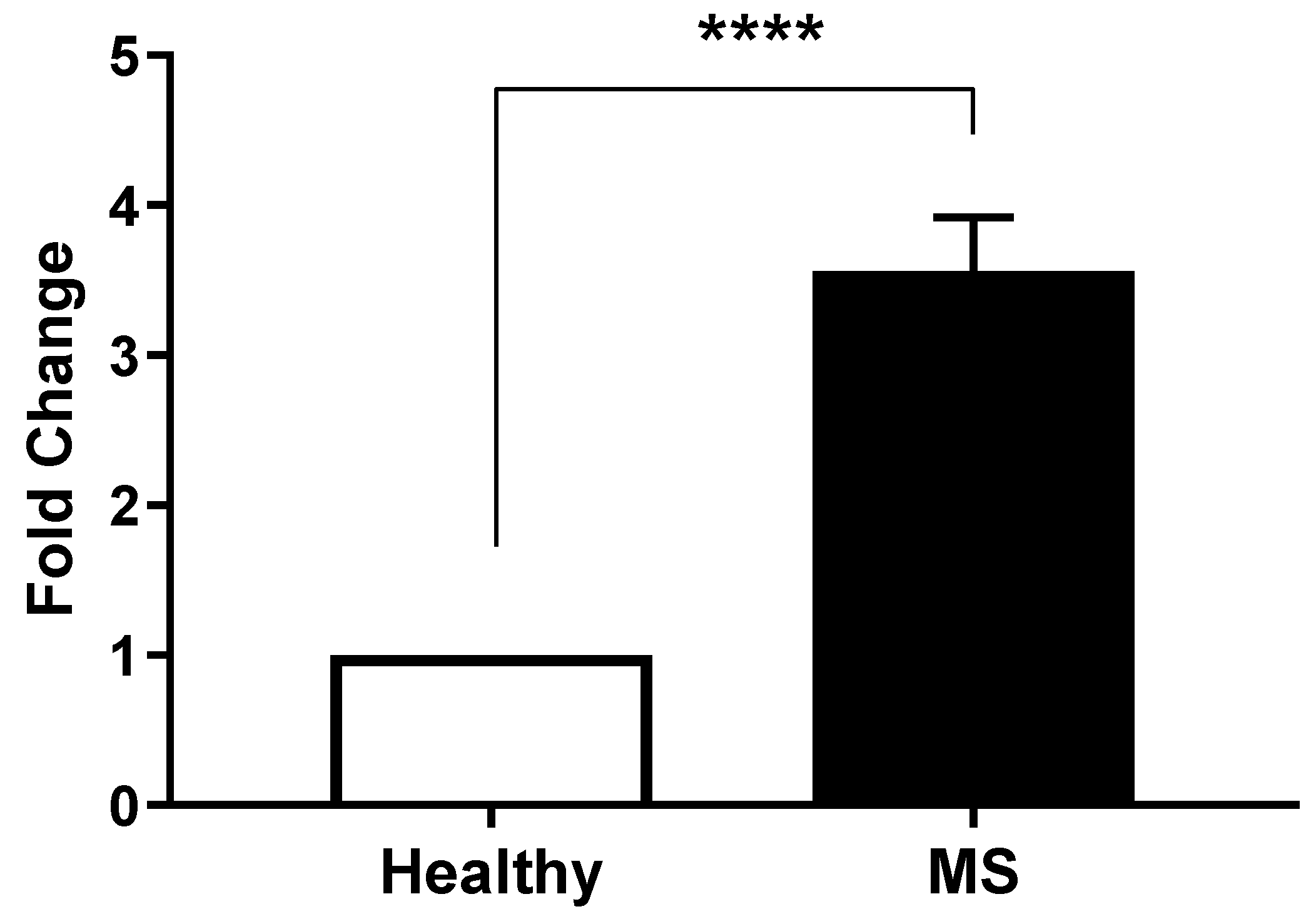
2.2. SARAF Gene Expression Level in MS Patients Compared to Healthy Controls Using qRT-PCR
2.3. Calcium Levels in Healthy Individuals Compared to MS Patients
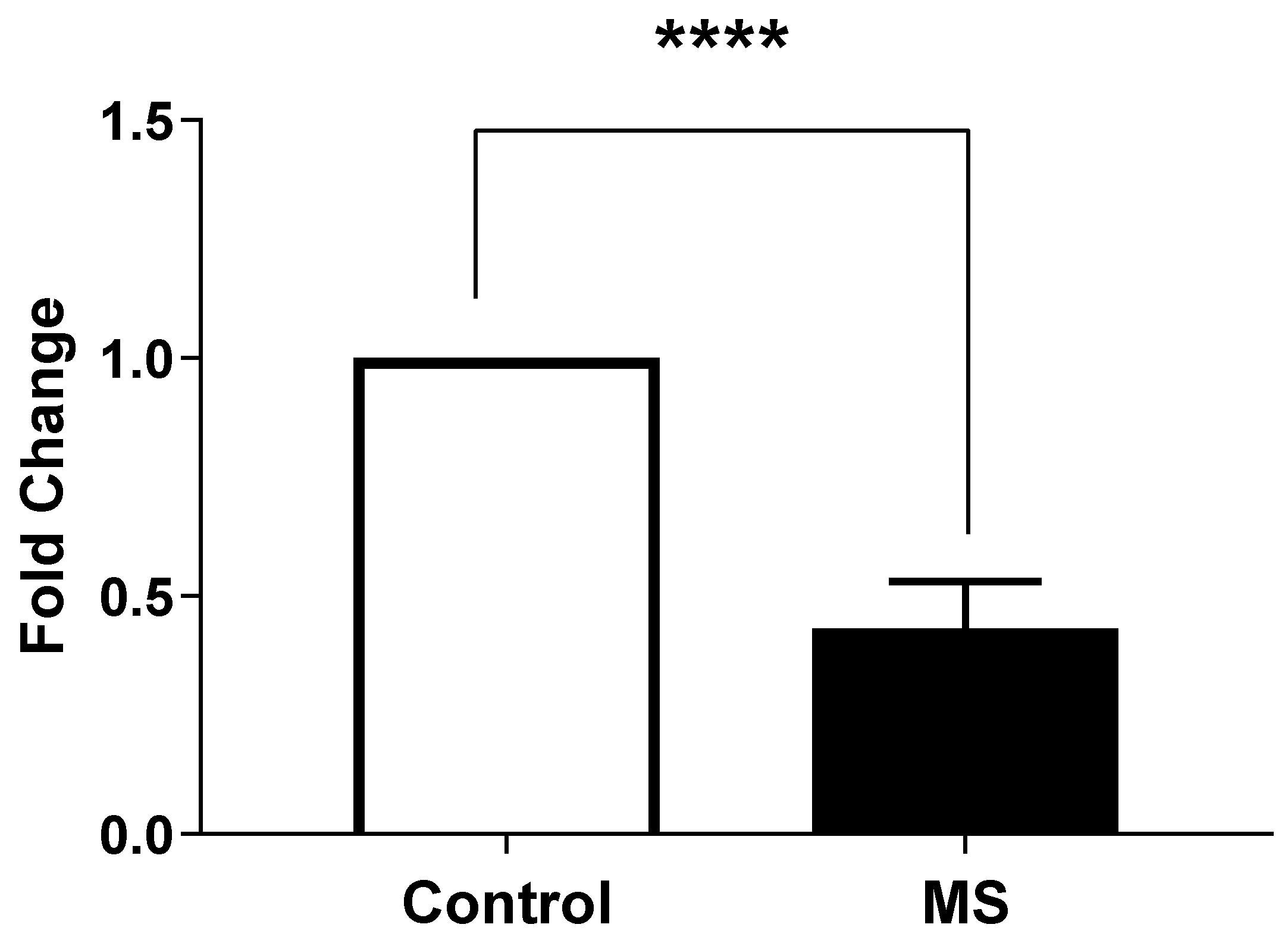
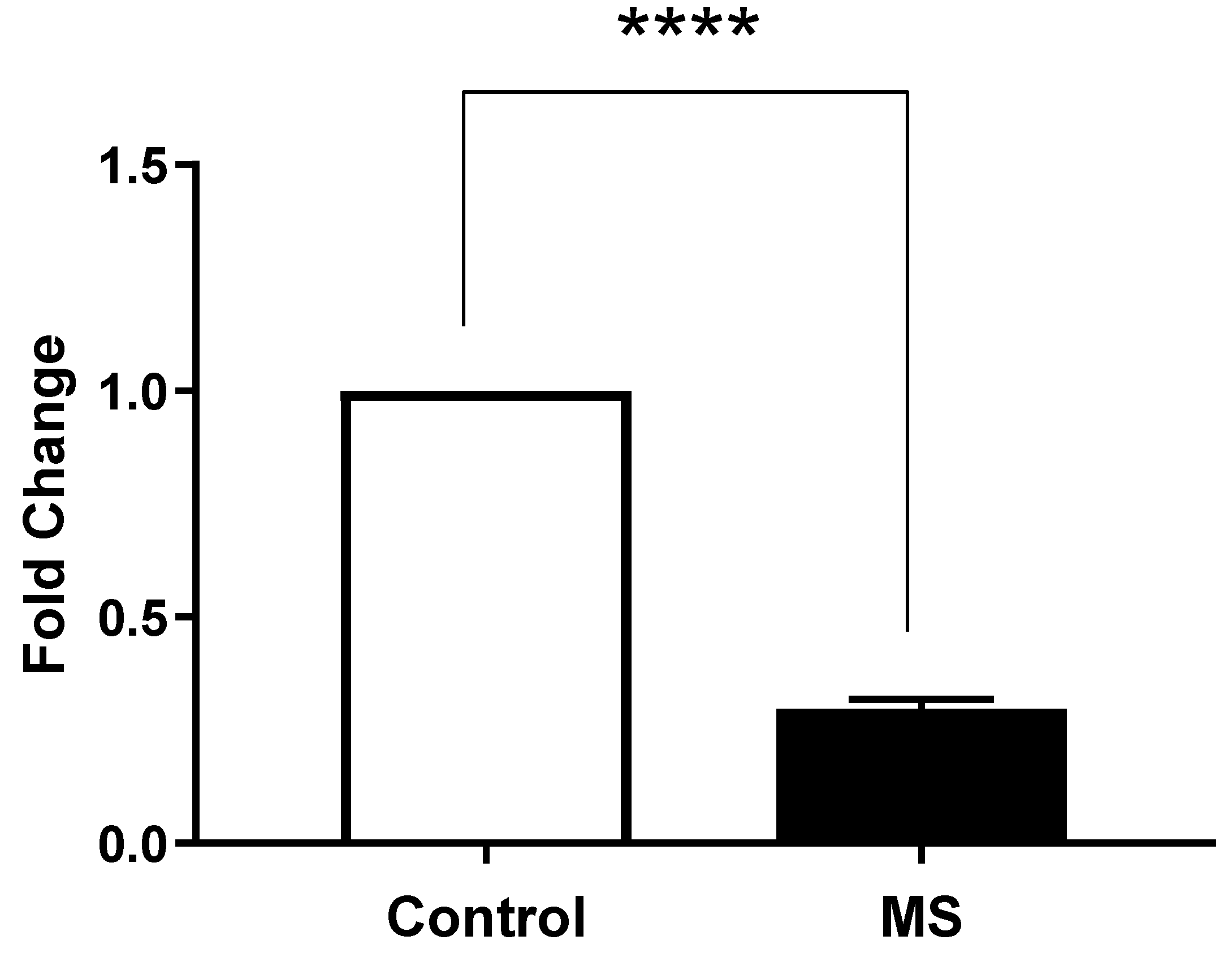
2.4. Measurement of Expression of STIM1 and ORAI1 by Real-Time PCR
3. Discussion
- Elevated SARAF Levels: MS patients exhibited a mean SARAF concentration of 307.4 ± 52.32 ng/mL, significantly higher than the 135.8 ± 10.15 ng/mL observed in healthy controls (p = 0.0021). This elevation suggests a potential role for SARAF in MS-related inflammatory processes [14].
- Correlation with Cellular Mechanisms: Real-time PCR analysis revealed a fold change in SARAF expression of 3.829 ± 0.04422 in peripheral blood mononuclear cells (PBMCs) from MS patients (p < 0.0001). This increase may influence calcium signaling pathways critical for T cell activation, which are often dysregulated in autoimmune conditions [1].
- Calcium Dysregulation: MS patients demonstrated significantly higher intracellular calcium levels (0.1758 ± 0.006399) compared to healthy controls (0.105 ± 0.008438; p < 0.0001). Additionally, lower expression levels of STIM1 (0.4324 ± 0.01471) and ORAI1 (0.2963 ± 0.02156) were noted, indicating impaired store-operated calcium entry (SOCE) mechanisms [15].
4. Materials and Methods
4.1. Subjects
4.2. Demographics and Clinical Characteristics
4.3. Blood Sample Collection
4.4. Measurement of SARAF Levels by ELISA
4.5. RNA Isolation and cDNA Synthesis
4.6. Measurement of Expression of SARAF, STIM1, and ORAI 1 by Real-Time PCR
4.7. Measurement of Intracellular Calcium Levels
4.8. Statistical Analysis
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARAF | Store-Operated Calcium Entry-Associated Regulatory Factor |
| SOCE | Store-operated calcium entry |
| Orai1 | Calcium Release-Activated Calcium Channel Protein 1 |
| STIM1 | Stromal Interaction Molecule 1 |
References
- Hafler, D.A. Multiple sclerosis. J. Clin. Investig. 2004, 113, 788–794, Erratum in J. Clin. Investig. 2004, 113, 1070. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 92, e1029–e1040, Erratum in Neurology 2019, 93, 688. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef]
- Robinson, D., Jr.; Eisenberg, D.; Nietert, P.J.; Doyle, M.; Bala, M.; Paramore, C.; Fraeman, K.; Renahan, K. Systemic sclerosis prevalence and comorbidities in the US, 2001–2002. Curr. Med. Res. Opin. 2008, 24, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Alsharoqi, I.; Alsaffar, M.; Almukhtar, B.; Abdulla, F.; Aljishi, A. Prevalence, demographics and clinical features of multiple sclerosis in Bahrain. Mult. Scler. Relat. Disord. 2014, 3, 761–762. [Google Scholar] [CrossRef]
- Lewis, R.S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 2001, 19, 497–521. [Google Scholar] [CrossRef]
- Robert, V.; Triffaux, E.; Savignac, M.; Pelletier, L. Singularities of calcium signaling in effector T-lymphocytes. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Ernst, I.M.A.; Fliegert, R.; Guse, A.H. Adenine dinucleotide second messengers and T-lymphocyte calcium signaling. Front. Immunol. 2013, 4, 259. [Google Scholar] [CrossRef]
- Guse, A.H.; Da Silva, C.P.; Berg, I.; Skapenko, A.L.; Weber, K.; Heyer, P.; Hohenegger, M.; Ashamu, G.A.; Schulze-Koops, H.; Potter, B.V.; et al. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature 1999, 398, 70–73. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. [Internet] 2015, 95, 1383–1436. Available online: www.prv.org (accessed on 15 January 2025). [CrossRef]
- Secondo, A.; Bagetta, G.; Amantea, D. On the role of store-operated calcium entry in acute and chronic neurodegenerative diseases. Front. Mol. Neurosci. 2018, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Gselman, S.; Fabjan, T.H.; Bizjak, A.; Potočnik, U.; Gorenjak, M. Cholecalciferol Supplementation Induced Up-Regulation of SARAF Gene and Down-Regulated miR-155-5p Expression in Slovenian Patients with Multiple Sclerosis. Genes 2023, 14, 1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Pan, H.; Liang, X.; Xie, J.; Han, W. The roles of transmembrane family proteins in the regulation of store-operated Ca2+ entry. Cell. Mol. Life Sci. 2022, 79, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.; Hu, H. Store-Operated Calcium Channels in Physiological and Pathological States of the Nervous System. Front. Cell. Neurosci. 2020, 14, 600758. [Google Scholar] [CrossRef]
- Enders, M.; Heider, T.; Ludwig, A.; Kuerten, S. Strategies for Neuroprotection in Multiple Sclerosis and the Role of Calcium. Int. J. Mol. Sci. 2020, 21, 1663. [Google Scholar] [CrossRef]
- Martino, G.; Grohovaz, F.; Brambilla, E.; Codazzi, F.; Consiglio, A.; Clementi, E.; Filippi, M.; Comi, G.; Grimaldi, L.M. Proinflammatory cytokines regulate antigen-independent T-cell activation by two separate calcium-signaling pathways in multiple sclerosis patients. Ann. Neurol. 1998, 43, 340–349. [Google Scholar] [CrossRef]
- Hundehege, P.; Epping, L.; Meuth, S.G. Calcium homeostasis in multiple sclerosis. Neurol. Int. Open 2017, 1, E127–E135. [Google Scholar] [CrossRef]
- Berna-Erro, A.; Granados, M.P.; Teruel-Montoya, R.; Ferrer-Marin, F.; Delgado, E.; Corbacho, A.J.; Fenández, E.; Vazquez-Godoy, M.T.; Tapia, J.A.; Redondo, P.C. SARAF overexpression impairs thrombin-induced Ca2+ homeostasis in neonatal platelets. Br. J. Haematol. 2023, 204, 988–1004. [Google Scholar] [CrossRef]
- Baraibar, A.M.; Colomer, T.; Moreno-García, A.; Bernal-Chico, A.; Sánchez-Martín, E.; Utrilla, C.; Serrat, R.; Soria-Gómez, E.; Rodríguez-Antigüedad, A.; Araque, A.; et al. Autoimmune inflammation triggers aberrant astrocytic calcium signaling to impair synaptic plasticity. Brain. Behav. Immun. 2024, 121, 192–210. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium signaling and cell proliferation. Cell Calcium 2014, 56, 243–253. [Google Scholar]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, R.D. Neuronal calcium sensor proteins: Generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosci. 2007, 8, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Lilienbaum, A.; Israël, A. From Calcium to NF-κB Signaling Pathways in Neurons. Mol. Cell. Biol. 2003, 23, 2680–2698. [Google Scholar] [CrossRef]
- Bading, H.; Ginty, D.D.; Greenberg, M.E. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 1993, 260, 181–186. [Google Scholar] [CrossRef]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease—A therapeutic opportunity? Biochem. Biophys. Res. Commun. 2017, 483, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Fjodorova, M.; Noakes, Z.; De La Fuente, D.C.; Errington, A.C.; Li, M. Dysfunction of cAMP–Protein Kinase A–Calcium Signaling Axis in Striatal Medium Spiny Neurons: A Role in Schizophrenia and Huntington’s Disease Neuropathology. Biol. Psychiatry Glob. Open Sci. 2023, 3, 418–429. [Google Scholar] [CrossRef]
- Albarran, L.; Lopez, J.J.; Amor, N.B.; Martin-Cano, F.E.; Berna-Erro, A.; Smani, T.; Salido, G.M.; Rosado, J.A. Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store-operated calcium entry. Sci. Rep. 2016, 6, 24452. [Google Scholar] [CrossRef]
- Jha, A.; Ahuja, M.; Maléth, J.; Moreno, C.M.; Yuan, J.P.; Kim, M.S.; Muallem, S. The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. J. Cell Biol. 2013, 202, 71–79. [Google Scholar] [CrossRef]
- Galeano-Otero, I.; Del Toro, R.; Khatib, A.M.; Rosado, J.A.; Ordóñez-Fernández, A.; Smani, T. SARAF and Orai1 Contribute to Endothelial Cell Activation and Angiogenesis. Front. Cell Dev. Biol. 2021, 9, 639952. [Google Scholar] [CrossRef]
- Martín-Bórnez, M.; Ávila-Medina, J.; Calderón-Sánchez, E.; Rosado, J.A.; Ordoñez-Fernández, A.; Smani, T. Essential role of Orai1 and SARAF in vascular remodeling: Calcium Signaling and Excitation–Contraction in Cardiac, Skeletal and Smooth Muscle. J. Gen. Physiol. 2022, 154, e2021ecc19. [Google Scholar] [CrossRef]
- Bodnar, D. The Role of EHD2 in Orai1-STIM1 Interaction. Biophys. J. 2017, 112, 538A. [Google Scholar] [CrossRef]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]

| Primer | Accession Number | Forward Primer | Reverse Primer | Product Length |
|---|---|---|---|---|
| GAPDH | NM_002046.7 | TCCCTGAGCTGAACGGGAAG | GGAGGAGTGGGTGTCGCTGT | 217 bp |
| SARAF | NM_016127.6 | TGGAACGACCCTGACAGAATG | ACCCATCCCAGCCTTTGTTC | 181 bp |
| Orai 1 | NM_032790.4 | AGTCGTGGTCAGCGTCCAGCT | AGGTGATGAGCCTCAACGAGCA | 159 bp |
| STIM1 | NM_003156.4 | TTGACAAGCCGGGTATCTCTG | CATCTGAGGAGGTTTGGGGG | 369 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, S.; Aljishi, M.; Sultan, A.; Al-Nashmi, M.E.; Bakhiet, M.; Spicuglia, S.; Belhocine, M. Progressive Elevation of Store-Operated Calcium Entry-Associated Regulatory Factor (SARAF) and Calcium Pathway Dysregulation in Multiple Sclerosis. Int. J. Mol. Sci. 2025, 26, 4520. https://doi.org/10.3390/ijms26104520
Taha S, Aljishi M, Sultan A, Al-Nashmi ME, Bakhiet M, Spicuglia S, Belhocine M. Progressive Elevation of Store-Operated Calcium Entry-Associated Regulatory Factor (SARAF) and Calcium Pathway Dysregulation in Multiple Sclerosis. International Journal of Molecular Sciences. 2025; 26(10):4520. https://doi.org/10.3390/ijms26104520
Chicago/Turabian StyleTaha, Safa, Muna Aljishi, Ameera Sultan, Moudi E. Al-Nashmi, Moiz Bakhiet, Salvatore Spicuglia, and Mohamed Belhocine. 2025. "Progressive Elevation of Store-Operated Calcium Entry-Associated Regulatory Factor (SARAF) and Calcium Pathway Dysregulation in Multiple Sclerosis" International Journal of Molecular Sciences 26, no. 10: 4520. https://doi.org/10.3390/ijms26104520
APA StyleTaha, S., Aljishi, M., Sultan, A., Al-Nashmi, M. E., Bakhiet, M., Spicuglia, S., & Belhocine, M. (2025). Progressive Elevation of Store-Operated Calcium Entry-Associated Regulatory Factor (SARAF) and Calcium Pathway Dysregulation in Multiple Sclerosis. International Journal of Molecular Sciences, 26(10), 4520. https://doi.org/10.3390/ijms26104520







