Nitazoxanide Analogs: Synthesis, In Vitro Giardicidal Activity, and Effects on Giardia lamblia Metabolic Gene Expression
Abstract
1. Introduction
2. Results and Discussion
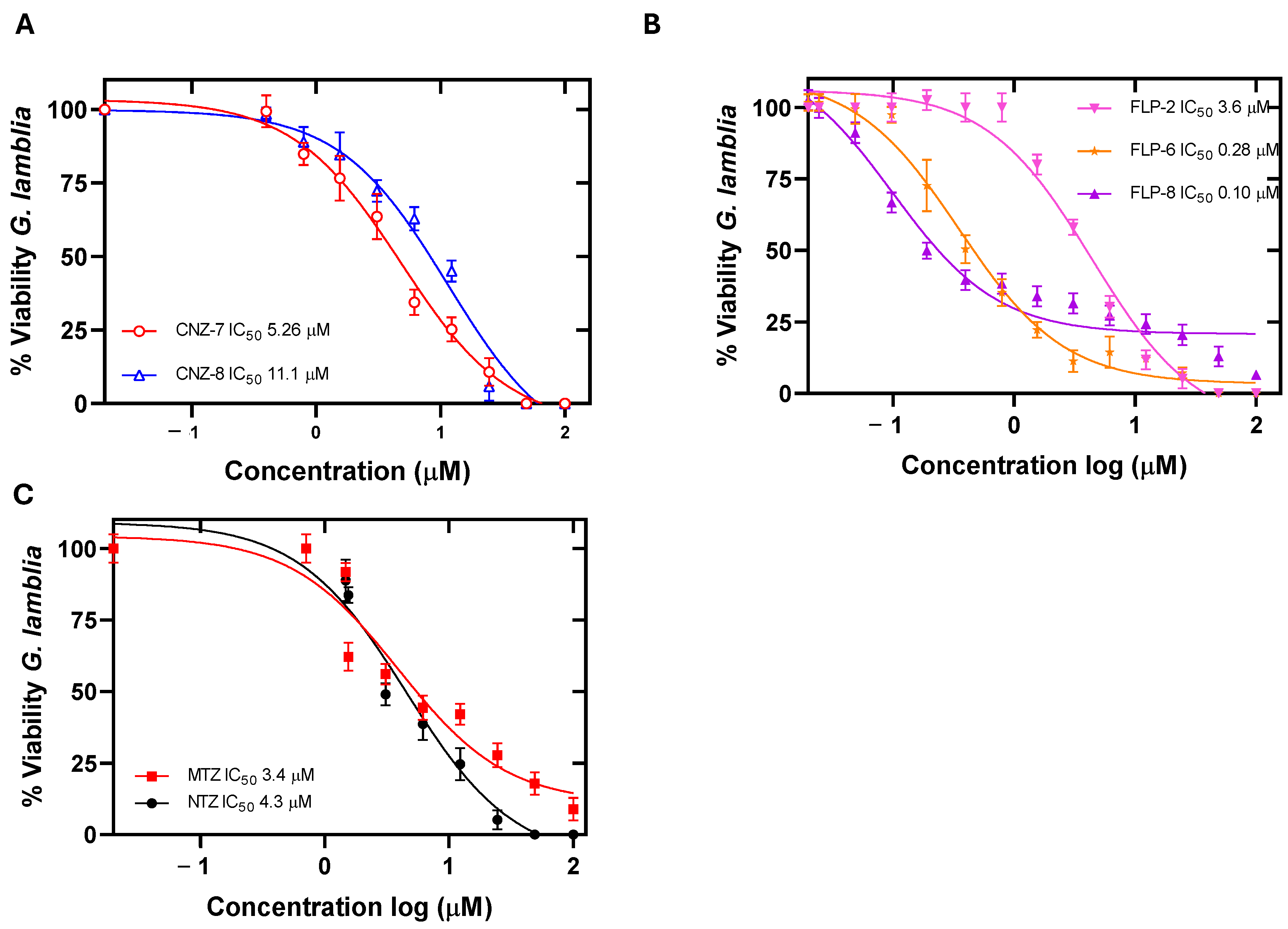
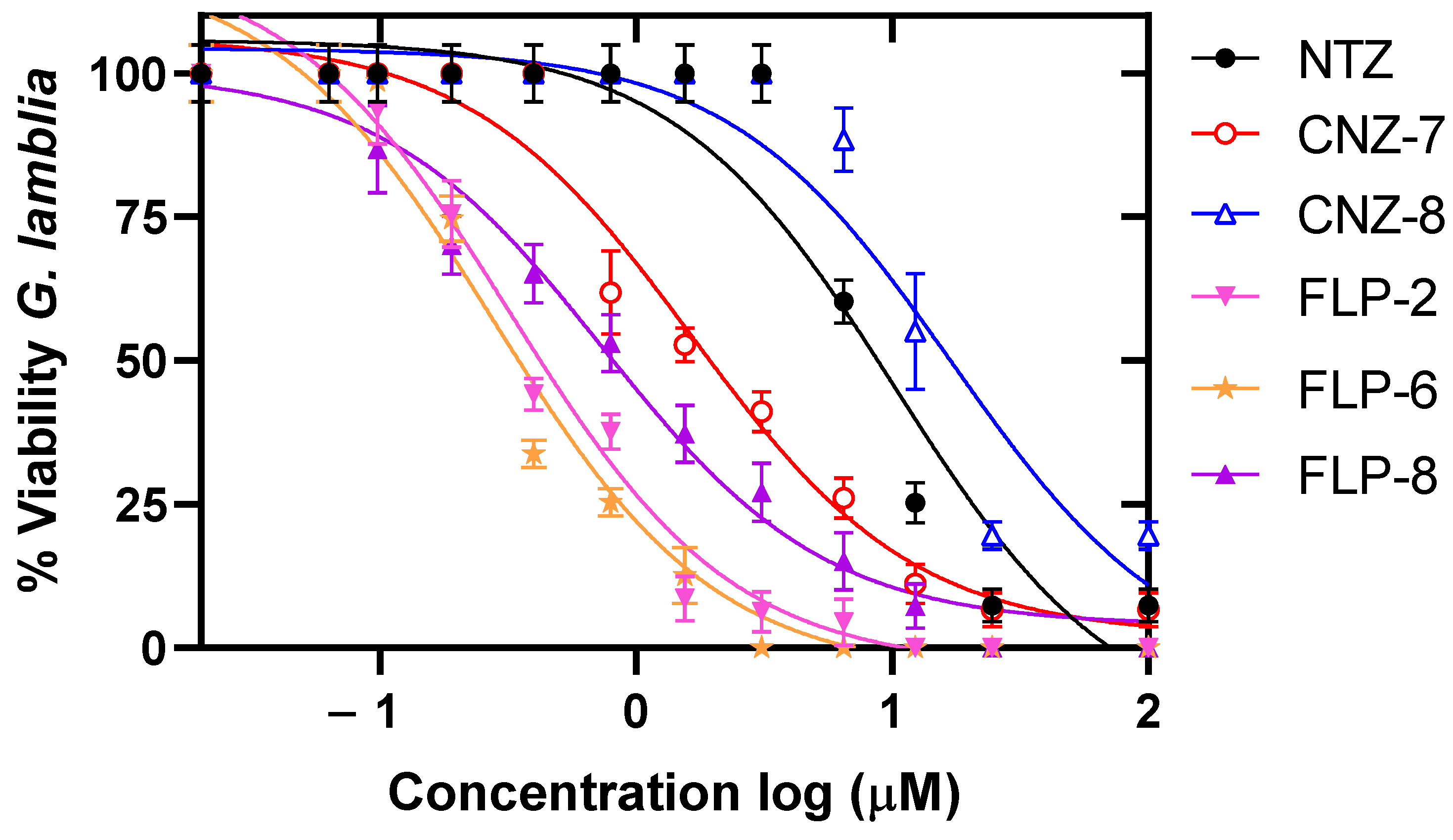
2.1. Evaluation of the Concentration–Response Effect of the Compounds on the Viability of Giardia lamblia
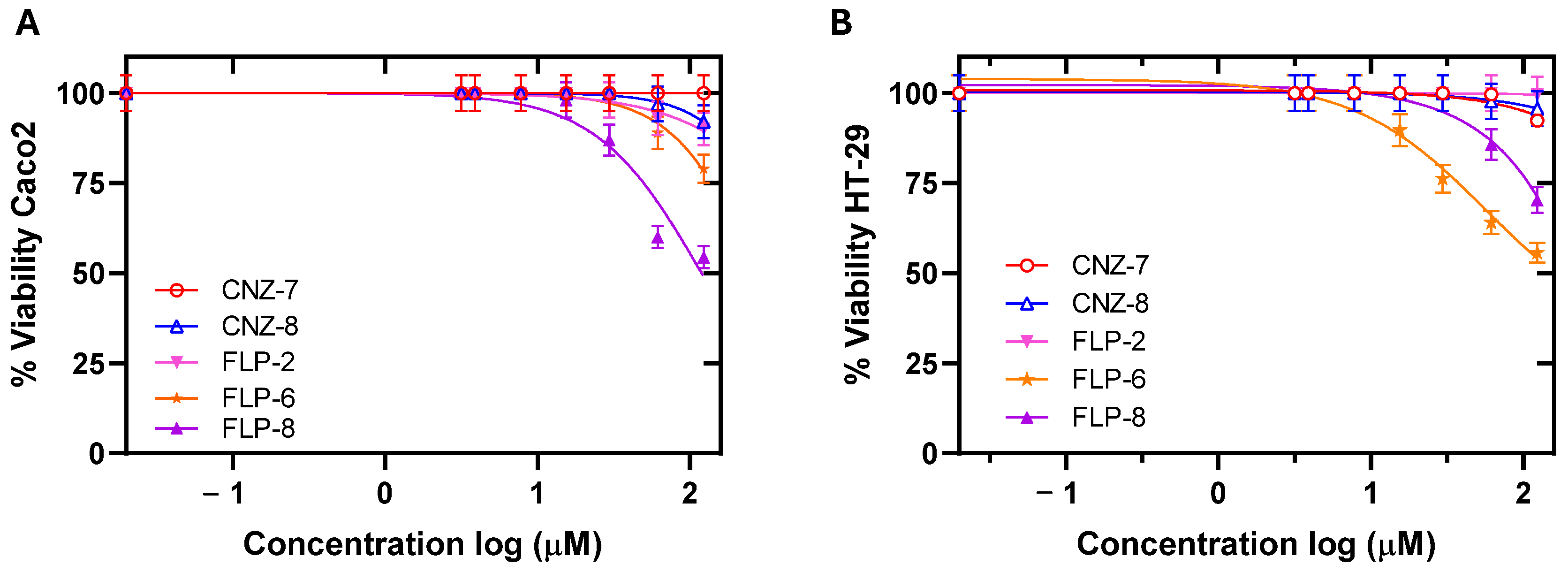
2.2. Cytotoxicity and ADMET Predictive Parameters of the Compounds
| Compound | IC50 (µM) | CC50 (µM) | ||
|---|---|---|---|---|
| G. lamblia | G. lamblia (NTZ Resistant) | HT-29 (SI) | Caco-2 (SI) | |
| CNZ-7 | 5.26 | 2.5 | 529 (100) | 640 (121) |
| CNZ-8 | 11.1 | 13.7 | 622 (56) | 633 (57) |
| FLP-2 | 3.6 | 0.37 | 1912 (531) | 3184 (884) |
| FLP-6 | 0.28 | 0.54 | 115.33 (411) | 178.18(636) |
| FLP-8 | 0.10 | 1.19 | 43.67 (436) | 46 (460) |
| MTZ | 3.4 | Not determined | 550 (161) [37] | 545 (160) [37] |
| NTZ | 4.3 | 7.29 | 634 (147) [37] | 580 (134) [37] |
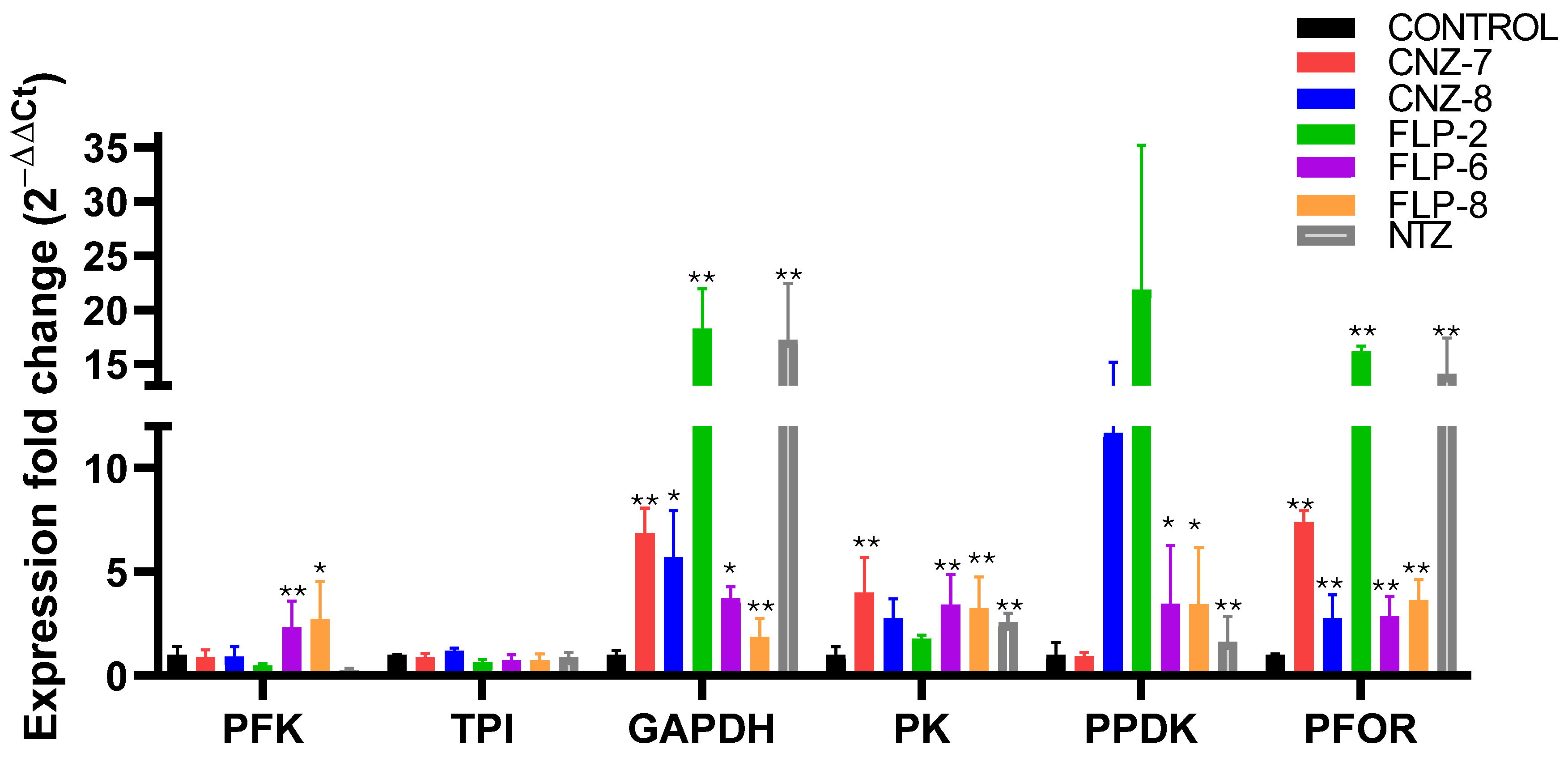
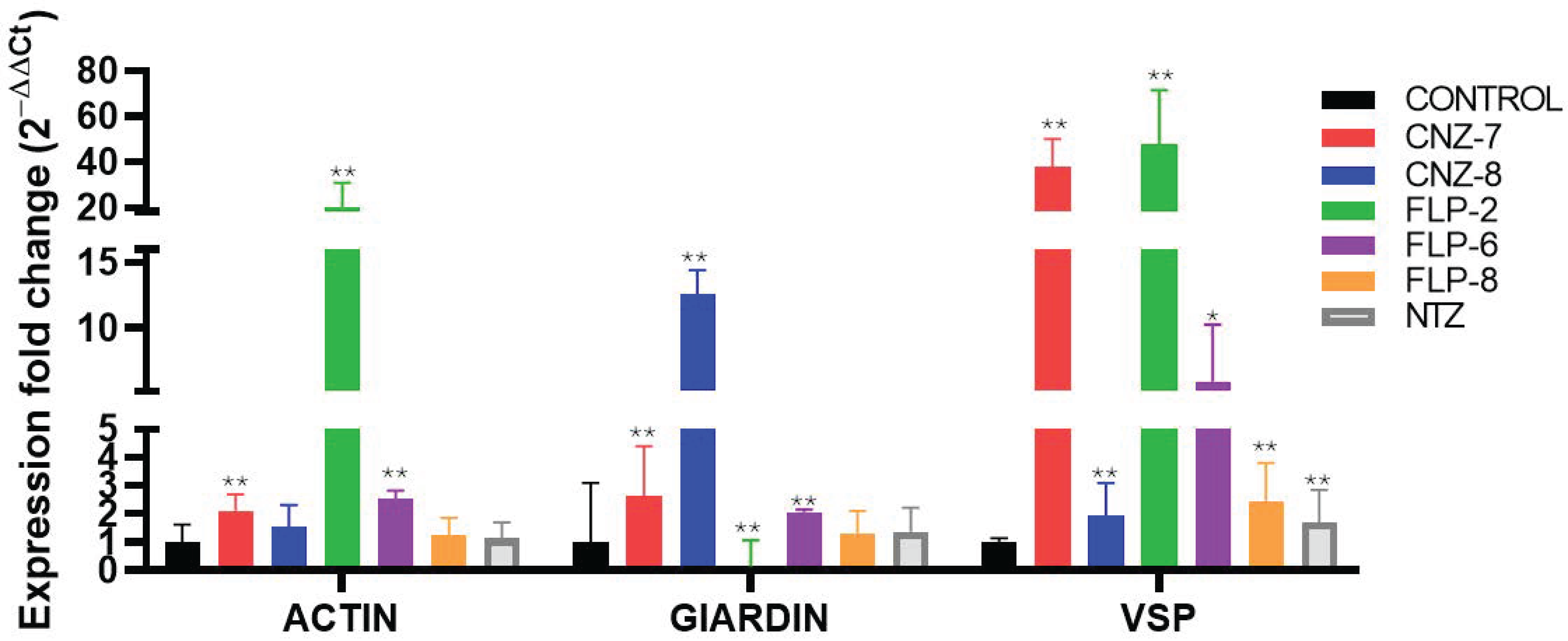
2.3. Effect on Metabolic Gene Expression Levels of Giardia lamblia
2.4. Cell Damage Caused by the Compound FLP-2 in Giardia lamblia Trophozoites
2.5. Elucidation of the Effect of Compounds on Drug-Resistant Strains
3. Materials and Methods
3.1. Synthesis of Compounds FLP-2, FLP-6, and FLP-8
3.2. Determination of the IC50 Value of Inhibitors of Giardia lamblia Culture
3.3. Cytotoxicity Assessment of Compounds in Caco-2 and HT-29 Cell Cultures
3.4. Evaluation of the Effect of Compounds on the Expression Levels of G. lamblia Genes Using Quantitative RT-qPCR
3.5. Evaluation of Pharmacokinetic and Physicochemical Parameters of Selected Antigiardial Compounds
3.6. Evaluation of Cell Damage Using Transmission Electron Microscopy
3.7. Giardicidal Activity of Selected Compounds in Nitazoxanide-Resistant Strain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mørch, K.; Hanevik, K. Giardiasis treatment: An update with a focus on refractory disease. Curr. Opin. Infect. Dis. 2020, 33, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Leung, A.A.; Wong, A.H.; Sergi, C.M.; Kam, J.K. Giardiasis: An Overview. Recent. Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fletcher, S.M.; Stark, D.; Harkness, J.; Ellis, J. Enteric protozoa in the developed world: A public health perspective. Clin. Microbiol. Rev. 2012, 25, 420–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahdavi, F.; Sadrebazzaz, A.; Chahardehi, A.M.; Badali, R.; Omidian, M.; Hassanipour, S.; Asghari, A. Global epidemiology of Giardia duodenalis infection in cancer patients: A systematic review and meta-analysis. Int. Health 2022, 14, 5–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogawski, E.T.; Bartelt, L.A.; Platts-Mills, J.A.; Seidman, J.C.; Samie, A.; Havt, A.; Babji, S.; Trigoso, D.R.; Qureshi, S.; Shakoor, S.; et al. Determinants and Impact of Giardia Infection in the First 2 Years of Life in the MAL-ED Birth Cohort. J. Pediatric Infect. Dis. Soc. 2017, 6, 153–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cernikova, L.; Faso, C.; Hehl, A.B. Five facts about Giardia lamblia. PLoS Pathog. 2018, 14, e1007250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Efstratiou, A.; Ongerth, J.; Karanis, P. Evolution of monitoring for Giardia and Cryptosporidium in water. Water Res. 2017, 15, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Lanata, C.F.; Fischer-Walker, C.L.; Olascoaga, A.C.; Torres, C.X.; Aryee, M.J.; Black, R.E. Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: A systematic review. PLoS ONE 2013, 8, e72788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.X.; Weller, P.F. Antiparasitic drugs. N. Engl. J. Med. 1996, 334, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adam, R.D. Giardia duodenalis: Biology and Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e0002419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Argüello-García, R.; Leitsch, D.; Skinner-Adams, T.; Ortega-Pierres, M.G. Drug resistance in Giardia: Mechanisms and alternative treatments for Giardiasis. Adv. Parasitol. 2020, 107, 201–282. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rosas, V.; Hernández-Ochoa, B.; Morales-Luna, L.; Ortega-Cuellar, D.; González-Valdez, A.; Arreguin-Espinosa, R.; Rufino-González, Y.; Calderón-Jaimes, E.; Castillo-Rodríguez, R.A.; Wong-Baeza, C.; et al. Nitazoxanide Inhibits the Bifunctional Enzyme GlG6PD::6PGL of Giardia lamblia: Biochemical and In Silico Characterization of a New Druggable Target. Int. J. Mol. Sci. 2023, 14, 11516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sørensen, C.G.; Karlsson, W.K.; Amin, F.M.; Lindelof, M. Metronidazole-induced encephalopathy: A systematic review. J. Neurol. 2020, 267, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.R.; Nabarro, L.E.; Hedley, L.; Chiodini, P.L. Nitroimidazole-refractory giardiasis: A growing problem requiring rational solutions. Clin. Microbiol. Infect. 2018, 24, 37–42. [Google Scholar] [CrossRef]
- Leitsch, D. Drug Resistance in the Microaerophilic Parasite Giardia lamblia. Curr. Trop. Med. Rep. 2015, 2, 128–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loderstädt, U.; Frickmann, H. Antimicrobial resistance of the enteric protozoon Giardia duodenalis—A narrative review. Eur. J. Microbiol. Immunol. 2021, 11, 29–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Nava-Zuazo, C.; Castillo-Villanueva, A.; Méndez, S.T.; Torres-Arroyo, A.; Gómez-Manzo, S.; Marcial-Quino, J.; Ponce-Macotela, M.; Rufino-González, Y.; et al. Novel giardicidal compounds bearing proton pump inhibitor scaffold proceeding through triosephosphate isomerase inactivation. Sci. Rep. 2017, 7, 7810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vique-Sánchez, J.L.; Caro-Gómez, L.A.; Brieba, L.G.; Benítez-Cardoza, C.G. Developing a new drug against trichomoniasis, new inhibitory compounds of the protein triosephosphate isomerase. Parasitol. Int. 2020, 76, 102086. [Google Scholar] [CrossRef]
- Raj, D.; Ganguly, S. Oxidative stress regulation in Giardia lamblia. In Oxidative Stress in Microbial Diseases; Chakraborti, S., Chakraborti, T., Chattopadhyay, D., Shaha, C., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, D.; Kuehne, A.; Estermann, A.; Fuhrer, T.; Lang, P.; Sauer, U. Reserve Flux Capacity in the Pentose Phosphate Pathway by NADPH Binding Is Conserved across Kingdoms. iScience 2019, 19, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Storm, J.; Perner, J.; Aparicio, I.; Patzewitz, E.-M.; Olszewski, K.; Llinas, M.; Engel, P.C.; Müller, S. Plasmodium falciparum glutamate dehydrogenase a is dispensable and not a drug target during erythrocytic development. Malar. J. 2011, 10, 193. [Google Scholar] [CrossRef]
- Morales-Luna, L.; Serrano-Posada, H.; González-Valdez, A.; Ortega-Cuellar, D.; Vanoye-Carlo, A.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Rufino-González, Y.; Castillo-Rodríguez, R.A.; de la Cruz, V.P.; et al. Biochemical Characterization and Structural Modeling of Fused Glucose-6-Phosphate Dehydrogenase-Phosphogluconolactonase from Giardia lamblia. Int. J. Mol. Sci. 2018, 19, 2518. [Google Scholar] [CrossRef]
- Morales-Luna, L.; González-Valdez, A.; Hernández-Ochoa, B.; Arreguin-Espinosa, R.; Ortega-Cuellar, D.; Castillo-Rodríguez, R.A.; Martínez-Rosas, V.; Cárdenas-Rodríguez, N.; Enríquez-Flores, S.; Canseco-Ávila, L.M.; et al. Glucose-6-Phosphate Dehydrogenase::6-Phosphogluconolactonase from the Parasite Giardia lamblia. A Molecular and Biochemical Perspective of a Fused Enzyme. Microorganisms 2021, 9, 1678. [Google Scholar] [CrossRef]
- Morales-Luna, L.; Vázquez-Bautista, M.; Martínez-Rosas, V.; Rojas-Alarcón, M.A.; Ortega-Cuellar, D.; González-Valdez, A.; de la Cruz, V.P.; Arreguin-Espinosa, R.; Rodríguez-Bustamante, E.; Rodríguez-Flores, E.; et al. Fused Enzyme Glucose-6-Phosphate Dehydrogenase::6-Phosphogluconolactonase (G6PD::6PGL) as a Potential Drug Target in Giardia lamblia, Trichomonas vaginalis, and Plasmodium falciparum. Microorganisms 2024, 12, 112. [Google Scholar] [CrossRef]
- Morales-Luna, L.; Hernández-Ochoa, B.; Martínez-Rosas, V.; Navarrete-Vázquez, G.; Ortega-Cuellar, D.; Rufino-González, Y.; González-Valdez, A.; Arreguin-Espinosa, R.; Franco-Vásquez, A.M.; Pérez de la Cruz, V.; et al. Giardia lamblia G6PD::6PGL Fused Protein Inhibitors Decrease Trophozoite Viability: A New Alternative against Giardiasis. Int. J. Mol. Sci. 2022, 23, 14358. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Chávez-Silva, F.; Colín-Lozano, B.; Estrada-Soto, S.; Hidalgo-Figueroa, S.; Guerrero-Álvarez, J.; Méndez, S.T.; Reyes-Vivas, H.; Oria-Hernández, J.; Canul-Canché, J.; et al. Synthesis of nitro(benzo)thiazole acetamides and in vitro antiprotozoal effect against amitochondriate parasites Giardia intestinalis and Trichomonas vaginalis. Bioorg Med. Chem. 2015, 23, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Auriostigue-Bautista, J.C.; Hernández-Vázquez, E.; González-Calderón, D.; Figueroa-Romero, J.L.; Castillo-Villanueva, A.; Torres-Arroyo, A.; Ponce-Macotela, M.; Rufino-González, Y.; Martínez-Gordillo, M.; Miranda, L.D.; et al. Discovery of Benzopyrrolizidines as Promising Antigiardiasic Agents. Front. Cell Infect. Microbiol. 2022, 11, 828100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rufino-González, Y.; Ponce-Macotela, M.; García-Ramos, J.C.; Martínez-Gordillo, M.N.; Galindo-Murillo, R.; González-Maciel, A.; Reynoso-Robles, R.; Tovar-Tovar, A.; Flores-Alamo, M.; Toledano-Magaña, Y.; et al. Antigiardiasic activity of Cu(II) coordination compounds: Redox imbalance and membrane damage after a short exposure time. J. Inorg. Biochem. 2019, 195, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Conde, C.; Colín-Lozano, B.; Gutiérrez-Hernández, A.; Hernández-Núñez, E.; Yépez-Mulia, L.; Colorado-Pablo, L.F.; Aguayo-Ortiz, R.; Escalante, J.; Rivera-Leyva, J.C.; Sánchez-Carranza, J.N.; et al. Enhancing Giardicidal Activity and Aqueous Solubility through the Development of “RetroABZ”, a Regioisomer of Albendazole: In Vitro, In Vivo, and In Silico Studies. Int. J. Mol. Sci. 2023, 24, 14949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cock, I.E.; Rayan, P. Ascorbic acid potentiates the Giardia duodenalis growth inhibitory activity of pure Terminalia ferdinandiana Exell compounds. Parasitol. Res. 2020, 119, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Popruk, S.; Tummatorn, J.; Sreesai, S.; Ampawong, S.; Thiangtrongjit, T.; Tipthara, P.; Tarning, J.; Thongsornkleeb, C.; Ruchirawat, S.; Reamtong, O. Inhibition of Giardia duodenalis by isocryptolepine -triazole adducts and derivatives. Int. J. Parasitol. Drugs Drug Resist. 2024, 26, 100561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Souza, J.B.; Tsantarlis, K.; Tonelli, R.R. Oxygen-dependent regulation of permeability in low resistance intestinal epithelial cells infected with Giardia lamblia. Exp. Parasitol. 2022, 240, 108329. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Laurent, F.; Langford, T.D.; Hetsko, M.L.; Smith, J.R.; Kagnoff, M.F.; Gillin, F.D. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 2000, 164, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rosas, V.; Navarrete-Vázquez, G.; Ortega-Cuellar, D.; Arreguin-Espinosa, R.; Pérez de la Cruz, V.; Calderón-Jaimes, E.; Enríquez-Flores, S.; Wong-Baeza, C.; Baeza-Ramírez, I.; Morales-Luna, L.; et al. Imidazole Carbamates as a Promising Alternative for Treating Trichomoniasis: In Vitro Effects on the Growth and Gene Expression of Trichomonas vaginalis. Molecules 2024, 29, 2585. [Google Scholar] [CrossRef]
- Feng, X.M.; Cao, L.J.; Adam, R.D.; Zhang, X.C.; Lu, S.Q. The catalyzing role of PPDK in Giardia lamblia. Biochem. Biophys. Res. Commun. 2008, 367, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.F.; Chen, B.; Wen, J.F. Giardiasis, drug resistance, and new target discovery. Infect. Disord. Drug Targets 2010, 10, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Schofield, P.J.; Edwards, M.R. Pyruvate kinase is present in Giardia intestinalis. Exp. Parasitol. 1997, 87, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, F.; Hong, C.Q.; Giuliano, A.E.; Cui, X.J.; Zhou, G.J.; Zhang, G.J.; Cui, Y.K. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biol. Med. 2015, 12, 10–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sirover, M.A. GAPDH and Hypoxia. In Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH); Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–165. ISBN 9780128098523. [Google Scholar]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Collins, L.J. Reconstruction of Sugar Metabolic Pathways of Giardia lamblia. Int. J. Proteomics. 2012, 2012, 980829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, X.; Yang, C.; Zheng, W.; Wen, J. Structural and evolutionary characteristics of pyruvate phosphate dikinase in Giardia lamblia and other amitochondriate protozoa. Chin. Med. J. 2014, 127, 4097–4103. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.Á. Phosphorylation and acylation transfer reactions: Clues to a dual origin of metabolism. Biosystems 2020, 198, 104260. [Google Scholar] [CrossRef] [PubMed]
- Baykov, A.A.; Malinen, A.M.; Luoto, H.H.; Lahti, R. Pyrophosphate-fueled Na+ and H+ transport in prokaryotes. Microbiol. Mol. Biol. Rev. 2013, 77, 267–276. [Google Scholar] [CrossRef]
- Belozersky, A.N.; Kulaev, I.S. Polyphosphates and their significance in the development of Aspergillus niger. Biochemistry 1957, 22, 27–36. [Google Scholar] [PubMed]
- Holm, N.G.; Baltscheffsky, H. Links between hydrothermal environments, pyrophosphate, Na+, and early evolution. Orig. Life Evol. Biosph. 2011, 41, 483–493. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. Pyrophosphate as an alternative energy currency in plants. Biochem. J. 2021, 478, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Ghosh, E.; Mukherjee, A.K.; Nozaki, T.; Ganguly, S. Differential gene expression in Giardia lamblia under oxidative stress: Significance in eukaryotic evolution. Gene 2014, 535, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Saini, P.; Nozaki, T.; Ganguly, S. Involvement of pyruvate on oxidative stress management in the microaerophilic protozoan parasite Giardia lamblia. Int. J. Adv. Res. 2015, 4, 1148–1166. [Google Scholar]
- Fox, L.M.; Saravolatz, L.D. Nitazoxanide: A new thiazolide antiparasitic agent. Clin. Infect. Dis. 2005, 40, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Begaydarova, R.; Yukhnevich, Y.; Babenko, D.; Kaliyeva, S.; Azizov, I.; Muldaeva, G.; Omarkulov, B. Determination of PFOR gene expression in strains of G. intestinalis with different inhibitory concentrations of metronidazole. J. Infect. Dev. Ctries 2015, 9, 519–523. [Google Scholar] [CrossRef] [PubMed]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moussaoui, M.; Misevičienė, L.; Anusevičius, Ž.; Marozienė, A.; Lederer, F.; Baciou, L.; Čėnas, N. Quinones and nitroaromatic compounds as subversive substrates of Staphylococcus aureus flavohemoglobin. Free Radic. Biol. Med. 2018, 123, 107–115. [Google Scholar] [CrossRef] [PubMed]
- APech-Santiago, E.O.; Argüello-García, R.; Arce-Cruz, G.; Angeles, E.; Ortega-Pierres, G. Giardia duodenalis flavohemoglobin is a target of 5-nitroheterocycle and benzimidazole compounds acting as enzymatic inhibitors or subversive substrates. Free Radic. Biol. Med. 2025, 227, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Villanueva, A.; Méndez, S.T.; Torres-Arroyo, A.; Reyes-Vivas, H.; Oria-Hernández, J. Cloning, Expression and Characterization of Recombinant, NADH Oxidase from Giardia lamblia. Protein J. 2016, 35, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Mastronicola, D.; Falabella, M.; Forte, E.; Testa, F.; Sarti, P.; Giuffrè, A. Antioxidant defence systems in the protozoan pathogen Giardia intestinalis. Mol. Biochem. Parasitol. 2016, 206, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Müller, J.; Müller, N. Evaluation of Giardia lamblia thioredoxin reductase as drug activating enzyme and as drug target. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 148–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma’ayeh, S.Y.; Knörr, L.; Svärd, S.G. Transcriptional profiling of Giardia intestinalis in response to oxidative stress. Int. J. Parasitol. 2015, 45, 925–938. [Google Scholar] [CrossRef]
- Leitsch, D.; Rout, S.; Lundström-Stadelmann, B.; Balmer, V.; Hehl, A.; Müller, N. Giardia lamblia: Missing evidence for a canonical thioredoxin system. Parasitol. Open 2017, 3, e13. [Google Scholar] [CrossRef]
- Leitsch, D.; Schlosser, S.; Burgess, A.; Duchêne, M. Nitroimidazole drugs vary in their mode of action in the human parasite Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.P.; Benchimol, M.; de Souza, W. The structural organization of Giardia intestinalis cytoskeleton. Adv. Parasitol. 2020, 107, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nava-Zuazo, C.; Chávez-Silva, F.; Moo-Puc, R.; Chan-Bacab, M.J.; Ortega-Morales, B.O.; Moreno-Díaz, H.; Díaz-Coutiño, D.; Hernández-Núñez, E.; Navarrete-Vázquez, G. 2-Acylamino-5-nitro-1,3-thiazoles: Preparation and in vitro bioevaluation against four neglected protozoan parasites. Bioorg. Med. Chem. 2014, 22, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Marcial-Quino, J.; Gómez-Manzo, S.; Fierro, F.; Vanoye-Carlo, A.; Rufino-González, Y.; Sierra-Palacios, E.; Castillo-Villanueva, A.; Castillo-Rodríguez, R.A.; Rodríguez-Bustamante, E.; Arreguin-Espinosa, R.; et al. Stem-Loop RT-qPCR as an Efficient Tool for the Detection and Quantification of Small RNAs in Giardia lamblia. Genes 2016, 7, 131. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Vivas, H.; de la Mora-de la Mora, I.; Castillo-Villanueva, A.; Yépez-Mulia, L.; Hernández-Alcántara, G.; Figueroa-Salazar, R.; García-Torres, I.; Gómez-Manzo, S.; Méndez, S.T.; Vanoye-Carlo, A.; et al. Giardial triosephosphate isomerase as possible target of the cytotoxic effect of omeprazole in Giardia lamblia. Antimicrob. Agents Chemother. 2014, 58, 7072–7082. [Google Scholar] [CrossRef]


| Model | Compounds | ||||||
|---|---|---|---|---|---|---|---|
| CNZ-7 | CNZ-8 | FLP-2 | FLP-6 | FLP-8 | NTZ | ||
| A | Gastrointestinal absorption | (---) | (---) | (---) | (---) | (---) | (---) |
| Caco-2 permeability | −4.839 | −4.686 | −4.798 | −4.789 | −4.908 | −5.038 | |
| D | Plasma protein binding | 98% | 93.5% | 96.4% | 97.8% | 96.6% | 97.2% |
| Blood–brain barrier permeability | (---) | (---) | (++) | (---) | (---) | (+++) | |
| Volume of distribution | 1.899 L/kg | 0.526 L/kg | 1.32 L/kg | 1.085 L/kg | 0.633 L/kg | 0.478 L/kg | |
| M | CYP3A4 substrate | (---) | (-) | (---) | (---) | (---) | (---) |
| CYP2D6 substrate | (+++) | (+++) | (+++) | (+) | (-) | (---) | |
| E | CL plasma | 3.832 mL/min/Kg | 1.697 mL/min/Kg | 4.826 mL/min/Kg | 4.381 mL/min/kg | 2.615 mL/min/kg | 2.529 mL/min/kg |
| Half-life (T½) | <3 h | <3 h | <3 h | <3 h | <3 h | <3 h | |
| T | Blockers hERG | (--) | (---) | (--) | (--) | (---) | (--) |
| Rat oral acute toxicity | (+++) | (+++) | (+++) | (+++) | (+++) | (+++) | |
| Carcinogenesis | (+++) | (+++) | (+++) | (+++) | (+++) | (++) | |
| Gene Symbol | Gene Name | Length (bp) | Function | Accession Number |
|---|---|---|---|---|
| PFK | Phosphofructokinase | 1635 | Transferase in glycolysis | XM_001707455.1 |
| TPI | Triose phosphate isomerase | 764 | Oxidoreductase in glycolysis | XM_001706778 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 1224 | Oxidoreductase in glycolysis | XM_001703983 |
| PK | Pyruvate kinase | 1662 | Oxidoreductase in glycolysis | XM_001709477.1 |
| PPDK | Pyruvate phosphate dikinase | 2655 | Oxidoreductase in glycolysis | XM_001705520.1 |
| ALDO | Aldolase | 972 | Oxidoreductase in glycolysis | XM_001709998 |
| G6PD | Glucose-6-phosphate dehydrogenase | 2229 | Oxidoreductase in PPP | XM_001704389.1 |
| 6PGDH | 6-Phosphogluconate dehydrogenase | 1416 | Oxidoreductase in PPP | XM_001704391.1 |
| TKT | Transketolase | 2160 | Transferase in PPP | XM_001704562.1 |
| NADHox | NADH oxidase | 1377 | O2 detoxifying enzyme | XM_001707922 |
| PFOR | Pyruvate:ferredoxin oxidoreductase | 3762 | Oxidoreductase | XM_001708652.1 |
| GIA | Giardin | 1091 | Cytoskeletal structural protein | AF331827 |
| ACT | Actin | 1128 | Cytoskeletal structural protein | AF331826 |
| VSP | Variant surface protein | 702 | Membrane protein | U89152 |
| Gene | 5′-3′ Sequence | Length (bp) | Function |
|---|---|---|---|
| δ-GIA, giardin [66] | Fw 5′ AGGACGACCAGGAGGAGAA-3′ Rv 5′ ACGGGTAAAGGCACAATTCA-3′ | 74 | Structural |
| ACT, actin [66] | Fw 5′ TTGCCGTACCTGCCTTCTAT Rv 5′ GCCCGGAACTGTAGAGAGC | 60 | Structural |
| VSP, variant-specific surface [66] | Fw 5′ GCGAAAGTGATAGCAATGGG Rv 5′ TGAGGTAACAGAGGACGGAGC | 60 | Structural |
| P-FOR, pyruvate oxidoreductase [54] | Fw 5′ CTACGACATTGACTTTGCTG-3′ Rv 5′ CCCATCTTCTTGTCCTTGAC-3′ | 180 | Energy production |
| NADH, oxidase [66] | Fw 5′ GCACCATATGGCTTCAACGG Rv 5′ CAGGCCTGTCCGTGTCATTA | 98 | Oxidative stress |
| ALD, Aldolase [66] | Fw 5′ GAGTCCGTGAAGATGGCGA Rv 5′ GTCCCAAGTTCAGCCTCCAC | 149 | Glycolysis |
| TPI, triose phosphate isomerase [66] | Fw 5′ AGGAGCTCGGAGAGTCCAA Rv 5′ ACACGGGCTCGTAAGCAAT | 60 | Glycolysis |
| GAPDH, glyceraldehyde-3-phosphate [66] | Fw 5′ CATGGAGCGTGCCTACTT Rv 5′ CACTCCAAGACCACATCC | 237 | Glycolysis |
| PPDK, pyruvate phosphate dikinase [66] | Fw 5′ TTGGAAACACAGGCGATGAC Rv 5′ TCATCATAGCACGCCTTCCA | 196 | Glycolysis |
| G6PD, glucose-6-phosphate dehydrogenase | Fw 5′- CTACCTTCACAAGGACAC-3′ Rv 5′- ATACCGTCCTTAATACGA -3′ | 87 | PPP |
| 6PDH, 6-phosphogluconate dehydrogenase | Fw 5′ CTCGACATGATCCAGACTG-3′ Rv 5′ TCATAGGTGTGAGCTCCAA-3′ | 80 | PPP |
| TKT, transketolase | Fw 5′ AAGATCACCATACACGGC-3′ Rv 5′ ACGGGATAGGCATACGATA-3′ | 96 | PPP |
| PFK, phosphofructokinase | Fw 5′ ATCTCTCAGATTGAAACG-3′ Rv 5′ AGTGATAGAGCGGAGTAA-3′ | 97 | Glycolysis |
| PK, pyruvate kinase | Fw 5′ AGGTGTGGATAAGAATCA-3′ Rv 5″ GATCATTCCTGCTATGAC-3′ | 97 | Glycolysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Luna, L.; Hernández-Ochoa, B.; González-Valdez, A.; Vázquez-Bautista, M.; Arreguin-Espinosa, R.; Pérez de la Cruz, V.; Enríquez-Flores, S.; De la Mora De la Mora, I.; Hernández-Urzúa, E.; Castillo-Rodríguez, R.A.; et al. Nitazoxanide Analogs: Synthesis, In Vitro Giardicidal Activity, and Effects on Giardia lamblia Metabolic Gene Expression. Int. J. Mol. Sci. 2025, 26, 4504. https://doi.org/10.3390/ijms26104504
Morales-Luna L, Hernández-Ochoa B, González-Valdez A, Vázquez-Bautista M, Arreguin-Espinosa R, Pérez de la Cruz V, Enríquez-Flores S, De la Mora De la Mora I, Hernández-Urzúa E, Castillo-Rodríguez RA, et al. Nitazoxanide Analogs: Synthesis, In Vitro Giardicidal Activity, and Effects on Giardia lamblia Metabolic Gene Expression. International Journal of Molecular Sciences. 2025; 26(10):4504. https://doi.org/10.3390/ijms26104504
Chicago/Turabian StyleMorales-Luna, Laura, Beatriz Hernández-Ochoa, Abigail González-Valdez, Montserrat Vázquez-Bautista, Roberto Arreguin-Espinosa, Verónica Pérez de la Cruz, Sergio Enríquez-Flores, Ignacio De la Mora De la Mora, Elizabeth Hernández-Urzúa, Rosa Angélica Castillo-Rodríguez, and et al. 2025. "Nitazoxanide Analogs: Synthesis, In Vitro Giardicidal Activity, and Effects on Giardia lamblia Metabolic Gene Expression" International Journal of Molecular Sciences 26, no. 10: 4504. https://doi.org/10.3390/ijms26104504
APA StyleMorales-Luna, L., Hernández-Ochoa, B., González-Valdez, A., Vázquez-Bautista, M., Arreguin-Espinosa, R., Pérez de la Cruz, V., Enríquez-Flores, S., De la Mora De la Mora, I., Hernández-Urzúa, E., Castillo-Rodríguez, R. A., Cárdenas-Rodríguez, N., Martínez-Rosas, V., Navarrete-Vázquez, G., & Gómez-Manzo, S. (2025). Nitazoxanide Analogs: Synthesis, In Vitro Giardicidal Activity, and Effects on Giardia lamblia Metabolic Gene Expression. International Journal of Molecular Sciences, 26(10), 4504. https://doi.org/10.3390/ijms26104504










