Characterization of the Antiproliferative and Antimetastatic Properties of Centrapalus pauciflorus Meroterpenoid Centrapalus Coumarin F
Abstract
1. Introduction
2. Results
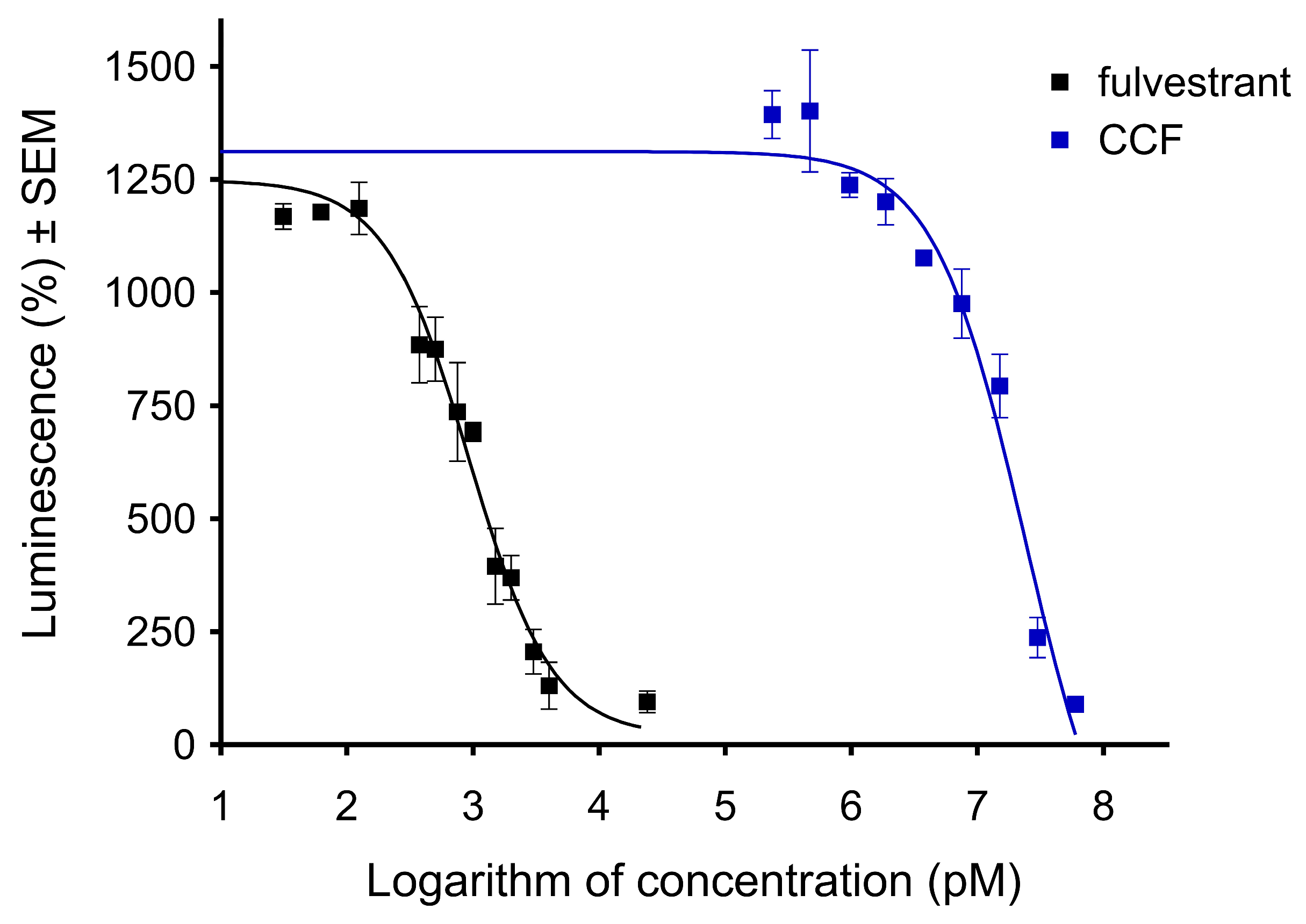
2.1. Antiproliferative Properties of CCF
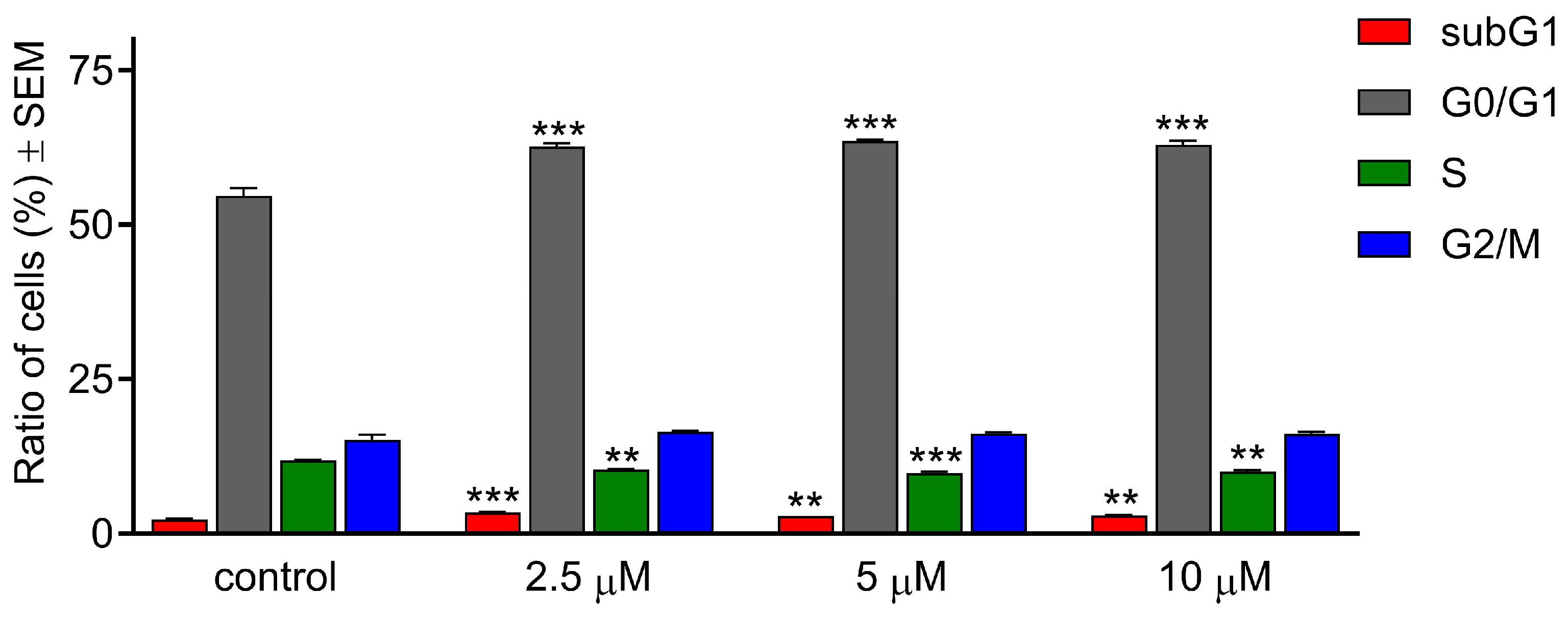
2.2. CCF Induced G0/G1 Cell Cycle Arrest
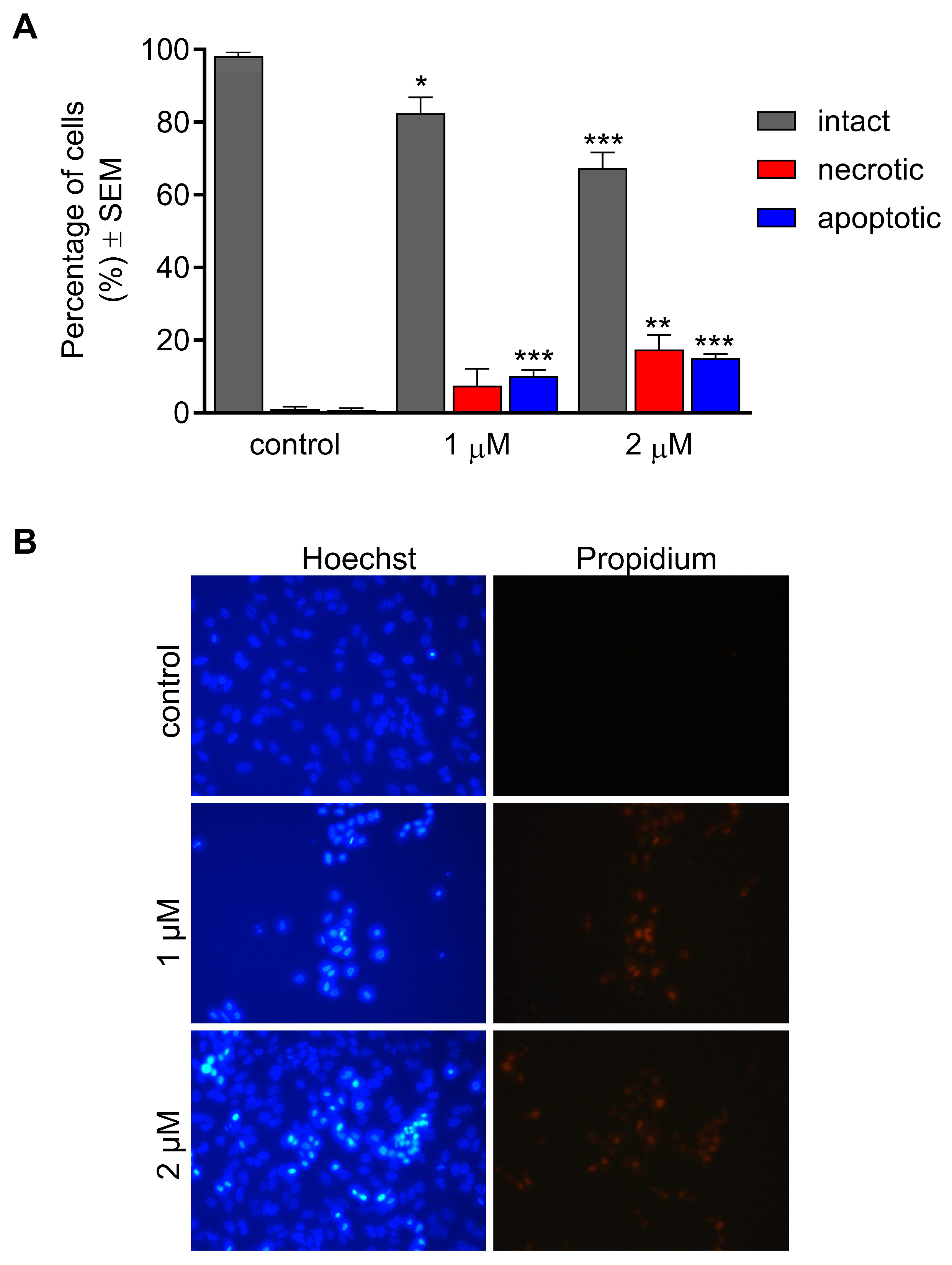
2.3. CCF Induced Morphological Changes
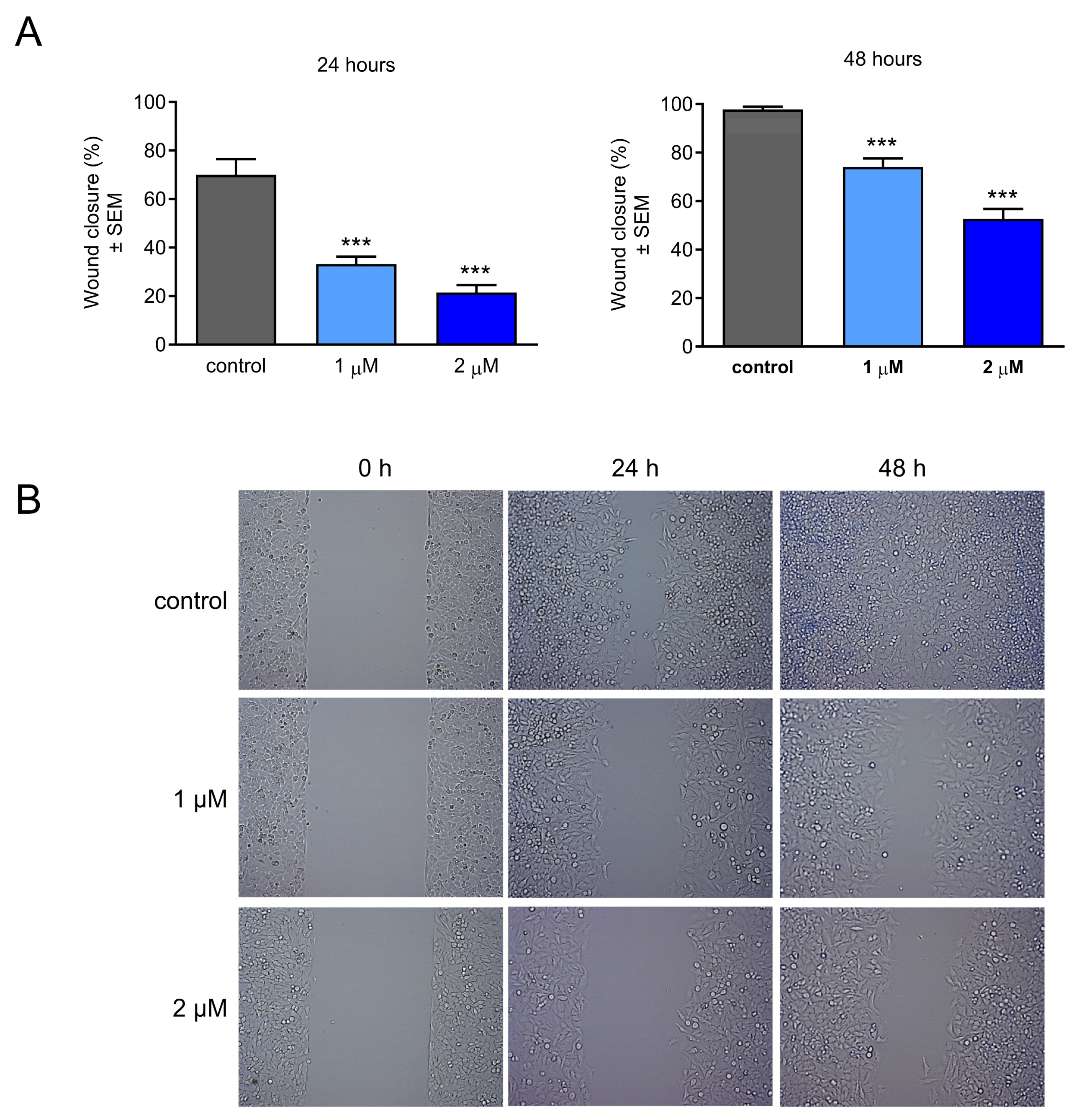
2.4. Effect of CCF on Cancer Cell Migration
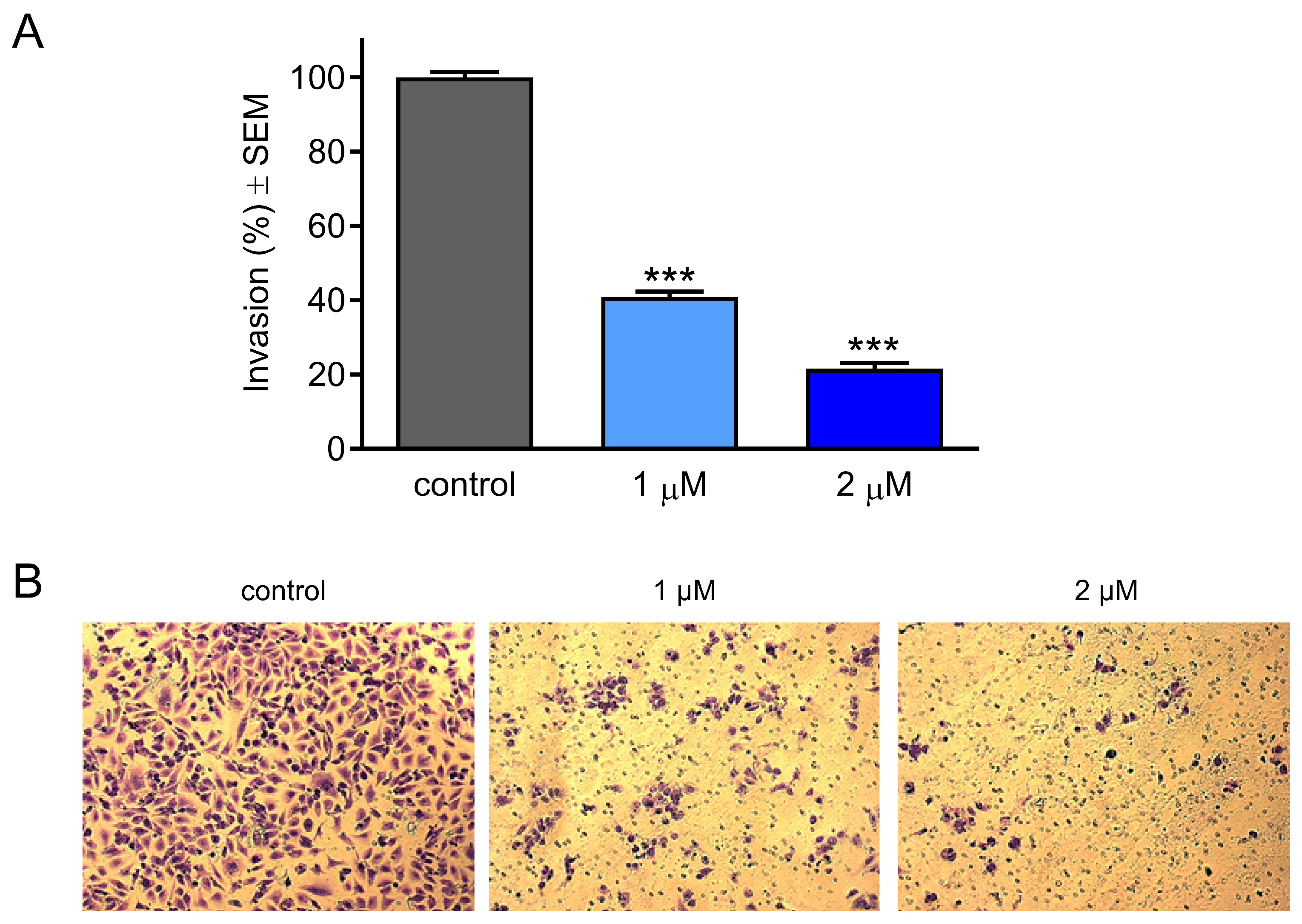
2.5. Effect of CCF on Cancer Cell Invasion
2.6. CCF Exerted an Antiestrogenic Effect
3. Discussion
4. Materials and Methods
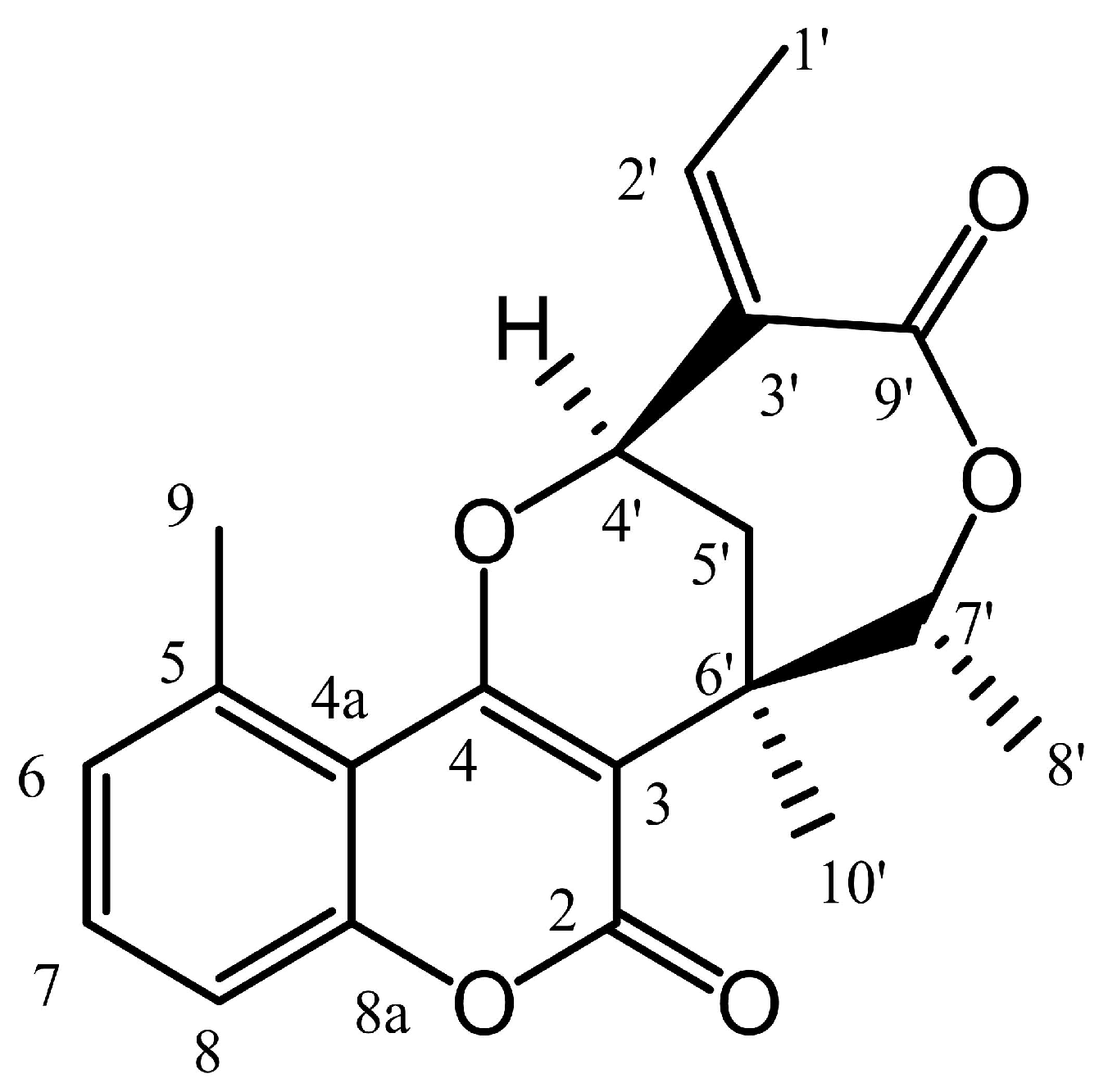
4.1. Test Compound
4.2. Tumor Cell Lines and Culture
4.3. Antiproliferative Assay
4.4. Cell Cycle Analysis
4.5. Hoechst and Propidium Iodide Fluorescent Double Staining
4.6. Cell Migration Assay
4.7. Cell Invasion Assay
4.8. Antiestrogenic Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCF | Centrapalus coumarin F |
| DMSO | Dimethylsulfoxide |
| FBS | Fetal bovine serum |
| HO | Hoechst 33258 |
| HPV | Human papillomavirus |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| SEM | Standard error of the mean |
References
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Bray, F.; Charvat, H.; Rajaraman, S.; Soerjomataram, I. The world cancer patient population (WCPP): An updated standard for international comparisons of population-based survival. Cancer Epidemiol. 2020, 69, 101802. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, C.E.; Clarke, W. Cancer chemotherapy: The case for therapeutic drug monitoring. Ther. Drug Monit. 2020, 42, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.H.; Menard, S.M.; Labedz, M.Z.; Krueger, C.D.; Sarna, K.V. Enteral tube administration of oral chemotherapy drugs. J. Oncol. Pharm. Pract. 2020, 26, 703–717. [Google Scholar] [CrossRef]
- Zraik, I.M.; Hess-Busch, Y. Management von Nebenwirkungen der Chemotherapie und deren Langzeitfolgen. Urologe 2021, 60, 862–871. [Google Scholar] [CrossRef]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody-drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef]
- Ciarimboli, G. Anticancer platinum drugs update. Biomolecules 2021, 11, 1637. [Google Scholar] [CrossRef]
- Efferth, T.; Saeed, M.E.M.; Kadioglu, O.; Seo, E.J.; Shirooie, S.; Mbaveng, A.T.; Nabavi, S.M.; Kuete, V. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol. Adv. 2020, 38, 107342. [Google Scholar] [CrossRef]
- Nazir, M.; Saleem, M.; Tousif, M.I.; Anwar, M.A.; Surup, F.; Ali, I.; Wang, D.; Mamadalieva, N.Z.; Alshammari, E.; Ashour, M.L.; et al. Meroterpenoids: A comprehensive update insight on structural diversity and biology. Biomolecules 2021, 11, 957. [Google Scholar] [CrossRef]
- Ahmed, M.; Jakupovic, J.; Bohlmann, F.; Mungai, M.G. A 5-methylcoumarin and glaucolides from Bothriocline-amplifolia. Phytochemistry 1991, 30, 2807–2808. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Ahmed, A.A.; Iinuma, M.; Tanaka, T. Further monoterpene 5-methylcoumarins and an acetophenone derivative from. Phytochemistry 1998, 48, 543–546. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer potential of coumarin and its derivatives. Mini Rev. Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-containing hybrids and their anticancer activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Singh, H.; Singh, J.V.; Bhagat, K.; Gulati, H.K.; Sanduja, M.; Kumar, N.; Kinarivala, N.; Sharma, S. Rational approaches, design strategies, structure activity relationship and mechanistic insights for therapeutic coumarin hybrids. Bioorg. Med. Chem. 2019, 27, 3477–3510. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, L.; Sun, X.; Zeng, F.; Pan, Q.; Xue, M. Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion and PI3K/AKT signalling pathway. J. BUON 2019, 24, 997–1002. [Google Scholar]
- Saidu, M.B.; Krstic, G.; Todorovic, N.; Berkecz, R.; Ali, H.; Zupko, I.; Hohmann, J.; Redei, D. Monoterpenoid 5-methylcoumarins from Centrapalus pauciflorus with antiproliferative activity. Arab. J. Chem. 2023, 16, 104777. [Google Scholar] [CrossRef]
- Huang, L.; Feng, Z.L.; Wang, Y.T.; Lin, L.G. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017, 15, 881–888. [Google Scholar] [CrossRef]
- Kostova, I. Studying plant-derived coumarins for their pharmacological and therapeutic properties as potential anticancer drugs. Expert Opin. Drug Discov. 2007, 2, 1605–1618. [Google Scholar] [CrossRef]
- Ma, Q.; Jiang, J.G.; Yuan, X.; Qiu, K.; Zhu, W. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC. Food Chem. Toxicol. 2019, 125, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Song, X.F.; Fan, J.; Liu, L.; Liu, X.F.; Gao, F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. 2020, 353, e2000025. [Google Scholar] [CrossRef] [PubMed]
- Moi, D.; Carradori, S.; Gallorini, M.; Mencarelli, N.; Deplano, A.; Angeli, A.; Vittorio, S.; Supuran, C.T.; Onnis, V. Investigation on human carbonic anhydrase IX and XII inhibitory activity and A549 antiproliferative activity of a new class of coumarinamides. Pharmaceuticals 2025, 18, 372. [Google Scholar] [CrossRef] [PubMed]
- Zengin Kurt, B.; Celebi, G.; Ozturk Civelek, D.; Angeli, A.; Akdemir, A.; Sonmez, F.; Supuran, C.T. Tail-approach-based design and synthesis of coumarin-monoterpenes as carbonic anhydrase inhibitors and anticancer agents. ACS Omega 2023, 8, 5787–5807. [Google Scholar] [CrossRef]
- Koley, M.; Han, J.; Soloshonok, V.A.; Mojumder, S.; Javahershenas, R.; Makarem, A. Latest developments in coumarin-based anticancer agents: Mechanism of action and structure–activity relationship studies. RSC Med. Chem. 2024, 15, 10–54. [Google Scholar] [CrossRef]
- Lepley, D.M.; Pelling, J.C. Induction of p21/WAF1 and G1 cell-cycle arrest by the chemopreventive agent apigenin. Mol. Carcinog. 1997, 19, 74–82. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Y.; Zhou, P.; Gui, S.; Wu, X.; Xia, Y.; Tu, J. Panduratin A inhibits cell proliferation by inducing G0/G1 phase cell cycle arrest and induces apoptosis in breast cancer cells. Biomol. Ther. 2018, 26, 328–334. [Google Scholar] [CrossRef]
- Noori, S.; Hassan, Z.M. Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radic. Biol. Med. 2012, 52, 1987–1999. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The apoptosis paradox in cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, L.; Ran, Q.; Wang, J.; Wang, C.; He, H.; Li, L.; Qi, H. Notopterol-induced apoptosis and differentiation in human acute myeloid leukemia HL-60 cells. Drug Des. Devel. Ther. 2019, 13, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Muyaba, M.; Lyu, W.; Tang, Z.; Zhao, R.; Xu, Q.; You, Q.; Xiang, H. Design, synthesis and biological evaluation of novel 3-substituted 4-anilino-coumarin derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2017, 27, 867–874. [Google Scholar] [CrossRef]
- Yan, G.; Luo, J.; Han, X.; Zhang, W.; Pu, C.; Zhou, M.; Zhong, X.; Hou, X.; Hou, M.Z.; Li, R. Design, synthesis and biological evaluation of 4, 6-coumarin derivatives as anti- cancer and apoptosis-inducing agents. Anticancer Agents Med. Chem. 2021, 21, 2351–2367. [Google Scholar] [CrossRef]
- Zhu, H.; Ying, S.; Zhou, B.; Hu, X.; Liang, X.; Li, W.; Wang, D.; Jin, H.; Pan, Y. Design, synthesis, and evaluation of novel coumarin-dithiocarbamate derivatives (IDs) as anti-colorectal cancer agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 593–604. [Google Scholar] [CrossRef]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R.; Kshitiz. Systems biology of cancer metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef]
- Clarke, R.; Kraikivski, P.; Jones, B.C.; Sevigny, C.M.; Sengupta, S.; Wang, Y. A systems biology approach to discovering pathway signaling dysregulation in metastasis. Cancer Metastasis Rev. 2020, 39, 903–918. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Ganesh, K.; Massague, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Scheele, C.; Maynard, C.; van Rheenen, J. Intravital insights into heterogeneity, metastasis, and therapy responses. Trends Cancer. 2016, 2, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Beatty, P.H.; Lewis, J.D. Novel therapeutic targets for cancer metastasis. Expert Rev. Anticancer Ther. 2020, 20, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shang, Y. Estrogen and cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.G.; Zeng, Q.; Tse, G.M. Estrogen and its receptors in cancer. Med. Res. Rev. 2008, 28, 954–974. [Google Scholar] [CrossRef]
- Germain, D. Estrogen carcinogenesis in breast cancer. Endocrinol. Metab. Clin. N. Am. 2011, 40, 473–484. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor b: An overview and update. Nucl. Recept. Signal. 2008, 6, e003. [Google Scholar] [CrossRef]
- Mihovic, N.; Tomasevic, N.; Matic, S.; Mitrovic, M.M.; Kostic, D.A.; Sabatino, M.; Antonini, L.; Ragno, R.; Mladenovic, M. Human estrogen receptor α antagonists. Part 1: 3-D QSAR-driven rational design of innovative coumarin-related antiestrogens as breast cancer suppressants through structure-based and ligand-based etudies. J. Chem. Inf. Model. 2021, 61, 5028–5053. [Google Scholar] [CrossRef]
- Bai, C.; Ren, S.; Wu, S.; Zhu, M.; Luo, G.; Xiang, H. Design and synthesis of novel benzothiophene analogs as selective estrogen receptor covalent antagonists against breast cancer. Eur. J. Med. Chem. 2021, 221, 113543. [Google Scholar] [CrossRef]
- Kurtanovic, N.; Tomasevic, N.; Matic, S.; Mitrovic, M.M.; Kostic, D.A.; Sabatino, M.; Antonini, L.; Ragno, R.; Mladenovic, M. Human estrogen receptor alpha antagonists, part 2: Synthesis driven by rational design, in vitro antiproliferative, and in vivo anticancer evaluation of innovative coumarin-related antiestrogens as breast cancer suppressants. Eur. J. Med. Chem. 2022, 227, 113869. [Google Scholar] [CrossRef]
- Selvaraj, J.; John, J.B.A.; Joghee, N.M.; Antony, J.; Wadhwani, A.; Natarajan, J. Coumarin-fatty acid conjugates as potential ERα/AKT-1 antagonists for ER positive breast cancer. Anticancer Agents Med. Chem. 2020, 20, 437–449. [Google Scholar] [CrossRef]
- Kotogány, E.; Balog, J.A.; Nagy, L.I.; Alföldi, R.; Bertagnolo, V.; Brugnoli, F.; Demjén, A.; Kovács, A.K.; Batár, P.; Mezei, G.; et al. Imidazo[1,2-b]pyrazole-7-carboxamide derivative induces differentiation-coupled apoptosis of immature myeloid cells such as acute myeloid leukemia and myeloid-derived suppressor cells. Int. J. Mol. Sci. 2020, 21, 5135. [Google Scholar] [CrossRef]
- Wilson, V.S.; Bobseine, K.; Gray, L.E., Jr. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol. Sci. 2004, 81, 69–77. [Google Scholar] [CrossRef]
| Cell Line | Growth Inhibition (% ± SEM) | Calculated IC50 Value (μM) | Cisplatin IC50 (μM) | |
|---|---|---|---|---|
| 10 μM | 30 μM | |||
| HeLa 1 | 69.48 ± 1.30 | 94.60 ± 0.55 | 2.28 | 12.43 |
| SiHa 1 | 20.83 ± 1.68 | 40.84 ± 1.49 | n.d. 2 | 4.29 |
| C33A | 37.32 ± 1.89 | 65.91 ± 0.67 | 15.69 | |
| MCF7 1 | 57.26 ± 1.05 | 69.71 ± 1.01 | 6.59 | 5.78 |
| MDA-MB-231 1 | <20 3 | 26.23 ± 1.38 | n.d. | 10.17 |
| T47D | 35.65 ± 1.53 | 53.06 ± 0.88 | 25.55 | |
| A2780 1 | 37.22 ± 3.02 | 67.96 ± 2.01 | 15.41 | 1.30 |
| UPCI-SCC-131 | 24.50 ± 3.21 | 38.01 ± 2.87 | n.d. | |
| UPCI-SCC-154 | 32.61 ± 2.20 | 52.21 ± 1.14 | 36.37 | |
| U-87 | 22.25 ± 3.02 | 62.60 ± 1.96 | 21.54 | |
| NIH/3T3 | 28.59 ± 2.18 | 51.38 ± 1.46 | 28.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.; Rasool, S.; Saidu, M.B.; Germán, P.; Szebeni, G.J.; Szabó, E.; Rédei, D.; Hohmann, J.; Zupkó, I. Characterization of the Antiproliferative and Antimetastatic Properties of Centrapalus pauciflorus Meroterpenoid Centrapalus Coumarin F. Int. J. Mol. Sci. 2025, 26, 4489. https://doi.org/10.3390/ijms26104489
Ali H, Rasool S, Saidu MB, Germán P, Szebeni GJ, Szabó E, Rédei D, Hohmann J, Zupkó I. Characterization of the Antiproliferative and Antimetastatic Properties of Centrapalus pauciflorus Meroterpenoid Centrapalus Coumarin F. International Journal of Molecular Sciences. 2025; 26(10):4489. https://doi.org/10.3390/ijms26104489
Chicago/Turabian StyleAli, Hazhmat, Shelan Rasool, Muhammad Bello Saidu, Péter Germán, Gábor J. Szebeni, Enikő Szabó, Dóra Rédei, Judit Hohmann, and István Zupkó. 2025. "Characterization of the Antiproliferative and Antimetastatic Properties of Centrapalus pauciflorus Meroterpenoid Centrapalus Coumarin F" International Journal of Molecular Sciences 26, no. 10: 4489. https://doi.org/10.3390/ijms26104489
APA StyleAli, H., Rasool, S., Saidu, M. B., Germán, P., Szebeni, G. J., Szabó, E., Rédei, D., Hohmann, J., & Zupkó, I. (2025). Characterization of the Antiproliferative and Antimetastatic Properties of Centrapalus pauciflorus Meroterpenoid Centrapalus Coumarin F. International Journal of Molecular Sciences, 26(10), 4489. https://doi.org/10.3390/ijms26104489







