Reactions of Lanthanide Ions with Glycolic Acid or Tartaric Acid in the Presence of Spermine: Potentiometric and Spectroscopic Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Equilibrium Study
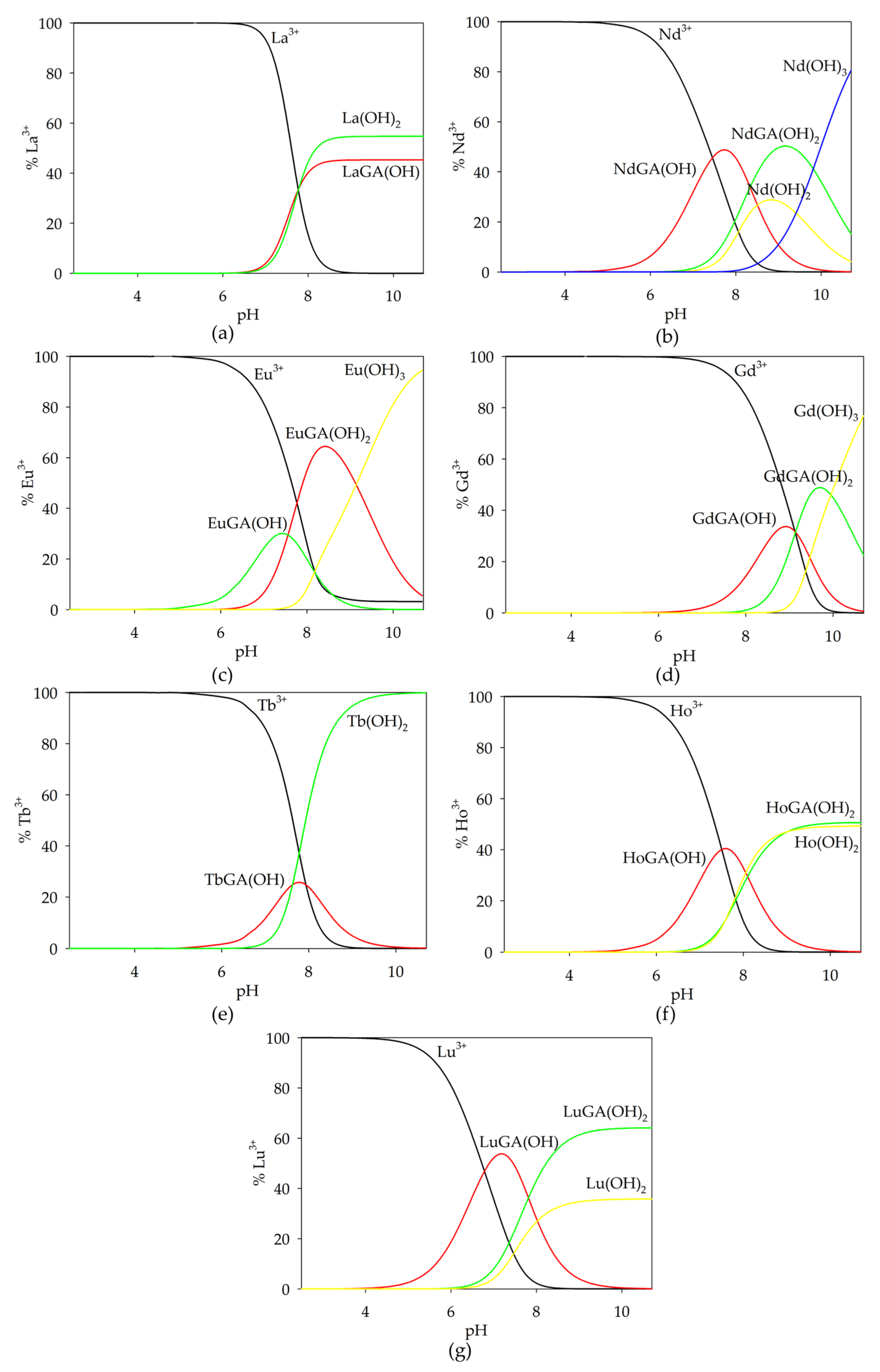
2.1.1. Binary Systems of Lanthanide(III) Ion/α-Hydroxyacids
2.1.2. Binary Systems of α-Hydroxyacids/Spermine
2.1.3. Ternary Systems of Ln(III)/Tar/Spm
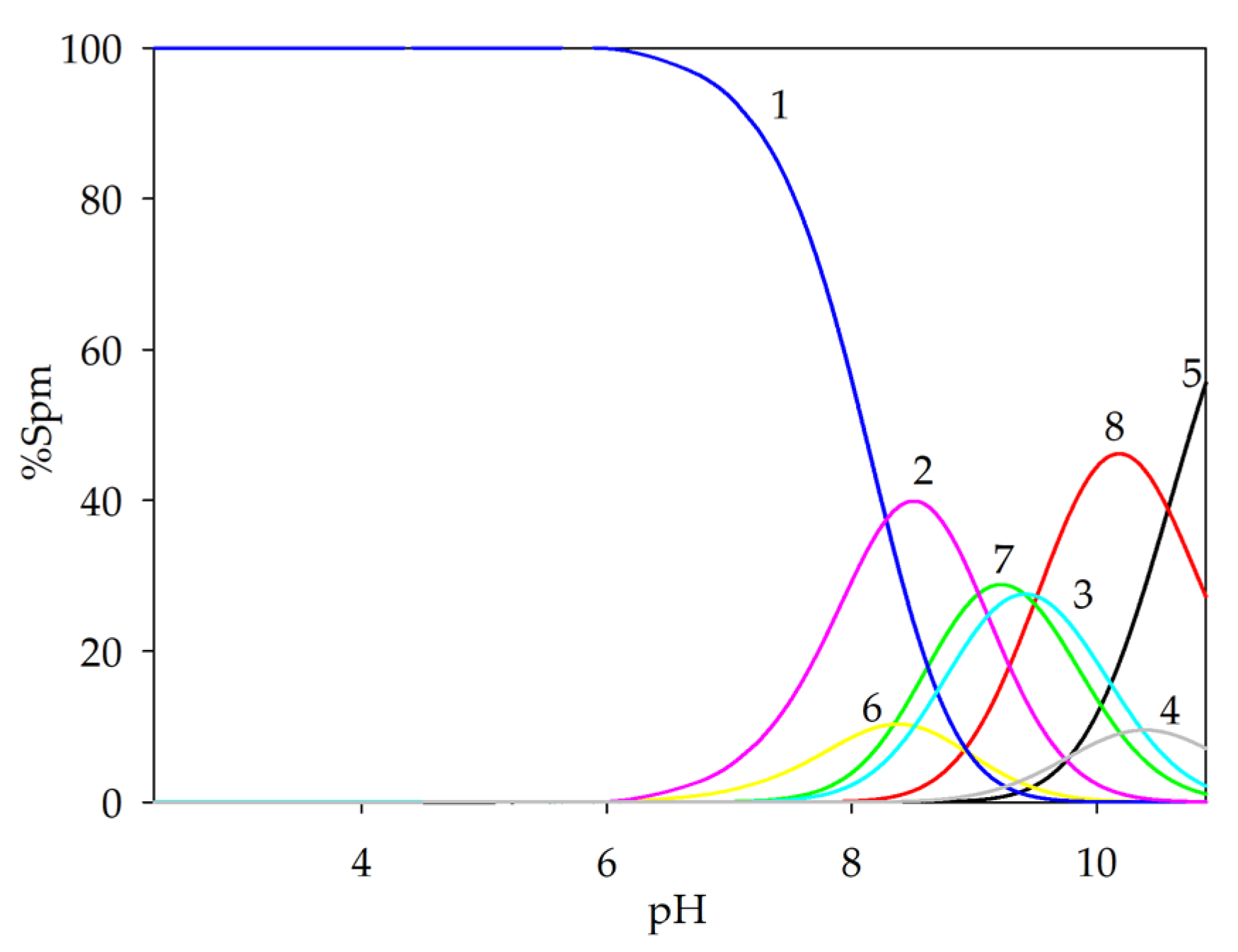
2.1.4. Ternary Systems of Ln(III)/GA/Spm
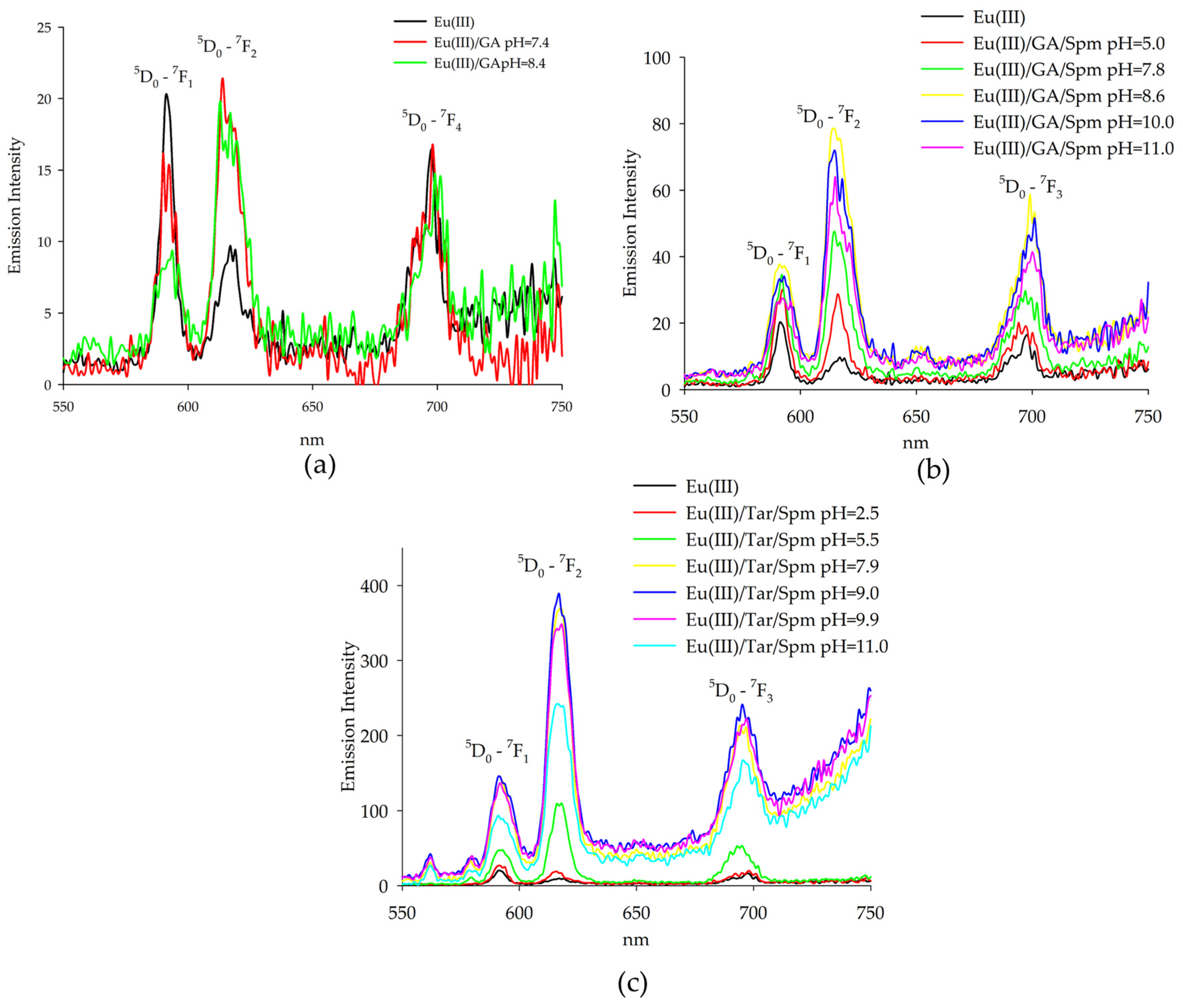
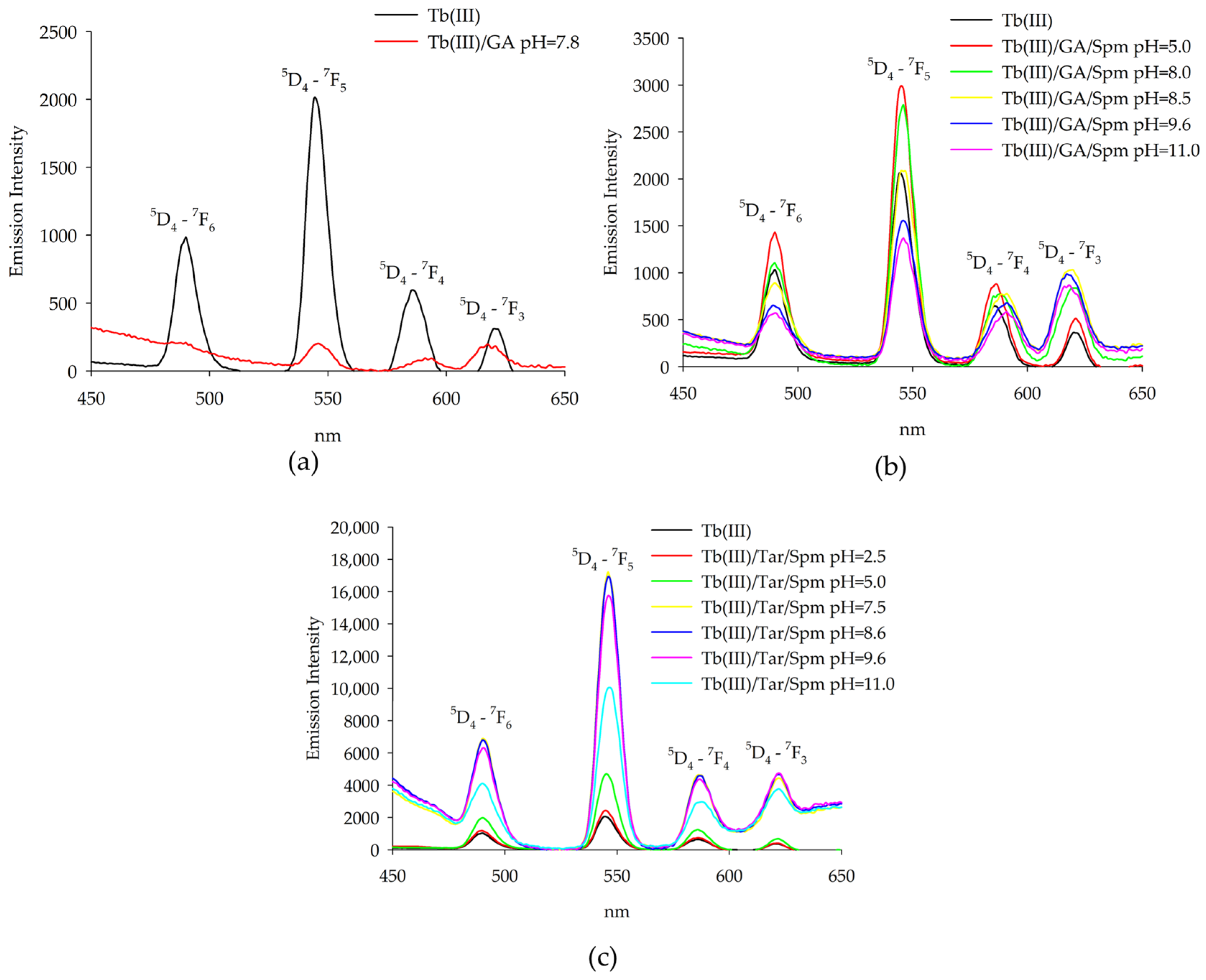
2.2. Luminescence Spectroscopy
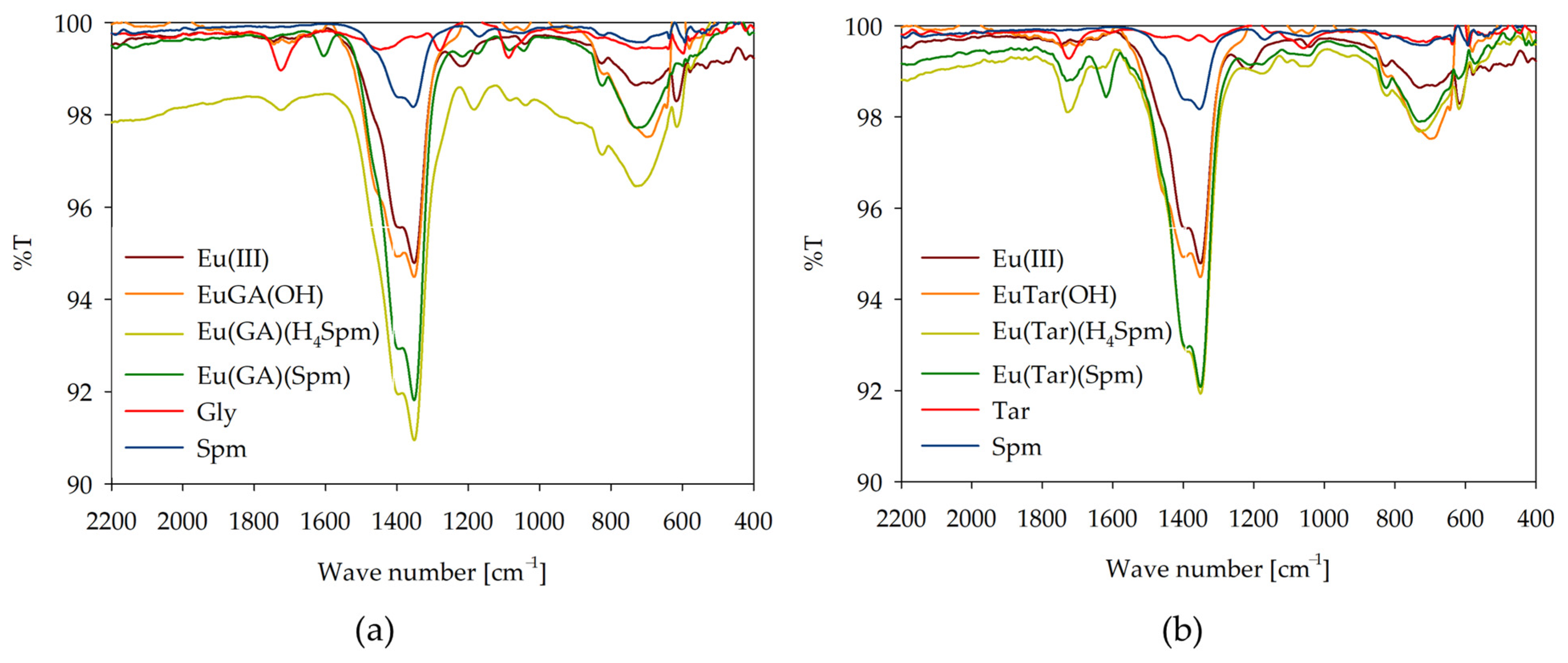
2.3. IR Spectroscopy
3. Materials and Methods
3.1. Materials
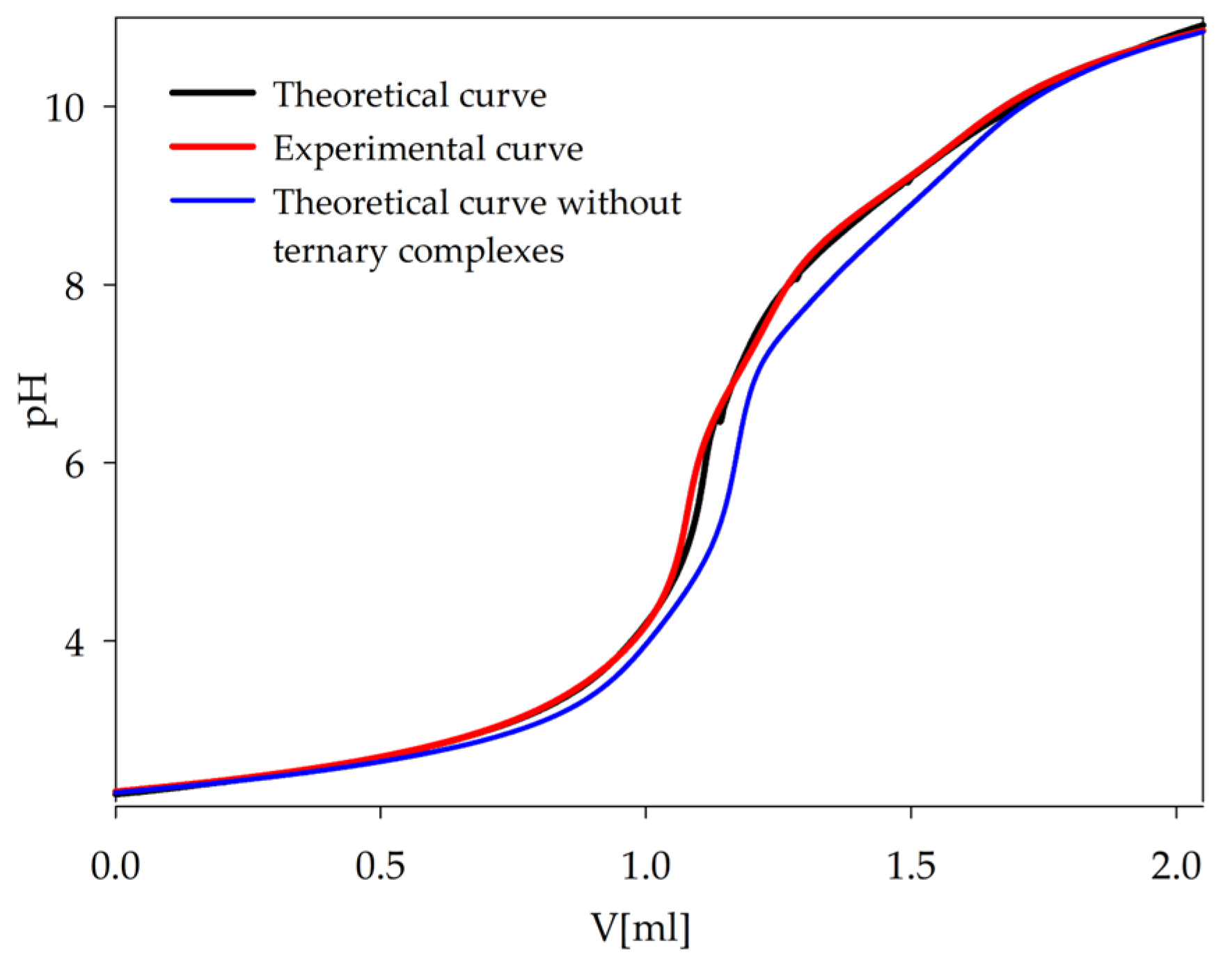
3.2. Equilibrium Study
3.3. Luminescence Spectroscopy
3.4. Infrared Spectroscopy (FT-IR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gálico, D.A.; Lahoud, M.G.; Davolos, M.R.; Frem, R.C.G.; Fraga-Silva, T.F.C.; Venturini, J.; Arruda, M.S.P.; Bannach, G. Spectroscopic, luminescence and in vitro biological studies of solid ketoprofen of heavier trivalent lanthanides and yttrium(III). J. Inorg. Biochem. 2014, 140, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Shurygin, A.V.; Vovna, V.I.; Korochentsev, V.V.; Mirochnik, A.G.; Kalinovskaya, I.V.; Sergienko, V.I. Electronic structure and optical properties of Ln(III) nitrate adducts with 1,10-phenanthroline. Spectrochim. Acta Part A 2019, 213, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bombieri, G.; Artali, R.; Mason, S.A.; McIntyre, G.J.; Mortillaro, A.; Aime, S. Inner-sphere water and hydrogen bonds in lanthanide DOTAM complexes. A neutron diffraction study. Inorg. Chim. Acta 2018, 470, 433–438. [Google Scholar] [CrossRef]
- Babič, A.; Vorobiev, V.; Xayaphoummine, C.; Lapicorey, G.; Chauvin, A.S.; Helm, L.; Allémann, E. Self-Assembled Nanomicelles as MRI Blood-Pool Contrast Agent. Chem. Eur. J. 2018, 24, 1348–1357. [Google Scholar] [CrossRef]
- Phukan, B.; Mukherjee, C.; Varshney, R. A new heptadentate picolinate-based ligand and its corresponding Gd(III) complex: The effect of pendant picolinate versus acetate on complex properties. Dalton Trans. 2018, 47, 135–142. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Goren, S.D.; Yudina, E.B.; Aleksenskii, A.E.; Ya Vul’, A.; Shames, A.I. Gd(III)-Grafted Detonation Nanodiamonds for MRI Contrast Enhancement. J. Phys. Chem. C 2019, 123, 2627–2631. [Google Scholar] [CrossRef]
- Tessitore, G.; Mandl, G.A.; Maurizio, S.L.; Kaur, M.; Capobianco, J.A. The Role of Lanthanide Luminescence in Advancing Technology. RSC Adv. 2023, 13, 17787–17811. [Google Scholar] [CrossRef]
- Sardaru, M.-C.; Marangoci, N.L.; Shova, S.; Bejan, D. Novel Lanthanide (III) Complexes Derived from an Imidazole–Biphenyl–Carboxylate Ligand: Synthesis, Structure and Luminescence Properties. Molecules 2021, 26, 6942. [Google Scholar] [CrossRef]
- Qin, X.; Wang, J.; Yuan, Q. Synthesis and Biomedical Applications of Lanthanides-Doped Persistent Luminescence Phosphors with NIR Emissions. Front. Chem. 2020, 8, 608578. [Google Scholar] [CrossRef]
- Xian, T.; Meng, Q.; Gao, F.; Hu, M.; Wang, X. Functionalization of Luminescent Lanthanide Complexes for Biomedical Applications. Coord. Chem. Rev. 2023, 474, 214866. [Google Scholar] [CrossRef]
- Jastrzab, R.; Kaczmarek, M.T.; Nowak, M.; Trojanowska, A.; Zabiszak, M. Complexes of polyamines and their derivatives as living system active compounds. Coord. Chem. Rev. 2017, 351, 32–44. [Google Scholar] [CrossRef]
- Łyszczek, R.; Mazur, L. Polynuclear complexes constructed by lanthanides and pyridine-3,5-dicarboxylate ligand: Structures, thermal and luminescent properties. Polyhedron 2012, 41, 7–19. [Google Scholar] [CrossRef]
- Wang, L.; Ni, L.; Yao, J. Synthesis, structures and fluorescent properties of two novel lanthanides [Ln=Ce(III), Pr(III)] coordination polymers based on 1,3-benzenedicarboxylateand 2-(4-methoxyphenyl)-1H-imidazo[4,5-f][1,10]phenanthroline ligands. Solid State Sci. 2012, 14, 1361–1366. [Google Scholar] [CrossRef]
- Ramos-e-Silva, M.; Celem, L.R.; Ramos-e-Silva, S.; Fucci-da-Costa, A.P. Anti-aging cosmetics: Facts and controversies. Clin. Dermatol. 2013, 31, 750–758. [Google Scholar] [CrossRef]
- Green, B.A.; Yu, R.J.; Van Scott, E.J. Clinical and cosmeceutical uses of hydroxyacids. Clin. Dermatol. 2009, 27, 495–501. [Google Scholar] [CrossRef]
- Tung, R.C.; Bergfeld, W.F.; Vidimos, A.T.; Remzi, B.K. α-Hydroxy acid-based cosmetic procedures: Guidelines for patient management. Am. J. Clin. Dermatol. 2000, 1, 81–88. [Google Scholar] [CrossRef]
- Rickman, B.H.; Matile, S.; Nakanishi, K.; Berova, N. A two-step chemical/chiroptical method for determining absolute configurations of α-hydroxy acids. Tetrahedron 1998, 54, 5041–5064. [Google Scholar] [CrossRef]
- Tamura, T.; Kawauchi, S.; Satoh, M. Infrared spectroscopic study and ab initio calculation for dissociation of poly(α-hydroxy acrylic acid) in aqueous solutions. Polymer 1997, 38, 2093–2098. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Raina, A.; Jänne, J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med. Biol. 1975, 53, 121–147. [Google Scholar] [PubMed]
- Yamamoto, S.; Koumoto, Y.; Shikami, S.; Shinoda, S. Effect of norspermidine and its related triamines on the cell-free polyphenylalanine synthesizing system from Vibrio parahaemolyticus. Microbiol. Immunol. 1990, 34, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Williams-Ashman, H.G.; Canellakis, Z.N. Polyamines in mammalian biology and medicine. Perspect. Biol. Med. 1979, 22, 421–453. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Ganem, B. New chemistry of naturally occurring polyamines. Acc. Chem. Res. 1982, 15, 290–298. [Google Scholar] [CrossRef]
- Bratek-Wiewiorowska, M.D.; Alejska, M.; Figlerowicz, M.; Barciszewski, J.; Wiewiorowski, M.; Jaskolski, M.; Zielenkiewicz, W.; Zielenkiewicz, A.; Kaminski, M. Interaction of polyamines, their protonated salts and metal complexes with nucleic acid fragments. Pure Appl. Chem. 1987, 59, 407–414. [Google Scholar] [CrossRef]
- Zabiszak, M.; Frymark, J.; Nowak, M.; Grajewski, J.; Stachowiak, K.; Kaczmarek, M.T.; Jastrząb, R. Influence of d-Electron Divalent Metal Ions in Complex Formation with L-Tartaric and L-Malic Acids. Molecules 2021, 26, 5290. [Google Scholar] [CrossRef]
- Zabiszak, M.; Nowak, M.; Gabryel, M.; Ogawa, K.; Kaczmarek, M.T.; Hnatejko, Z.; Jastrzab, R. New coordination compounds of citric acid and polyamines with lanthanide ions—Potential application in monitoring the treatment of cancer diseases. J. Inorg. Biochem. 2019, 198, 110715. [Google Scholar] [CrossRef]
- Daniele, P.G.; Rigano, C.; Sammartano, S. Ionic strength dependence of formation constants—I: Protonation constants of organic and inorganic acids. Talanta 1983, 30, 81–87. [Google Scholar] [CrossRef]
- Sukhno, I.V.; Gavrilyuk, M.B.; Buz’ko, V.Y.; Panyushkin, V.T. Complexation of Cerium Group Lanthanides with L-Malic Acid under an Excess of Metal Ions. Russ. J. Coord. Chem. 2004, 30, 520–525. [Google Scholar] [CrossRef]
- Zabiszak, M.; Nowak, M.; Hnatejko, Z.; Grajewski, J.; Ogawa, K.; Kaczmarek, M.T.; Jastrząb, R. Thermodynamic and Spectroscopic Studies of the Complexes Formed in Tartaric Acid and Lanthanide(III) Ions Binary Systems. Molecules 2020, 25, 1121. [Google Scholar] [CrossRef] [PubMed]
- Frymark, J.; Zabiszak, M.; Grajewski, J.; Hnatejko, Z.; Kołodyńska, D.; Kaczmarek, M.T.; Jastrzab, R. Excess of Polyamine as a Factor Influencing the Mode of Coordination in the Eu(III)/α-Hydroxy Acid/Spermine System. Polyhedron 2022, 221, 115853. [Google Scholar] [CrossRef]
- Glasoe, P.K.; Long, F.A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 1960, 64, 188–190. [Google Scholar] [CrossRef]
- Jastrzab, R. Phosphoserine and specific types of its coordination in copper(II) and adenosine nucleotides systems—Potentiometric and spectroscopic studies. J. Inorg. Biochem. 2009, 103, 766–773. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
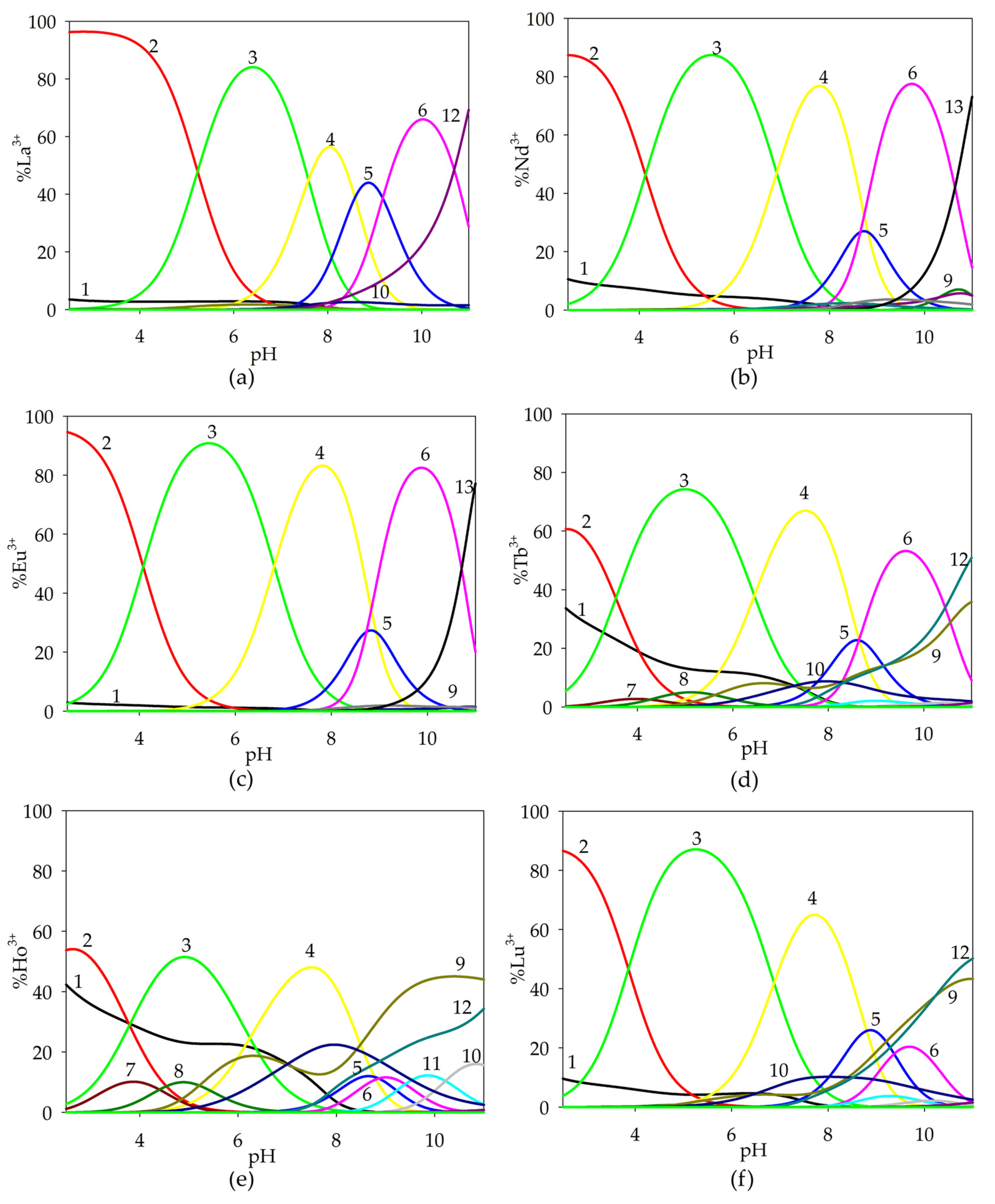
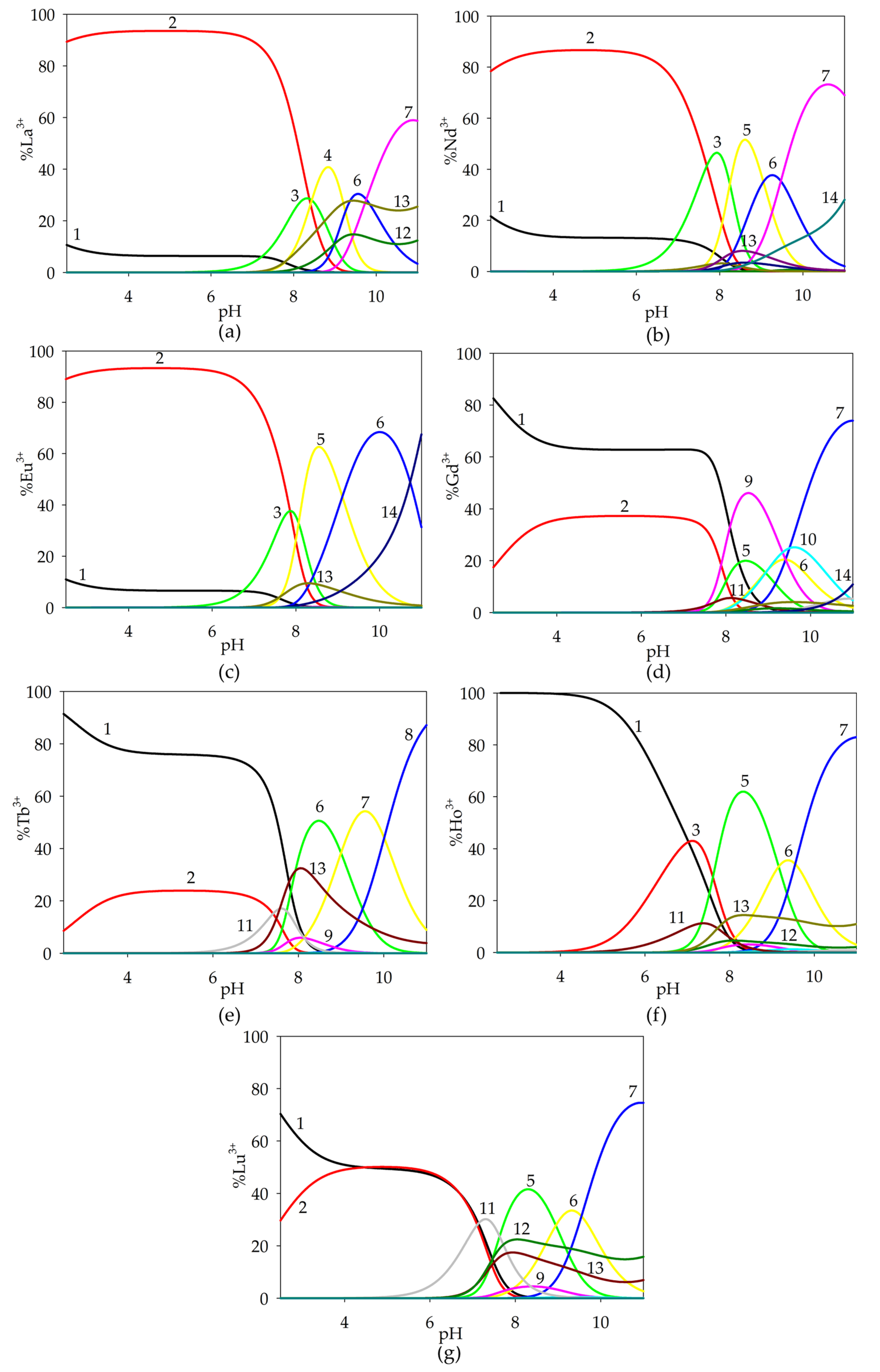
| Species | Overall Stability Constants (logβ) | Reaction | Equilibrium Constants (logKe) |
|---|---|---|---|
| LaGA(OH)2 | −12.36 (1) | La3+ + GA− + 2H2O ⇆ LaGA(OH)2 + 2H+ | 15.17 |
| NdGA(OH) | −4.28 (6) | Nd3+ + GA− + H2O ⇆ [NdGA(OH)]+ + H+ | 9.48 |
| NdGA(OH)2 | −12.54 (6) | [NdGA(OH)]+ + H2O ⇆ NdGA(OH)2 + H+ | 5.50 |
| EuGA(OH) | −4.25 (9) | Eu3+ + GA− + H2O ⇆ [EuGA(OH)]+ + H+ | 9.51 |
| EuGA(OH)2 | −11.81 (4) | [EuGA(OH)]+ + H2O ⇆ EuGA(OH)2 + H+ | 6.21 |
| GdGA(OH) | −5.70 (6) | Gd3+ + GA− + H2O ⇆ [GdGA(OH)]+ + H+ | 8.06 |
| GdGA(OH)2 | −14.84 (3) | Gd3+ + GA− + 2H2O ⇆ GdGA(OH)2 + 2H+ | 4.62 |
| TbGA(OH) | −4.81 (7) | Tb3+ + GA− + 2H2O ⇆ TbGA(OH)2 + 2H+ | 8.95 |
| HoGA(OH) | −4.31 (7) | Ho3+ + GA− + H2O ⇆ [HoGA(OH)]+ + H+ | 9.45 |
| HoGA(OH)2 | −12.41 (7) | [HoGA(OH)]+ + H2O ⇆ HoGA(OH)2 + H+ | 5.67 |
| LuGA(OH) | −3.68 (7) | Lu3+ + GA− + H2O ⇆ [LuGA(OH)]+ + H+ | 10.08 |
| LuGA(OH)2 | −11.49 (7) | [LuGA(OH)]+ + H2O ⇆ LuGA(OH)2 + H+ | 5.95 |
| Species | Overall Stability Constants (logβ) | Reaction | Equilibrium Constants (logKe) |
|---|---|---|---|
| La(HTar)(H4Spm) | 54.26 (5) | [LaHTar]2+ + H4Spm ⇆ [La(HTar)]2+·(H4Spm) | 7.49 |
| La(Tar)(H4Spm) | 49.03 (4) | [LaTar]+ + H4Spm ⇆ [La(Tar)]+·(H4Spm) | 6.01 |
| La(Tar)(H3Spm) | 41.44 (3) | [LaTar]+ + H3Spm− ⇆ [La(Tar)(H3Spm)] | 6.70 |
| La(Tar)(H2Spm) | 32.86 (3) | [LaTar]+ + H2Spm2− ⇆ [La(Tar)(H2Spm)]− | 7.23 |
| La(Tar)(HSpm) | 23.69 (3) | [LaTar]+ + HSpm3− ⇆ [La(Tar)(HSpm)]2− | 8.43 |
| Nd(HTar)(H4Spm) | 52.80 (5) | Nd3+ + HTar− + H4Spm ⇆ [Nd(HTar)]2+·(H4Spm) | 9.22 |
| Nd(Tar)(H4Spm) | 48.67 (5) | Nd3+ + [(Tar)(H4Spm)]2− ⇆ [Nd(Tar)]+·(H4Spm) | 6.52 |
| Nd(Tar)(H3Spm) | 41.78 (3) | Nd3+ + [(Tar)(H3Spm)]3− ⇆ [Nd(Tar)(H3Spm)] | 8.33 |
| Nd(Tar)(H2Spm) | 32.97 (4) | Nd3+ + [(Tar)(H2Spm)]4− ⇆ [Nd(Tar)(H2Spm)]− | 8.53 |
| Nd(Tar)(HSpm) | 24.32 (2) | Nd3+ + [(Tar)(HSpm)]5− ⇆ [Nd(Tar)(HSpm)]2− | 9.88 |
| Eu(HTar)(H4Spm) | 54.52 (5) | Eu3+ + HTar− + H4Spm ⇆ [Eu(HTar)]2+·(H4Spm) | 10.94 |
| Eu(Tar)(H4Spm) | 50.44 (4) | Eu3+ + [(Tar)(H4Spm)]2− ⇆ [Eu(Tar)]+·(H4Spm) | 8.29 |
| Eu(Tar)(H3Spm) | 43.63 (3) | Eu3+ + [(Tar)(H3Spm)]3− ⇆ [Eu(Tar)(H3Spm)] | 10.18 |
| Eu(Tar)(H2Spm) | 34.70 (4) | Eu3+ + [(Tar)(H2Spm)]4− ⇆ [Eu(Tar)(H2Spm)]− | 10.26 |
| Eu(Tar)(HSpm) | 25.97 (3) | Eu3+ + [(Tar)(HSpm)]5− ⇆ [Eu(Tar)(HSpm)]2− | 11.53 |
| Tb(HTar)(H4Spm) | 51.13 (8) | Tb3+ + HTar− + H4Spm ⇆ [Tb(HTar)]2+·(H4Spm) | 7.55 |
| Tb(Tar)(H4Spm) | 47.58 (6) | Tb3+ + [(Tar)(H4Spm)]2− ⇆ [Tb(Tar)]+·(H4Spm) | 5.43 |
| Tb(Tar)(H3Spm) | 41.13 (4) | Tb3+ + [(Tar)(H3Spm)]3− ⇆ [Tb(Tar)(H3Spm)] | 7.68 |
| Tb(Tar)(H2Spm) | 32.48 (6) | Tb3+ + [(Tar)(H2Spm)]4− ⇆ [Tb(Tar)(H2Spm)]− | 8.04 |
| Tb(Tar)(HSpm) | 23.84 (4) | Tb3+ + [(Tar)(HSpm)]5− ⇆ [Tb(Tar)(HSpm)]2− | 9.40 |
| Ho(HTar)(H4Spm) | 50.78 (3) | Ho3+ + HTar− + H4Spm ⇆ [Ho(HTar)]2+·(H4Spm) | 7.20 |
| Ho(Tar)(H4Spm) | 46.99 (3) | Ho3+ + [(Tar)(H4Spm)]2− ⇆ [Ho(Tar)]+·(H4Spm) | 4.84 |
| Ho(Tar)(H3Spm) | 40.75 (1) | Ho3+ + [(Tar)(H3Spm)]3− ⇆ [Ho(Tar)(H3Spm)] | 7.3 |
| Ho(Tar)(H2Spm) | 31.97 (4) | Ho3+ + [(Tar)(H2Spm)]4− ⇆ [Ho(Tar)(H2Spm)]− | 7.53 |
| Lu(HTar)(H4Spm) | 52.92 (2) | Lu3+ + HTar− + H4Spm ⇆ [Lu(HTar)]2+·(H4Spm) | 9.24 |
| Lu(Tar)(H4Spm) | 49.06 (1) | Lu3+ + [(Tar)(H4Spm)]2− ⇆ [Lu(Tar)]+·(H4Spm) | 6.91 |
| Lu(Tar)(H3Spm) | 42.18 (1) | Lu3+ + [(Tar)(H3Spm)]3− ⇆ [Lu(Tar)(H3Spm)] | 8.73 |
| Lu(Tar)(H2Spm) | 33.38 (1) | Lu3+ + [(Tar)(H2Spm)]4− ⇆ [Lu(Tar)(H2Spm)]− | 8.94 |
| Lu(Tar)(HSpm) | 24.01 (2) | Lu3+ + [(Tar)(HSpm)]5− ⇆ [Lu(Tar)(HSpm)]2− | 9.57 |
| Species | Overall Stability Constants (logβ) | Reaction | Equilibrium Constants (logKe) |
|---|---|---|---|
| La(GA)(H4Spm) | 48.23 (2) | La3+ + GA− + H4Spm ⇆ [La(GA)]2+·(H4Spm) | 9.56 |
| La(GA)(H3Spm) | 39.85 (2) | La3+ + [(GA)(H3Spm)]2− ⇆ [La(GA)(H3Spm)]+ | 6.76 |
| La(GA)(H2Spm) | 31.41 (2) | La3+ + [(GA)(H2Spm)]3− ⇆ [La(GA)(H2Spm)] | 6.79 |
| La(GA)(Spm) | 12.90 (5) | [LaSpm]− + GA− ⇆ [La(GA)(Spm)]2− | 6.75 |
| La(GA)(Spm)(OH) | 3.14 (3) | [La(GA)(Spm)]2− + H2O ⇆ [La(GA)(Spm)(OH)]3− + H+ | 4.01 |
| Nd(GA)(H4Spm) | 47.25 (8) | Nd3+ + GA− + H4Spm ⇆ [Nd(GA)]2+·(H4Spm) | 8.58 |
| Nd(GA)(H3Spm) | 39.56 (9) | Nd3+ + [(GA)(H3Spm)]2− ⇆ [Nd(GA)(H3Spm)]+ | 6.47 |
| Nd(GA)(HSpm) | 23.07 (9) | Nd3+ + [(GA)(HSpm)]4− ⇆ [Nd(GA)(HSpm)]− | 7.96 |
| Nd(GA)(Spm) | 14.02 (8) | [NdSpm]− + GA− ⇆ [Nd(GA)(Spm)]2− | 6.31 |
| Nd(GA)(Spm)(OH) | 4.55 (9) | [Nd(GA)(Spm)]2− + H2O ⇆ [Nd(GA)(Spm)(OH)]3− + H+ | 4.30 |
| Eu(GA)(H4Spm) | 48.19 (3) | Eu3+ + GA− + H4Spm ⇆ [Eu(GA)]2+·(H4Spm) | 9.52 |
| Eu(GA)(H3Spm) | 40.33 (4) | Eu3+ + [(GA)(H3Spm)]2− ⇆ [Eu(GA)(H3Spm)]+ | 7.24 |
| Eu(GA)(HSpm) | 24.20 (3) | Eu3+ + [(GA)(HSpm)]4− ⇆ [Eu(GA)(HSpm)]− | 9.09 |
| Eu(GA)(Spm) | 15.09 (3) | [EuSpm]− + GA− ⇆ [Eu(GA)(Spm)]2− | 6.74 |
| Gd(GA)(H4Spm) | 44.85 (5) | Gd3+ + GA− + H4Spm ⇆ [Gd(GA)]2+·(H4Spm) | 6.18 |
| Gd(GA)(HSpm) | 20.84 (7) | Gd3+ + [(GA)(HSpm)]4− ⇆ [Gd(GA)(HSpm)]− | 5.73 |
| Gd(GA)(Spm) | 11.91 (7) | [GdSpm]− + GA− ⇆ [Gd(GA)(Spm)]2− | 3.23 |
| Gd(GA)(Spm)(OH) | 2.50 (4) | [Gd(GA)(Spm)]2− + H2O ⇆ [Gd(GA)(Spm)(OH)]3− + H+ | 4.35 |
| Tb(GA)(H4Spm) | 44.41 (6) | Tb3+ + GA− + H4Spm ⇆ [Tb(GA)]2+·(H4Spm) | 5.74 |
| Tb(GA)(Spm) | 13.93 (2) | [TbSpm]− + GA− ⇆ [Tb(GA)(Spm)]2− | 5.96 |
| Tb(GA)(Spm)(OH) | 4.93 (1) | [Tb(GA)(Spm)]2− + H2O ⇆ [Tb(GA)(Spm)(OH)]3− + H+ | 4.77 |
| Tb(GA)(Spm)(OH)2 | −5.08 (2) | [Tb(GA)(Spm)(OH)]3− + H2O ⇆ [Tb(GA)(Spm)(OH)2]4− + H+ | 3.74 |
| Ho(GA)(H3Spm) | 38.29 (4) | Ho3+ + [(GA)(H3Spm)]2− ⇆ [Ho(GA)(H3Spm)]+ | 5.20 |
| Ho(GA)(HSpm) | 23.08 (2) | Ho3+ + [(GA)(HSpm)]4− ⇆ [Ho(GA)(HSpm)]− | 7.38 |
| Ho(GA)(Spm) | 13.92 (3) | [HoSpm]− + GA− ⇆ [Ho(GA)(Spm)]2− | 5.27 |
| Ho(GA)(Spm)(OH) | 4.37 (3) | [Ho(GA)(Spm)]2− + H2O ⇆ [Ho(GA)(Spm)(OH)]3− + H+ | 4.21 |
| Lu(GA)(H4Spm) | 45.29 (4) | Lu3+ + [(GA)(H4Spm)]2− ⇆ [Lu(GA)]2+·(H4Spm) | 6.62 |
| Lu(GA)(HSpm) | 22.99 (3) | Lu3+ + [(GA)(HSpm)]4− ⇆ [Lu(GA)(HSpm)]− | 7.88 |
| Lu(GA)(Spm) | 14.04 (3) | [LuSpm]− + GA− ⇆ [Lu(GA)(Spm)]2− | 5.55 |
| Lu(GA)(Spm)(OH) | 4.49 (3) | [Lu(GA)(Spm)]2− + H2O ⇆ [Lu(GA)(Spm)(OH)]3− + H+ | 4.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabiszak, M.; Frymark, J.; Skrobanska, M.; Kaczmarek, M.T.; Jastrzab, R. Reactions of Lanthanide Ions with Glycolic Acid or Tartaric Acid in the Presence of Spermine: Potentiometric and Spectroscopic Studies. Int. J. Mol. Sci. 2025, 26, 4477. https://doi.org/10.3390/ijms26104477
Zabiszak M, Frymark J, Skrobanska M, Kaczmarek MT, Jastrzab R. Reactions of Lanthanide Ions with Glycolic Acid or Tartaric Acid in the Presence of Spermine: Potentiometric and Spectroscopic Studies. International Journal of Molecular Sciences. 2025; 26(10):4477. https://doi.org/10.3390/ijms26104477
Chicago/Turabian StyleZabiszak, Michał, Justyna Frymark, Monika Skrobanska, Malgorzata T. Kaczmarek, and Renata Jastrzab. 2025. "Reactions of Lanthanide Ions with Glycolic Acid or Tartaric Acid in the Presence of Spermine: Potentiometric and Spectroscopic Studies" International Journal of Molecular Sciences 26, no. 10: 4477. https://doi.org/10.3390/ijms26104477
APA StyleZabiszak, M., Frymark, J., Skrobanska, M., Kaczmarek, M. T., & Jastrzab, R. (2025). Reactions of Lanthanide Ions with Glycolic Acid or Tartaric Acid in the Presence of Spermine: Potentiometric and Spectroscopic Studies. International Journal of Molecular Sciences, 26(10), 4477. https://doi.org/10.3390/ijms26104477






