Gene Expression Differences Based on Low Total 25(OH)D and Low VDBP Status with a Preterm Birth
Abstract
1. Introduction
2. Results
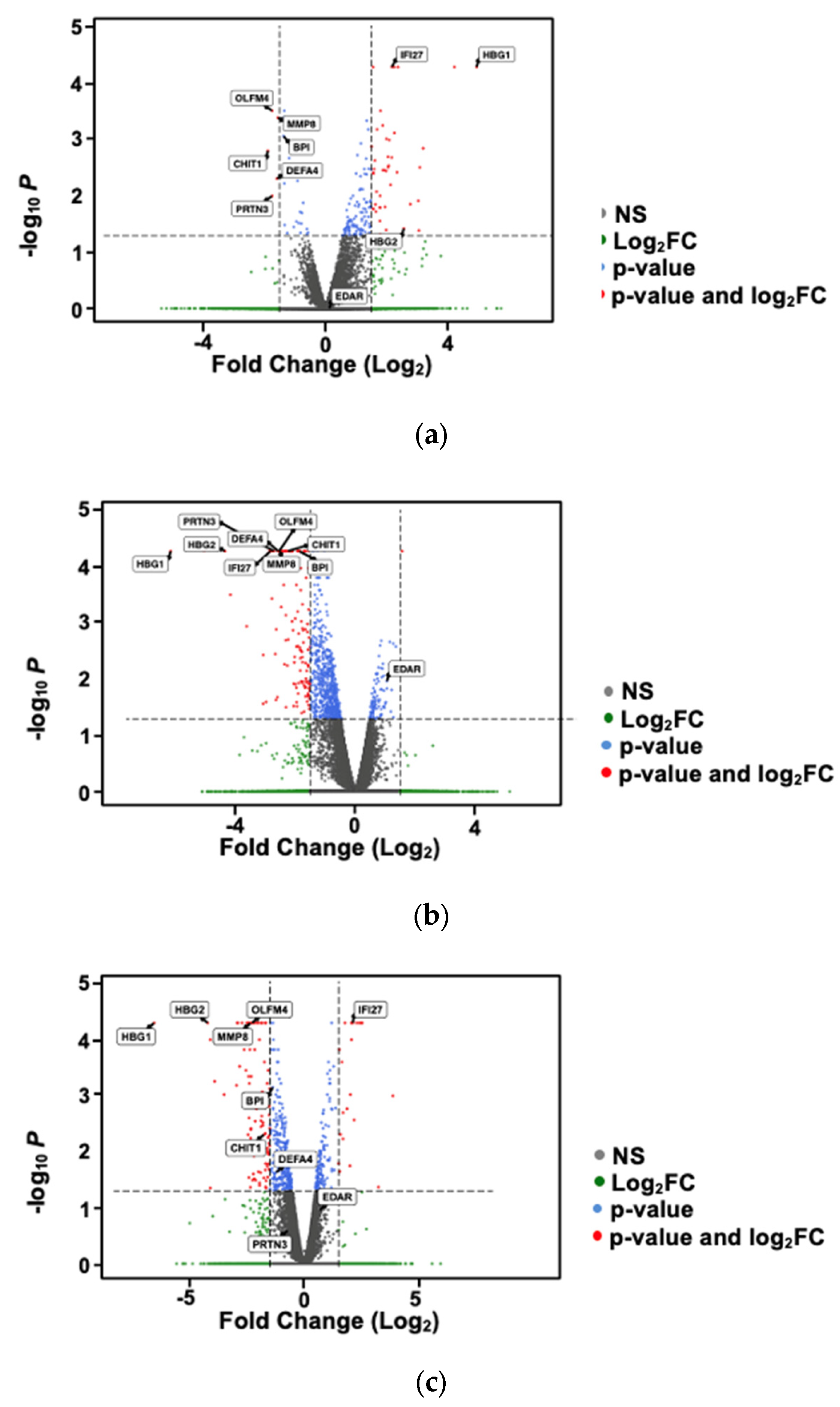
2.1. Differential Gene Expression Analysis Based on Preterm vs. Full-Term Births
2.2. Differential Gene Expression Analysis Based on Vitamin D Status
2.3. Differential Gene Expression Analysis Based on Vitamin D-Binding Protein Status
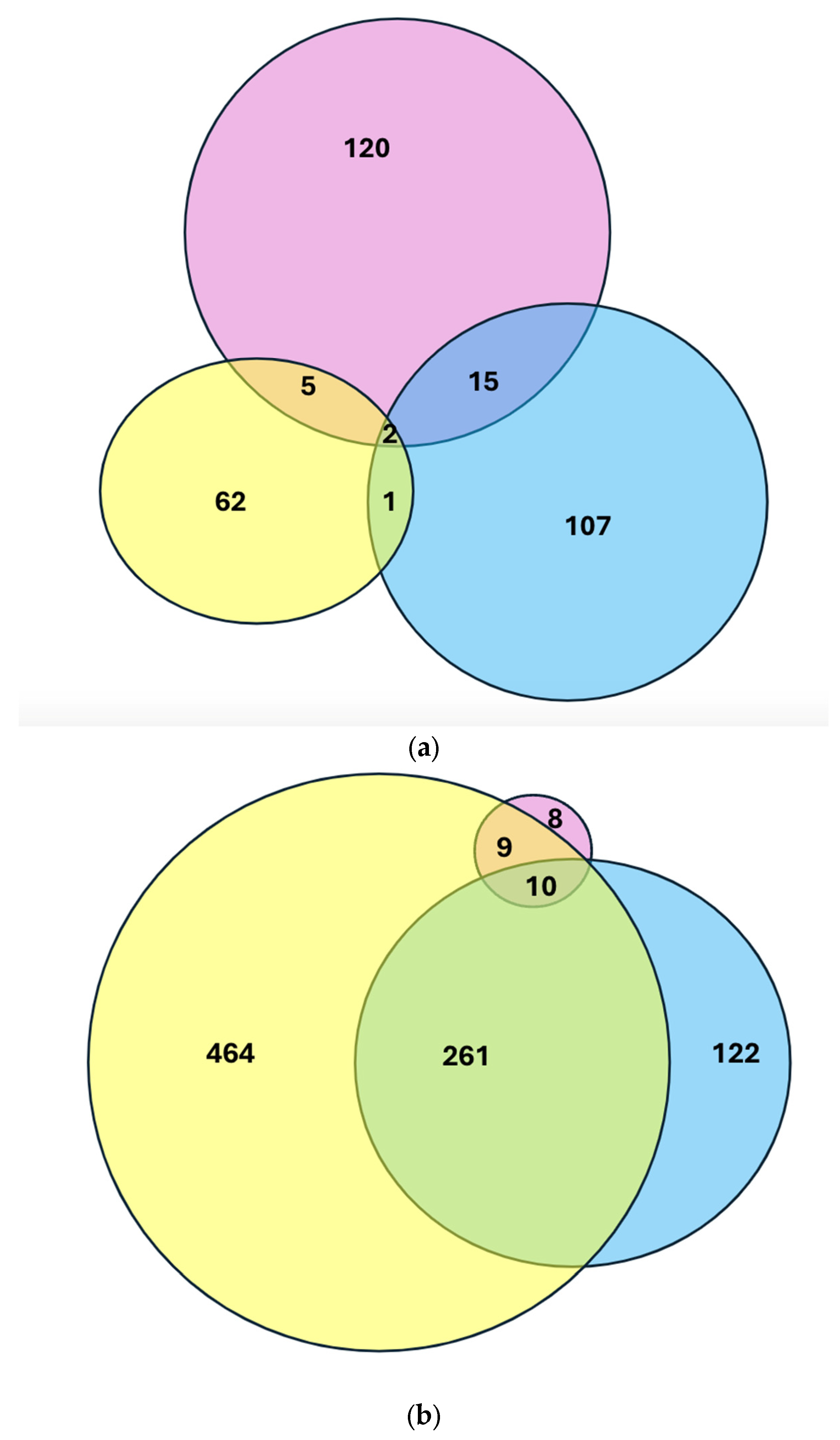
2.4. Intersection of Differential Gene Expression Analysis Among Groups
Gene Ontology Based on DGE
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sample Collection
4.3. Plasma 25(OH)D Analysis
4.4. Vitamin D-Binding Protein Analysis
4.5. RNA Extraction and Library Creation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronstein, J.M.; Wingate, M.S.; Brisendine, A.E. Why Is the U.S. Preterm Birth Rate So Much Higher Than the Rates in Canada, Great Britain, and Western Europe? Int. J. Health Serv. 2018, 48, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2022: Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/nchs/data/nvsr/nvsr73/nvsr73-02.pdf (accessed on 1 September 2024).
- SMFM. Society for Maternal-Fetal Medicine Statement: Response to the Food and Drug Administration’s withdrawal of 17-alpha hydroxyprogesterone caproate. Am. J. Obstet. Gynecol. 2023, 229, B2–B6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Green, C.A.; Vladutiu, C.J.; Manuck, T.A. Racial Disparities in Prematurity Persist among Women of High Socioeconomic Status. Am. J. Obstet. Gynecol. MFM 2020, 2, 100104. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Robinson, A.M.; Zucchi, F.C.R.; Robbins, J.C.; Babenko, O.; Kovalchuk, O.; Kovalchuk, I.; Olson, D.M.; Metz, G.A.S. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med. 2014, 12, 121. [Google Scholar] [CrossRef]
- Chae, D.H.; Clouston, S.; Martz, C.D.; Hatzenbuehler, M.L.; Cooper, H.L.F.; Turpin, R.; Stephens-Davidowitz, S.; Kramer, M.R. Area racism and birth outcomes among Blacks in the United States. Soc. Sci. Med. 2018, 199, 49–55. [Google Scholar] [CrossRef]
- Burris, H.H.; Collins, J.W., Jr. Commentary: Race and preterm birth-the case for epigenetic inquiry. Ethn. Dis. 2010, 20, 296–299. [Google Scholar]
- Bhattacharjee, E.; Maitra, A. Spontaneous preterm birth: The underpinnings in the maternal and fetal genomes. npj Genom. Med. 2021, 6, 43. [Google Scholar] [CrossRef]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Ferrero, D.M.; Larson, J.; Jacobsson, B.; Di Renzo, G.C.; Norman, J.E.; Martin, J.N., Jr.; D’alton, M.; Castelazo, E.; Howson, C.P.; Sengpiel, V. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS ONE 2016, 11, e0162506. [Google Scholar] [CrossRef]
- Paquette, A.G.; MacDonald, J.; Bammler, T.; Day, D.B.; Loftus, C.T.; Buth, E.; Mason, W.A.; Bush, N.R.; Lewinn, K.Z.; Marsit, C. Placental transcriptomic signatures of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2023, 228, 73.e71–73.e18. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary reference intakes for calcium and vitamin D. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. The National Academies Press: Washington, DC, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56070/ (accessed on 15 April 2025). [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Optimize dietary intake of vitamin D: An epigenetic perspective. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Guffey, T.; Dailey, R.; Misra, D.; Giurgescu, C. Vitamin D Status as an Important Predictor of Preterm Birth in a Cohort of Black Women. Nutrients 2023, 15, 4637. [Google Scholar] [CrossRef]
- Amegah, A.K.; Klevor, M.K.; Wagner, C.L. Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: A systematic review and meta-analysis of longitudinal studies. PLoS ONE 2017, 12, e0173605. [Google Scholar] [CrossRef]
- Kiely, M.E.; Wagner, C.; Roth, D. Vitamin D in pregnancy: Where we are and where we should go. J. Steroid Biochem. Mol. Biol. 2020, 201, 105669. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017, 5, 17030. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal-fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef]
- Avila, E.; Noriega-Mejía, B.J.; González-Macías, J.; Cortes-Hernández, U.; García-Quiroz, J.; García-Becerra, R.; Díaz, L. The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 8665. [Google Scholar] [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef]
- Mitro, S.D.; Waetjen, L.E.; Hedderson, M.M. Fibroids and vitamin D: Another piece of the puzzle. Fertil. Steril. 2022, 118, 1137–1138. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. 2012, 72, 92–102. [Google Scholar]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C.; Pressman, E.K.; Cooper, E.; Queenan, R.A.; Pillittere, J.; O’Brien, K.O. Low vitamin D is associated with infections and proinflammatory cytokines during pregnancy. Reprod. Sci. 2018, 25, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, K.R.; Peters, R.M.; Li, J.; Rao, S.D.; Woodcroft, K.J.; Cassidy-Bushrow, A.E. Early pregnancy vitamin D and patterns of antenatal inflammation in African–American women. J. Reprod. Immunol. 2015, 107, 52–58. [Google Scholar] [CrossRef]
- Fernando, M.; Ellery, S.J.; Marquina, C.; Lim, S.; Naderpoor, N.; Mousa, A. Vitamin D-binding protein in pregnancy and reproductive health. Nutrients 2020, 12, 1489. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Fakhoury, H.; Kotsa, K. Deconvoluting the biological roles of vitamin D-binding protein during pregnancy: A both clinical and theoretical challenge. Front. Endocrinol. 2018, 9, 373103. [Google Scholar] [CrossRef]
- Bikle, D.; Bouillon, R.; Thadhani, R.; Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25 (OH) D to assess vitamin D status? J. Steroid Biochem. Mol. Biol. 2017, 173, 105–116. [Google Scholar] [CrossRef]
- D’Silva, A.M.; Hyett, J.A.; Coorssen, J.R. First Trimester Protein Biomarkers for Risk of Spontaneous Preterm Birth: Identifying a Critical Need for More Rigorous Approaches to Biomarker Identification and Validation. Fetal Diagn. Ther. 2020, 47, 497–506. [Google Scholar] [CrossRef]
- Cho, M.-C.; Cho, I.A.; Seo, H.K.; Kang, M.J.; Jo, J.Y.; Shin, J.K.; Lee, S.A.; Kim, S.C.; Kim, R.-B.; Choi, W.J. Serum vitamin D-binding protein (VDBP) concentration and rs7041 genotype may be associated with preterm labor. J. Matern.-Fetal Neonatal Med. 2022, 35, 9422–9429. [Google Scholar] [CrossRef]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams 2015. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 15 April 2025).
- Liu, W.; Rodgers, G.P. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Cancer Metastasis Rev. 2016, 35, 201–212. [Google Scholar] [CrossRef]
- Liu, W.; Rodgers, G.P. Olfactomedin 4 Is a Biomarker for the Severity of Infectious Diseases. Open Forum Infect. Dis. 2022, 9, ofac061. [Google Scholar] [CrossRef] [PubMed]
- Cooley, A.; Madhukaran, S.; Stroebele, E.; Caraballo, M.C.; Wang, L.; Akgul, Y.; Hon, G.C.; Mahendroo, M. Dynamic states of cervical epithelia during pregnancy and epithelial barrier disruption. iScience 2023, 26, 105953. [Google Scholar] [CrossRef] [PubMed]
- Kuno, R.; Ito, G.; Kawamoto, A.; Hiraguri, Y.; Sugihara, H.Y.; Takeoka, S.; Nagata, S.; Takahashi, J.; Tsuchiya, M.; Anzai, S. Notch and TNF-α signaling promote cytoplasmic accumulation of OLFM4 in intestinal epithelium cells and exhibit a cell protective role in the inflamed mucosa of IBD patients. Biochem. Biophys. Rep. 2021, 25, 100906. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Jayyosi, C.; Lee, N.; Mahendroo, M.; Myers, K.M. Mechanics of cervical remodelling: Insights from rodent models of pregnancy. Interface Focus 2019, 9, 20190026. [Google Scholar] [CrossRef]
- Akgul, Y.; Holt, R.; Mummert, M.; Word, A.; Mahendroo, M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology 2012, 153, 3493–3503. [Google Scholar] [CrossRef]
- GeneCards The Human Gene Database. DEF4A: Defensin Alpha 4. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=DEFA4 (accessed on 10 January 2023).
- Strauss, J.F., 3rd; Romero, R.; Gomez-Lopez, N.; Haymond-Thornburg, H.; Modi, B.P.; Teves, M.E.; Pearson, L.N.; York, T.P.; Schenkein, H.A. Spontaneous preterm birth: Advances toward the discovery of genetic predisposition. Am. J. Obstet. Gynecol. 2018, 218, 294–314.e292. [Google Scholar] [CrossRef]
- Wright, M.L.; Goin, D.E.; Smed, M.K.; Jewell, N.P.; Nelson, J.L.; Olsen, J.; Hetland, M.L.; Zoffmann, V.; Jawaheer, D. Pregnancy-associated systemic gene expression compared to a pre-pregnancy baseline, among healthy women with term pregnancies. Front. Immunol. 2023, 14, 1161084. [Google Scholar] [CrossRef]
- Yadama, A.P.; Mirzakhani, H.; McElrath, T.F.; Litonjua, A.A.; Weiss, S.T. Transcriptome analysis of early pregnancy vitamin D status and spontaneous preterm birth. PLoS ONE 2020, 15, e0227193. [Google Scholar] [CrossRef]
- Mohan, A.R.; Loudon, J.A.; Bennett, P.R. Molecular and biochemical mechanisms of preterm labour. Semin. Fetal Neonatal Med. 2004, 9, 437–444. [Google Scholar] [CrossRef]
- Alifu, X.; Si, S.; Qiu, Y.; Cheng, H.; Huang, Y.; Chi, P.; Zhuang, Y.; Zhou, H.; Zhang, L.; Ainiwan, D.; et al. The Association of Vitamin D during Pregnancy and mRNA Expression Levels of Inflammatory Factors with Preterm Birth and Prelabor Rupture of Membranes. Nutrients 2023, 15, 3423. [Google Scholar] [CrossRef]
- Kemp, M.W.; Saito, M.; Newnham, J.P.; Nitsos, I.; Okamura, K.; Kallapur, S.G. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod. Sci. 2010, 17, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Ariyakumar, G.; Morris, J.M.; McKelvey, K.J.; Ashton, A.W.; McCracken, S.A. NF-κB regulation in maternal immunity during normal and IUGR pregnancies. Sci. Rep. 2021, 11, 20971. [Google Scholar] [CrossRef] [PubMed]
- Lindström, T.M.; Bennett, P.R. The role of nuclear factor kappa B in human labour. Reproduction 2005, 130, 569–581. [Google Scholar] [CrossRef]
- Talmor, Y.; Bernheim, J.; Klein, O.; Green, J.; Rashid, G. Calcitriol blunts pro-atherosclerotic parameters through NFκB and p38 in vitro. Eur. J. Clin. Investig. 2008, 38, 548–554. [Google Scholar] [CrossRef]
- Cohen-Lahav, M.; Shany, S.; Tobvin, D.; Chaimovitz, C.; Douvdevani, A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol. Dial. Transplant. 2006, 21, 889–897. [Google Scholar] [CrossRef]
- Barrera, D.; Díaz, L.; Noyola-Martínez, N.; Halhali, A. Vitamin D and Inflammatory Cytokines in Healthy and Preeclamptic Pregnancies. Nutrients 2015, 7, 6465–6490. [Google Scholar] [CrossRef]
- Veerapen, M.K. The Genetics of Preterm Birth; University of Miami: Coral Gables, FL, USA, 2016. [Google Scholar]
- Gudicha, D.W.; Romero, R.; Gomez-Lopez, N.; Galaz, J.; Bhatti, G.; Done, B.; Jung, E.; Gallo, D.M.; Bosco, M.; Suksai, M. The amniotic fluid proteome predicts imminent preterm delivery in asymptomatic women with a short cervix. Sci. Rep. 2022, 12, 11781. [Google Scholar] [CrossRef]
- Chaemsaithong, P.; Romero, R.; Docheva, N.; Chaiyasit, N.; Bhatti, G.; Pacora, P.; Hassan, S.S.; Yeo, L.; Erez, O. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes. J. Matern.-Fetal Neonatal Med. 2018, 31, 228–244. [Google Scholar] [CrossRef]
- Yadama, A.P.; Maiorino, E.; Carey, V.J.; McElrath, T.F.; Litonjua, A.A.; Loscalzo, J.; Weiss, S.T.; Mirzakhani, H. Early-pregnancy transcriptome signatures of preeclampsia: From peripheral blood to placenta. Sci. Rep. 2020, 10, 17029. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Wood, A.M.; Qureshi, O.S.; Hou, T.Z.; Gardner, D.; Briggs, Z.; Kaur, S.; Raza, K.; Sansom, D.M. Availability of 25-Hydroxyvitamin D3 to APCs Controls the Balance between Regulatory and Inflammatory T Cell Responses. J. Immunol. 2012, 189, 5155–5164. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Ji, M.; Song, J.; Moon, H.-W.; Hur, M.; Yun, Y.-M. Clinical utility of measurement of vitamin D-binding protein and calculation of bioavailable vitamin D in assessment of vitamin D status. Ann. Lab. Med. 2017, 37, 34–38. [Google Scholar] [CrossRef] [PubMed]
- BIKLE, D.D.; Gee, E.; Halloran, B.; KOWALSKI, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.C.; Blankenstein, M.A.; Kema, I.P.; Buijs, M.M. Accuracy of 6 routine 25-hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clin. Chem. 2012, 58, 543–548. [Google Scholar] [CrossRef]
- Heng, Y.J.; Pennell, C.E.; Chua, H.N.; Perkins, J.E.; Lye, S.J. Whole blood gene expression profile associated with spontaneous preterm birth in women with threatened preterm labor. PLoS ONE 2014, 9, e96901. [Google Scholar] [CrossRef]
- Wang, J.; Luo, X.; Pan, J.; Dong, X.; Tian, X.; Tu, Z.; Ju, W.; Zhang, M.; Zhong, M.; De Chen, C. (Epi) genetic variants of the sarcomere-desmosome are associated with premature utero-contraction in spontaneous preterm labor. Environ. Int. 2021, 148, 106382. [Google Scholar] [CrossRef]
- Ono, C.; Tanaka, S.; Myouzen, K.; Iwasaki, T.; Ueda, M.; Oda, Y.; Yamamoto, K.; Kochi, Y.; Baba, Y. Upregulated Fcrl5 disrupts B cell anergy and causes autoimmune disease. Front. Immunol. 2023, 14, 1276014. [Google Scholar] [CrossRef]
- Lee, J.; Romero, R.; Chaiworapongsa, T.; Dong, Z.; Tarca, A.L.; Xu, Y.; Chiang, P.J.; Kusanovic, J.P.; Hassan, S.S.; Yeo, L. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: Evidence of a distinct and novel type of human fetal systemic inflammatory response. Am. J. Reprod. Immunol. 2013, 70, 265–284. [Google Scholar] [CrossRef]
- Newton, D.A.; Baatz, J.E.; Kindy, M.S.; Gattoni-Celli, S.; Shary, J.R.; Hollis, B.W.; Wagner, C.L. Vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatr. Res. 2019, 86, 662–669. [Google Scholar] [CrossRef]
- Yao, P.; Sun, L.; Lu, L.; Ding, H.; Chen, X.; Tang, L.; Xu, X.; Liu, G.; Hu, Y.; Ma, Y.; et al. Effects of Genetic and Nongenetic Factors on Total and Bioavailable 25(OH)D Responses to Vitamin D Supplementation. J. Clin. Endocrinol. Metab. 2017, 102, 100–110. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Mohammed, A.K.; Bukhari, I.; Rikli, M.; Abdi, S.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alenad, A.; Al-Saleh, Y.; et al. Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition 2019, 63–64, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, D.; Płomiński, J.; Augustyn, K.; Cieślińska, A. rs7041 and rs4588 Polymorphisms in Vitamin D Binding Protein Gene (VDBP) and the Risk of Diseases. Int. J. Mol. Sci. 2022, 23, 933. [Google Scholar] [CrossRef] [PubMed]
- Sablok, A.; Batra, A.; Thariani, K.; Batra, A.; Bharti, R.; Aggarwal, A.R.; Kabi, B.C.; Chellani, H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. 2015, 83, 536–541. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Niño, J.F.; et al. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef]
- Dominguez, T.P. Adverse birth outcomes in African American women: The social context of persistent reproductive disadvantage. Soc. Work Public Health 2011, 26, 3–16. [Google Scholar] [CrossRef]
- Olson, D.M.; Severson, E.M.; Verstraeten, B.S.; Ng, J.W.; McCreary, J.K.; Metz, G.A. Allostatic Load and Preterm Birth. Int. J. Mol. Sci. 2015, 16, 29856–29874. [Google Scholar] [CrossRef]
- Nowak, A.L.; Saadat, N.; Sun, J.; Forsman, A.M.; Liang, X.; Joyce, C.; Woo, J.; Engeland, C.G.; Misra, D.P.; Giurgescu, C.; et al. Preterm Birth in African American Women: A Multi-Omic Pilot Study in Early Pregnancy. Biol. Res. Nurs. 2024, 27, 205–215. [Google Scholar] [CrossRef]
| Characteristics | Preterm Birth (n = 13) | Full-Term Birth (n = 6) | Vitamin D Deficiency (n = 12) | Vitamin D Sufficiency (n = 7) | High VDBP (n = 6) | Low VDBP (n = 13) |
|---|---|---|---|---|---|---|
| Maternal age, years, M ± SD | 27.54 ± 6.2 | 30.17 ± 7.19 | 28.17 ± 5.9 | 28.7 ± 7.8 | 27.2 ± 6.4 | 28.9 ± 6.6 |
| Gestational age at blood draw, weeks, M ± SD | 14.92 ± 3.79 | 11.33 ± 3.07 | 12.83 ± 3.09 | 15.3 ± 4.79 | 12.0 ± 2.1 | 14.6 ± 4.3 |
| Level of education, N (%) | ||||||
| Less than high school | 3 (23.1) | 1 (16.6) | 2 (16.6) | 2 (28.6) | 1 (16.6) | 3 (23) |
| High school or equivalent | 4 (30.7) | 2 (33.3) | 5 (41.7) | 1 (14.3) | 2 (33.3) | 4 (30.7) |
| > High school education | 5 (38.4) | 3 (50) | 5 (41.7) | 4 (57.1) | 3 (50) | 6 (46.3) |
| Marital status, N (%) | ||||||
| Married/lives with partner | 4 (30.7) | 3 (50) | 4 (33.3) | 3 (42.8) | 2 (33.3) | 5 (38.4) |
| Work status N (%) | ||||||
| Currently working | 6 (38.5) | 3 (50) | 6 (50) | 3 (42.8) | 3 (50) | 7 (54) |
| Health insurance N (%) | ||||||
| Medicaid * | 7 (53.8) | 3 (50) | 5 (41.6) | 6 (85.7) | 2 (33.3) | 8 (61.7) |
| Medicare | 3 (23.1) | 1 (16.6) | 4 (33.3) | 0 (0) | 2 (33.3) | 1 (7.6) |
| Medicaid + Medicare | 1 (7.6) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 1(7.6) |
| Private or other | 1 (7.6) | 1 (16.6) | 0 (0) | 1 (14.3) | 2 (33.3) | 3 (23.1) |
| HDP diagnosis ** N (%) | 7 (53.8) | 1 (16.6) | 3 (25) | 4 (57.1) | 4 (66.6) | 3 (23.1) |
| Obese (BMI ≥ 30 kg/m2), N (%) | 7 (53.8) | 3 (50) | 4 (33.3) | 3 (44.6) | 3 (50) | 7 (53.8) |
| Log2 Fold-Change Up 1 | Upregulated Genes | Log2 Fold-Change Down 1 | Downregulated Genes |
|---|---|---|---|
| 4.93 | HBG1 | −1.87 | CHIT1 |
| 4.19 | SERINC2 | −1.73 | OLFM4 |
| 3.18 | MAN1A2 | −1.72 | PRTN3 |
| 3.08 | LY96 | −1.58 | DEFA4 |
| 3.05 | LINC01506 | −1.55 | MMP8 |
| 3.02 | RSAD2 | −1.35 | BPI |
| 2.76 | CLEC2B | −1.34 | TMEM180 |
| 2.55 | HBG2 | −1.33 | ORM1 |
| 2.36 | RPS24 | −1.32 | RNASE3 |
| 2.33 | RPL31 | −1.31 | CTSG |
| Log2 Fold-Change Up 1 | Upregulated Genes | Log2 Fold-Change Down 1 | Downregulated Genes |
|---|---|---|---|
| 1.56 | FCRL5 | −6.128 | HBG1 |
| 1.35 | LYPD2 | −5.02 | SERINC2 |
| 1.263 | IL12RB2 | −4.32 | HBG2 |
| 1.260 | S100A8 | −2.85 | ABCC13 |
| 1.20 | PACSIN1 | −2.82 | IFI27 |
| 1.18 | MZB1 | −2.80 | S100A8 |
| 1.16 | RRM2 | −2.76 | HRAT92 |
| 1.14 | TDRD9 | −2.68 | PRTN3 |
| 1.06 | EDAR | −2.54 | DEFA4 |
| 1.05 | FBLN2 | −2.53 | OLFM4 |
| Log2 Fold-Change Up 1 | Upregulated Genes | Log2 Fold-Change Down 1 | Downregulated Genes |
|---|---|---|---|
| 3.88 | AP2B2 | −6.55 | HBG1 |
| 3.25 | RSAD2 | −4.21 | HBG2 |
| 2.544 | CMPK2 | −4.10 | SERINC2 |
| 2.49 | SIGLEC1 | −2.92 | COX7B |
| 2.41 | HERC5 | −2.72 | RPS24 |
| 2.29 | IFI44L | −2.49 | CD177 |
| 2.18 | IFIT3 | −2.44 | CCDC34 |
| 2.12 | IFI27 | −2.40 | DNAJC25, DNAJC25-GNG10 |
| 2.069 | OASL | −2.32 | MMP8 |
| 2.068 | USP18 | −2.28 | OLFM4 |
| Gene Ontology Term | Related Genes | p-Value | FDR (Benjamini–Hochberg) |
|---|---|---|---|
| Defense Response to Gram-negative bacterium (GO:0050829) | BPI, DEFA4, and RNASE3 | 0.00081 | 0.044 |
| Innate Immune Response in Mucosa (GO:0002227) | DEFA4 and RNASE3 | 0.017 | 0.32 |
| Innate immune response (GO:0045087) | BPI, DEFA4, and RNASE3 | 0.023 | 0.32 |
| Negative regulation of interleukin-6 production (GO:0032715) | BPI and ORM1 | 0.031 | 0.32 |
| Antibacterial humoral response (GO:0019731) | DEFA4 and RNASE3 | 0.034 | 0.32 |
| Negative Regulation of Tumor Necrosis factor production (GO: 0032720) | BPI and ORM1 | 0.035 | 0.32 |
| Antimicrobial humoral immune response mediated by antimicrobial peptide (GO:0061844) | DEFA4 and RNASE3 | 0.049 | 0.34 |
| Positive Regulation of tumor necrosis factors production (GO:0032760) | MMP8 and ORM1 | 0.050 | 0.34 |
| Defense response to Gram-positive bacterium (GO:0050830) | DEFA4 and RNASE3 | 0.058 | 0.35 |
| Carbohydrate metabolic process (GO:0005975) | CHIT1 and PGM5 | 0.067 | 0.36 |
| Cellular Response to Lipopolysaccharide (GO:0071222) | DEFA4 and MMP8 | 0.08 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, J.; Nandu, T.; Nowak, A.; Forsman, A.; Giurgescu, C. Gene Expression Differences Based on Low Total 25(OH)D and Low VDBP Status with a Preterm Birth. Int. J. Mol. Sci. 2025, 26, 4475. https://doi.org/10.3390/ijms26104475
Woo J, Nandu T, Nowak A, Forsman A, Giurgescu C. Gene Expression Differences Based on Low Total 25(OH)D and Low VDBP Status with a Preterm Birth. International Journal of Molecular Sciences. 2025; 26(10):4475. https://doi.org/10.3390/ijms26104475
Chicago/Turabian StyleWoo, Jennifer, Tulip Nandu, Alexandra Nowak, Anna Forsman, and Carmen Giurgescu. 2025. "Gene Expression Differences Based on Low Total 25(OH)D and Low VDBP Status with a Preterm Birth" International Journal of Molecular Sciences 26, no. 10: 4475. https://doi.org/10.3390/ijms26104475
APA StyleWoo, J., Nandu, T., Nowak, A., Forsman, A., & Giurgescu, C. (2025). Gene Expression Differences Based on Low Total 25(OH)D and Low VDBP Status with a Preterm Birth. International Journal of Molecular Sciences, 26(10), 4475. https://doi.org/10.3390/ijms26104475






