Identification of ALOX12B Gene Expression, Evolution, and Potential Functional Sites in Horn Development of Sheep
Abstract
1. Introduction
2. Results
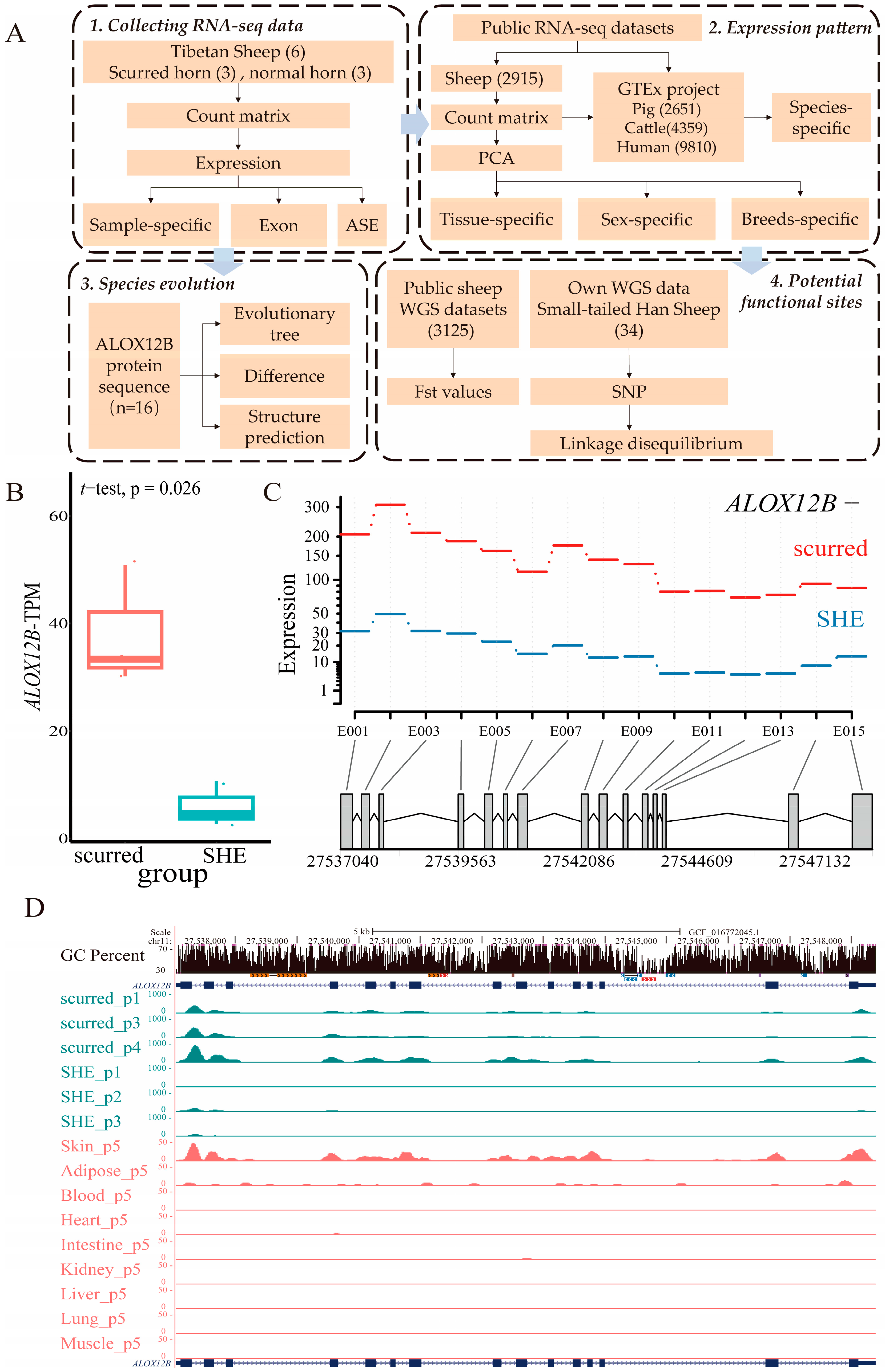
2.1. Sample-Specific Expression of ALOX12B in Different Groups
2.2. Differential Expression of ALOX12B in Tissues, Species, Sex, and Breeds
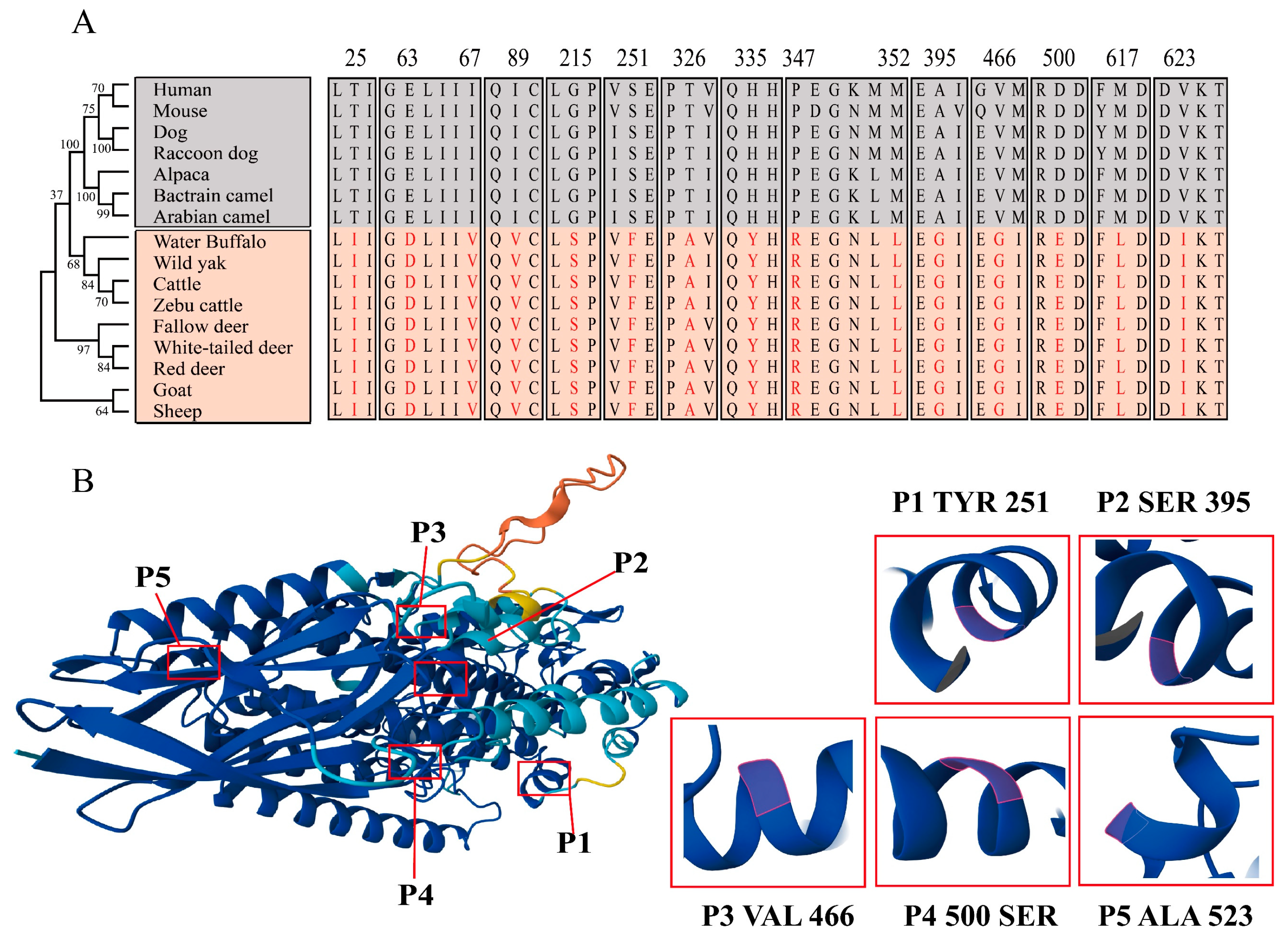
2.3. Species Evolution and Structure Prediction of the ALOX12B Protein
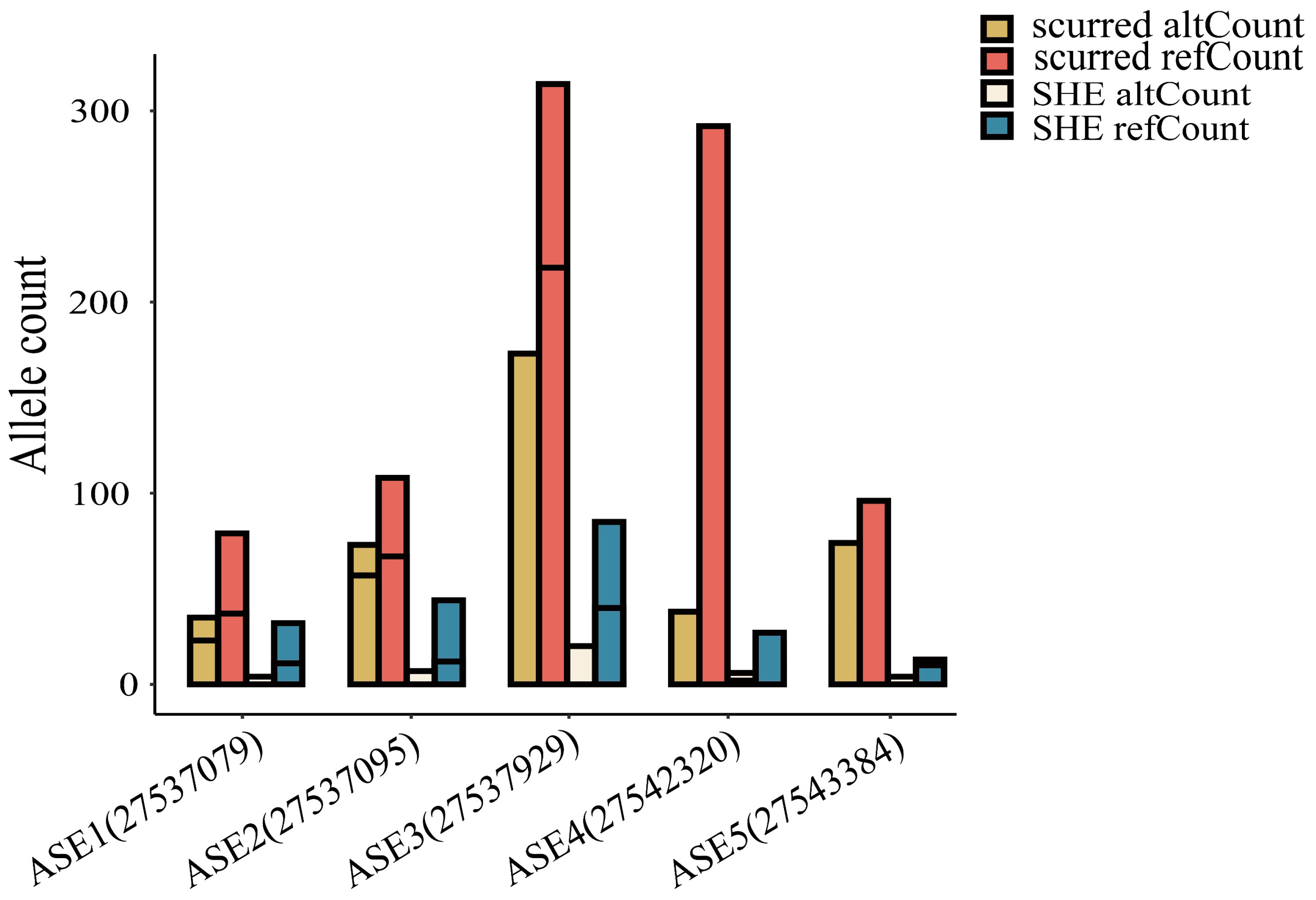
2.4. Allele-Specific Expression Analysis of ALOX12B
2.5. Identification of Potential Functional Sites in ALOX12B
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal and Sample Collection
4.3. RNA-Seq Data Analysis
4.4. Expression Pattern Analysis of the ALOX12B Gene
4.5. Cross-Species Comparison of ALOX12B Protein
4.6. Identifying SNP Loci from WGS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Z.; Zhang, Z.; Ji, J.; Li, H.; Sun, A.; Li, F.; Wang, H.; Chu, M.; Lü, S. Tissue Expression Pattern and Functional SNP Analysis of INSL3 Gene in Sheep. Acta Vet. Zootech. Sin. 2020, 51, 679–687. [Google Scholar]
- Zhou, L.; Yan, S.; Zhang, Y.; He, J.; Pan, Q.; Dong, H. Research Progress of Sheep Horn Morphology and Genetic Regulation Mechanism. Acta Vet. Zootech. Sin. 2021, 52, 2073–2082. [Google Scholar]
- Sun, H.; Zhang, H.; Liu, Y.; Dun, W.; Wang, K.; Li, X. Advances on Horn Type and Related Genes of Sheep. China Herbiv. Sci. 2021, 41, 54–57. [Google Scholar]
- Wang, Y. Comparative Genomics Analysis of Origin and Evolution of Ruminant Headgear; Northwest A&F University: Xianyang, China, 2019. [Google Scholar]
- He, X.; Jiang, L.; Pu, Y.; Zhao, Q.; Ma, Y. Progress on genetic mapping and genetic mechanism of cattle and sheep horns. Hereditas 2021, 43, 40–51. [Google Scholar] [PubMed]
- Zhang, L. Inheritance of Horn and Economic Traits in Inner Mongolia Cashmere Goat and Screening and Identification of Candidate Genes; Inner Mongolia Agricultural University: Hohhot, China, 2021. [Google Scholar]
- Lühken, G.; Krebs, S.; Rothammer, S.; Küpper, J.; Mioč, B.; Russ, I.; Medugorac, I. The 1.78-kb insertion in the 3′-untranslated region of RXFP2 does not segregate with horn status in sheep breeds with variable horn status. Genet. Sel. Evol. 2016, 48, 78. [Google Scholar] [CrossRef]
- Johnston, S.E.; McEwan, J.C.; Pickering, N.K.; Kijas, J.W.; Beraldi, D.; Pilkington, J.G.; Pemberton, J.M.; Slate, J. Genome-wide association mapping identifies the genetic basis of discrete and quantitative variation in sexual weaponry in a wild sheep population. Mol. Ecol. 2011, 20, 2555–2566. [Google Scholar] [CrossRef]
- He, X.; Zhou, Z.; Pu, Y.; Chen, X.; Ma, Y.; Jiang, L. Mapping the four-horned locus and testing the polled locus in three Chinese sheep breeds. Anim. Genet. 2016, 47, 623–627. [Google Scholar] [CrossRef]
- Luan, Y.; Wu, S.; Wang, M.; Pu, Y.; Zhao, Q.; Ma, Y.; Jiang, L.; He, X. Identification of Critical Genes for Ovine Horn Development Based on Transcriptome during the Embryonic Period. Biology 2023, 12, 591. [Google Scholar] [CrossRef]
- Chegini, M.; Eslami, M.; Motavaf, M.; Memarsadeghi, O.; Hoseini, A.; Torab, E.; Hoseininasab, F.; Amiri, H.; Ramandi, S.; Mostofinezhad, N.; et al. Whole exome sequencing identifies novel pathogenic variants in TGM1 and ALOX12B in patients with hereditary ichthyosis. Arch. Dermatol. Res. 2023, 316, 24. [Google Scholar] [CrossRef]
- Joosten, M.D.W.; Clabbers, J.M.K.; Jonca, N.; Mazereeuw-Hautier, J.; Gostyński, A.H. New developments in the molecular treatment of ichthyosis: Review of the literature. Orphanet J. Rare Dis. 2022, 17, 269. [Google Scholar] [CrossRef]
- Vahlquist, A.; Fischer, J.; Törmä, H. Inherited Nonsyndromic Ichthyoses: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2018, 19, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; McDonnell, M.; Chen, X.S.; Lakkis, M.M.; Li, H.; Isaacs, S.N.; Elsea, S.H.; Patel, P.I.; Funk, C.D. Human 12(R)-lipoxygenase and the mouse ortholog. Molecular cloning, expression, and gene chromosomal assignment. J. Biol. Chem. 1998, 273, 33540–33547. [Google Scholar] [CrossRef] [PubMed]
- Hotz, A.; Kopp, J.; Bourrat, E.; Oji, V.; Komlosi, K.; Giehl, K.; Bouadjar, B.; Bygum, A.; Tantcheva-Poor, I.; Pigg, M.H.; et al. Meta-Analysis of Mutations in ALOX12B or ALOXE3 Identified in a Large Cohort of 224 Patients. Genes 2021, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.; Liang, D.; Jiang, F.; Zhang, L.; Song, W.; Wan, B.; Xia, C.; Lu, Q. Solanine induces ferroptosis in colorectal cancer cells through ALOX12B/ADCY4 molecular axis. J. Pharm. Pharmacol. 2024, 76, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, B.; Li, Y.M.; Yang, Q.Y.; Tu, K.J.; Li, L.Y. ALOX12B promotes carcinogenesis in cervical cancer by regulating the PI3K/ERK1 signaling pathway. Oncol. Lett. 2020, 20, 1360–1368. [Google Scholar] [CrossRef]
- Gao, Y.; Xi, S.; Cai, B.; Wu, T.; Wang, Q.; Kalds, P.; Huang, S.; Wang, Y.; Han, S.; Pan, M.; et al. Sheep with Partial RXFP2 Knockout Exhibit Normal Horn Phenotype but Unilateral Cryptorchidism1. J. Integr. Agric. 2023; in press. [Google Scholar]
- Zhang, G.; Chu, M.; Yang, H.; Li, H.; Shi, J.; Feng, P.; Wang, S.; Pan, Z. Expression, Polymorphism, and Potential Functional Sites of the BMPR1A Gene in the Sheep Horn. Genes 2024, 15, 376. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, H.; Boeglin, W.E.; Elias, P.M.; Crumrine, D.; Beier, D.R.; Brash, A.R. Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: A proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope. J. Biol. Chem. 2011, 286, 24046–24056. [Google Scholar] [CrossRef]
- Doering, T.; Brade, H.; Sandhoff, K. Sphingolipid metabolism during epidermal barrier development in mice. J. Lipid Res. 2002, 43, 1727–1733. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Covalently bound omega-hydroxyacylsphingosine in the stratum corneum. Biochim. Biophys. Acta 1987, 917, 108–111. [Google Scholar] [CrossRef]
- Oczkowicz, M.; Szmatoła, T.; Piórkowska, K.; Ropka-Molik, K. Variant calling from RNA-seq data of the brain transcriptome of pigs and its application for allele-specific expression and imprinting analysis. Gene 2018, 641, 367–375. [Google Scholar] [CrossRef]
- Chitwood, J.L.; Rincon, G.; Kaiser, G.G.; Medrano, J.F.; Ross, P.J. RNA-seq analysis of single bovine blastocysts. BMC Genom. 2013, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Gregg, C.; Zhang, J.; Weissbourd, B.; Luo, S.; Schroth, G.P.; Haig, D.; Dulac, C. High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Mouse Brain. Science 2010, 329, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, X.; Li, X.; Feng, P.; Chu, M.; Jin, Y.; Pan, Z. Genetic diversity, tissue-specific expression, and functional analysis of the ATP7A gene in sheep. Front. Genet. 2023, 14, 1239979. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Van-Der-Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Angel, G.D.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Liu, S.; Gao, Y.; Canela-Xandri, O.; Wang, S.; Yu, Y.; Cai, W.; Li, B.; Xiang, R.; Chamberlain, A.J.; Pairo-Castineira, E.; et al. A multi-tissue atlas of regulatory variants in cattle. Nat. Genet. 2022, 54, 1438–1447. [Google Scholar] [CrossRef]
- Consortium, G. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Consortium, G. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Wang, J.; Hua, G.; Cai, G.; Ma, Y.; Yang, X.; Zhang, L.; Li, R.; Liu, J.; Ma, Q.; Wu, K.; et al. Genome-wide DNA methylation and transcriptome analyses reveal the key gene for wool type variation in sheep. J. Anim. Sci. Biotechnol. 2023, 14, 88. [Google Scholar] [CrossRef]
- Marina, H.; Gutiérrez-Gil, B.; Esteban-Blanco, C.; Suárez-Vega, A.; Pelayo, R.; Arranz, J.J. Analysis of Whole Genome Resequencing Datasets from a Worldwide Sample of Sheep Breeds to Identify Potential Causal Mutations Influencing Milk Composition Traits. Animals 2020, 10, 1542. [Google Scholar] [CrossRef]
- Schultz, D.T.; Haddock, S.H.D.; Bredeson, J.V.; Green, R.E.; Simakov, O.; Rokhsar, D.S. Ancient gene linkages support ctenophores as sister to other animals. Nature 2023, 618, 110–117. [Google Scholar] [CrossRef]
- Qiao, G.; Xu, P.; Guo, T.; Wu, Y.; Lu, X.; Zhang, Q.; He, X.; Zhu, S.; Zhao, H.; Lei, Z.; et al. Genetic Basis of Dorper Sheep (Ovis aries) Revealed by Long-Read De Novo Genome Assembly. Front. Genet. 2022, 13, 846449. [Google Scholar] [CrossRef]
- Luo, R.; Dai, X.; Zhang, L.; Li, G.; Zheng, Z. Genome-Wide DNA Methylation Patterns of Muscle and Tail-Fat in DairyMeade Sheep and Mongolian Sheep. Animals 2022, 12, 1399. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Li, F.; Zhang, D.; Zhang, Y.; Li, X.; Song, Q.; Zhou, B.; Zhao, L.; Wang, J.; et al. Whole Genome Sequencing Analysis to Identify Candidate Genes Associated With the rib eye Muscle Area in Hu Sheep. Front. Genet. 2022, 13, 824742. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, J.; Lv, C.; Wang, Y.; Wu, G.; Ding, X.; Quan, G. Sequencing Reveals Population Structure and Selection Signatures for Reproductive Traits in Yunnan Semi-Fine Wool Sheep (Ovis aries). Front. Genet. 2022, 13, 812753. [Google Scholar] [CrossRef]
- Allais-Bonnet, A.; Hintermann, A.; Deloche, M.C.; Cornette, R.; Bardou, P.; Naval-Sanchez, M.; Pinton, A.; Haruda, A.; Grohs, C.; Zakany, J.; et al. Analysis of Polycerate Mutants Reveals the Evolutionary Co-option of HOXD1 for Horn Patterning in Bovidae. Mol. Biol. Evol. 2021, 38, 2260–2272. [Google Scholar] [CrossRef] [PubMed]
- Posbergh, C.J.; Staiger, E.A.; Huson, H.J. A Stop-Gain Mutation within MLPH Is Responsible for the Lilac Dilution Observed in Jacob Sheep. Genes 2020, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Shen, M.; Xie, X.L.; Liu, G.J.; Xu, Y.X.; Lv, F.H.; Yang, H.; Yang, Y.L.; Liu, C.B.; et al. Whole-genome resequencing of wild and domestic sheep identifies genes associated with morphological and agronomic traits. Nat. Commun. 2020, 11, 2815. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Li, X.; Ni, W.; Xu, Y.; Yao, R.; Wei, B.; Zhang, M.; Li, H.; Zhao, Y.; et al. Whole-Genome Resequencing Reveals Loci Associated With Thoracic Vertebrae Number in Sheep. Front. Genet. 2019, 10, 674. [Google Scholar] [CrossRef]
- Pan, Z.; Li, S.; Liu, Q.; Wang, Z.; Zhou, Z.; Di, R.; Miao, B.; Hu, W.; Wang, X.; Hu, X.; et al. Whole-genome sequences of 89 Chinese sheep suggest role of RXFP2 in the development of unique horn phenotype as response to semi-feralization. Gigascience 2018, 7, giy019. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Dong, S.; He, W.; Ji, J.; Zhang, C.; Guo, Y.; Yang, T. LDBlockShow: A fast and convenient tool for visualizing linkage disequilibrium and haplotype blocks based on variant call format files. Brief. Bioinform. 2020, 22, bbaa227. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, R.; Zhang, G.; Li, H.; Shi, J.; Meng, Z.; Lu, X.; Shan, M.; Yang, J.; Pan, Z. Identification of ALOX12B Gene Expression, Evolution, and Potential Functional Sites in Horn Development of Sheep. Int. J. Mol. Sci. 2025, 26, 79. https://doi.org/10.3390/ijms26010079
Lv R, Zhang G, Li H, Shi J, Meng Z, Lu X, Shan M, Yang J, Pan Z. Identification of ALOX12B Gene Expression, Evolution, and Potential Functional Sites in Horn Development of Sheep. International Journal of Molecular Sciences. 2025; 26(1):79. https://doi.org/10.3390/ijms26010079
Chicago/Turabian StyleLv, Ran, Guoqing Zhang, Hao Li, Jianxin Shi, Zhu Meng, Xiaoning Lu, Mingzhu Shan, Jie Yang, and Zhangyuan Pan. 2025. "Identification of ALOX12B Gene Expression, Evolution, and Potential Functional Sites in Horn Development of Sheep" International Journal of Molecular Sciences 26, no. 1: 79. https://doi.org/10.3390/ijms26010079
APA StyleLv, R., Zhang, G., Li, H., Shi, J., Meng, Z., Lu, X., Shan, M., Yang, J., & Pan, Z. (2025). Identification of ALOX12B Gene Expression, Evolution, and Potential Functional Sites in Horn Development of Sheep. International Journal of Molecular Sciences, 26(1), 79. https://doi.org/10.3390/ijms26010079





