Can α-Mangostin and Photodynamic Therapy Support Ciprofloxacin in the Inactivation of Uropathogenic Escherichia coli and Staphylococcus aureus Strains?
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Testing
2.2. Identification of Antibiotic Resistance Genes
2.3. MIC Evaluation
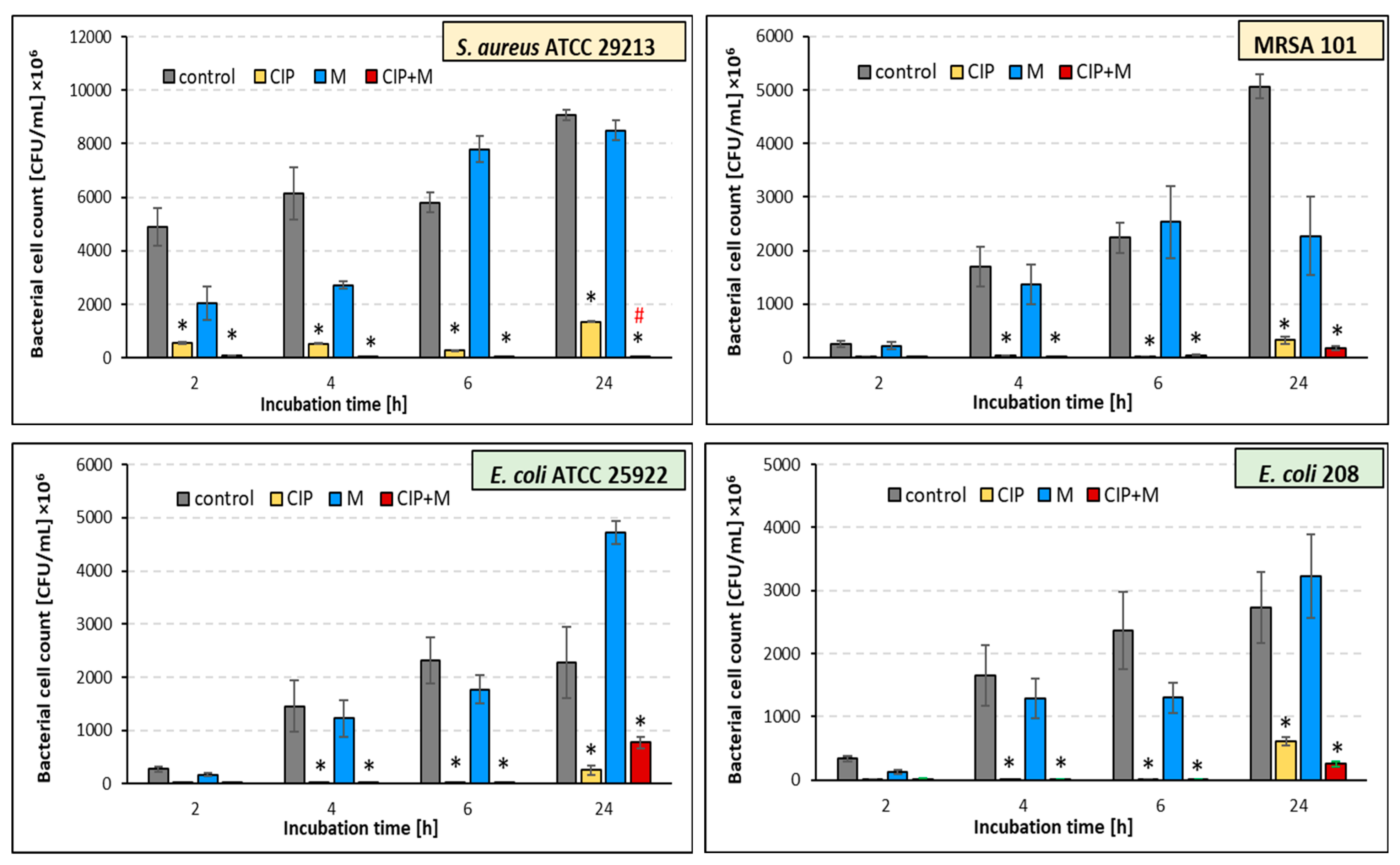
2.4. Effect of the Ciprofloxacin and α-Mangostin Combination on Bacterial Survival
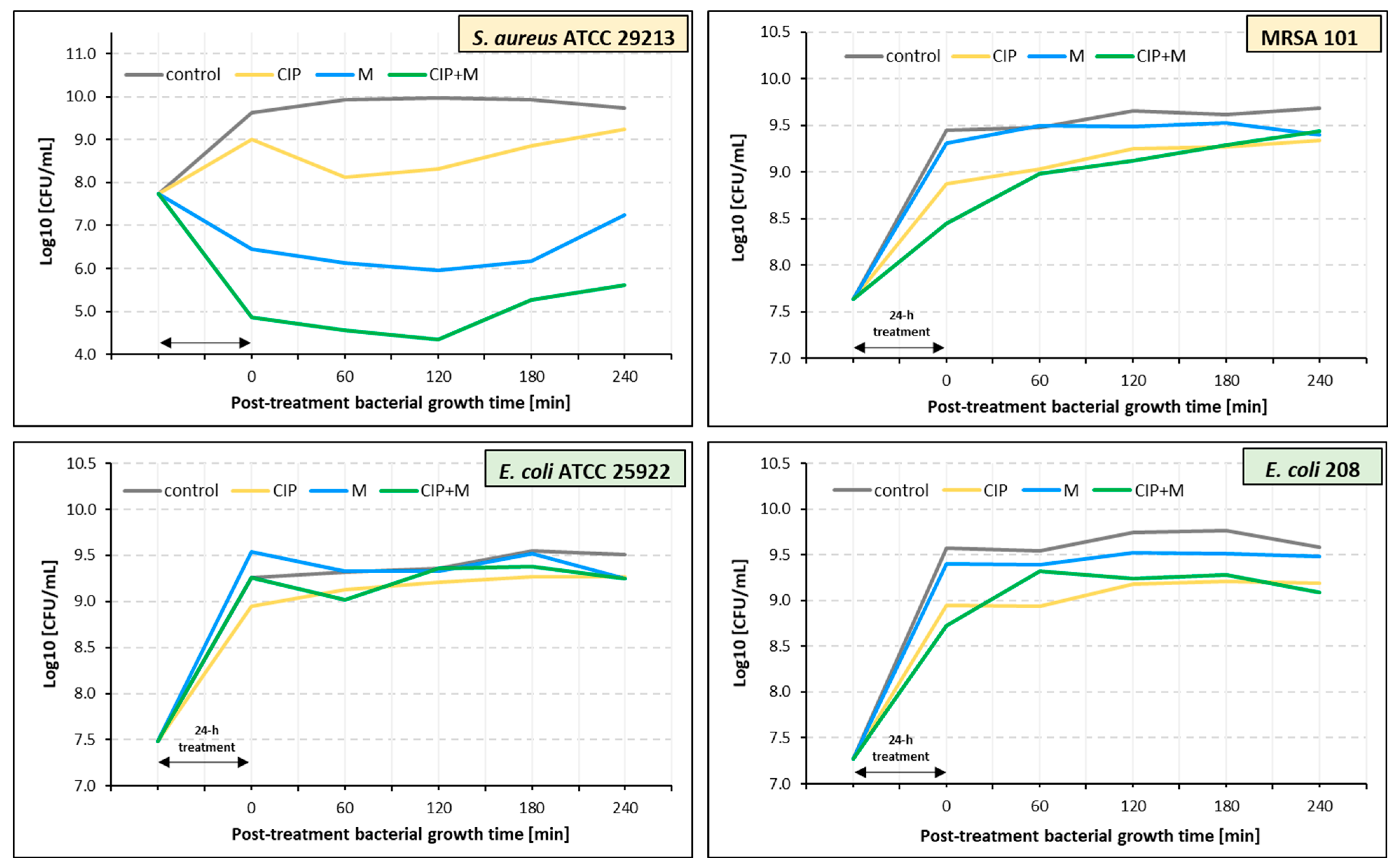
2.5. Estimation of Post-Treatment Duration Time Effects of Ciprofloxacin, α-Mangostin, and Their Combination
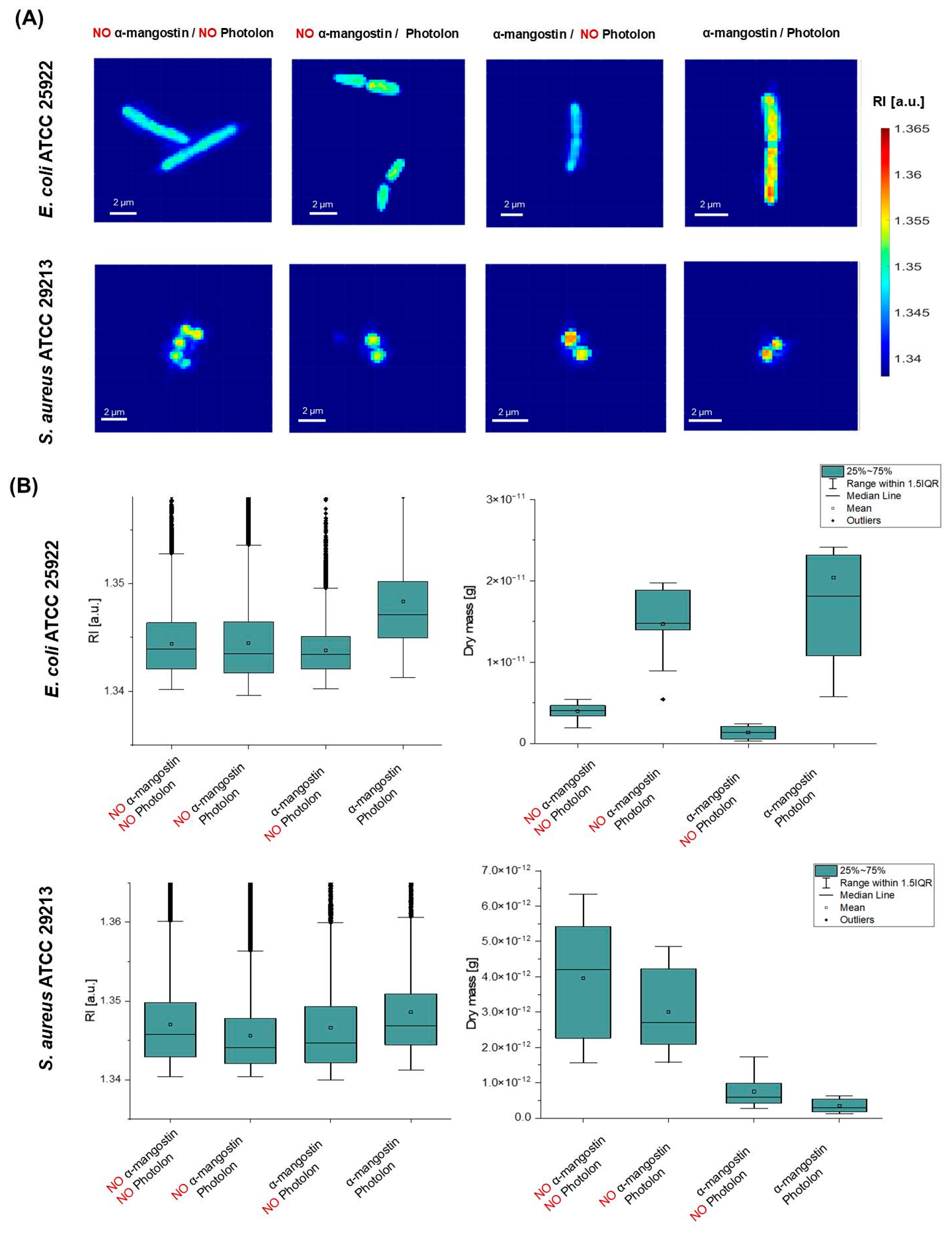
2.6. α-Mangostin-Induced Changes in Bacterial Cells’ Penetration by a Photosensitizer
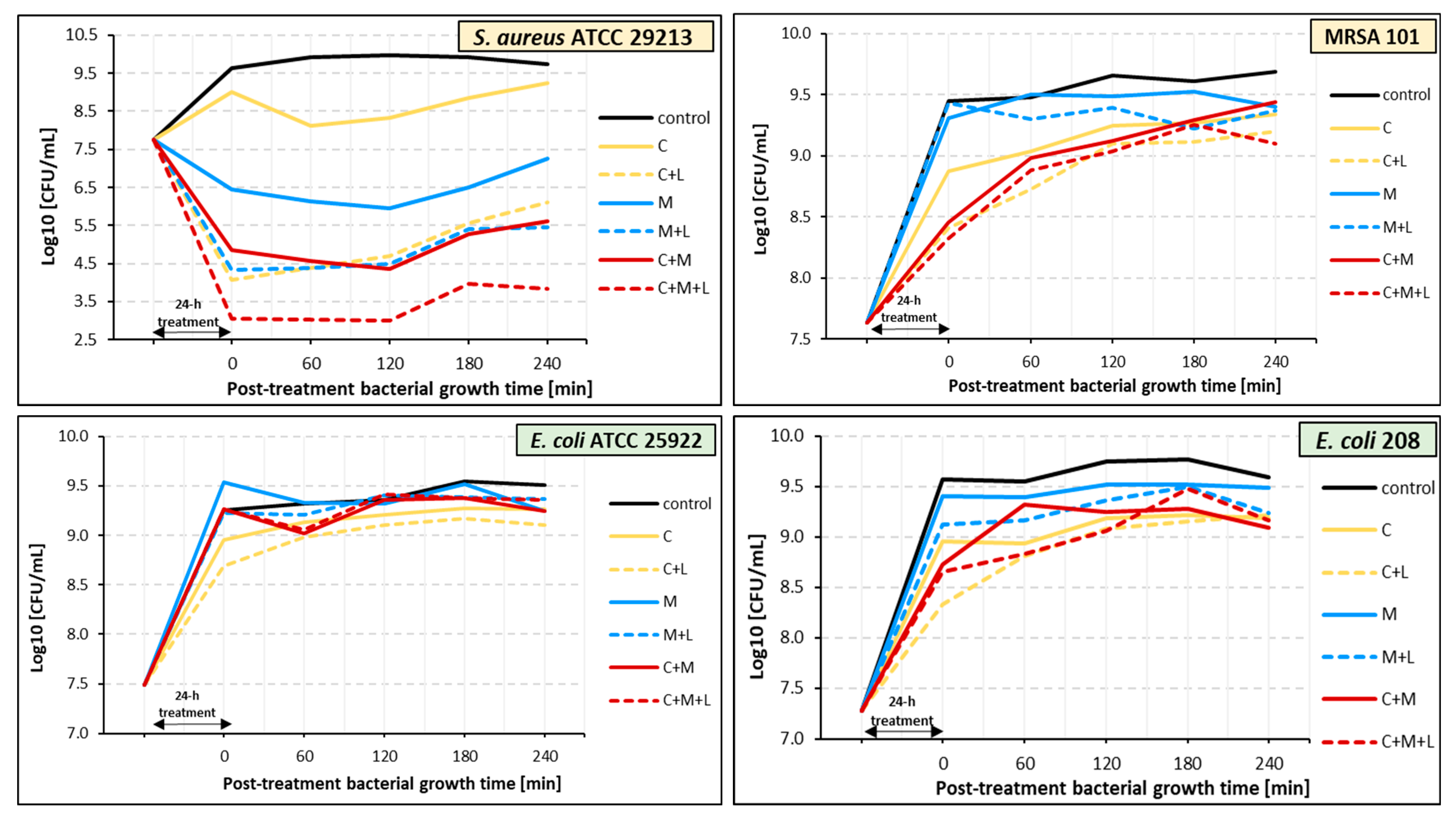
2.7. Effect of PDT on Bacteria Pre-Treated with Ciprofloxacin, α-Mangostin, and Ciprofloxacin with α-Mangostin
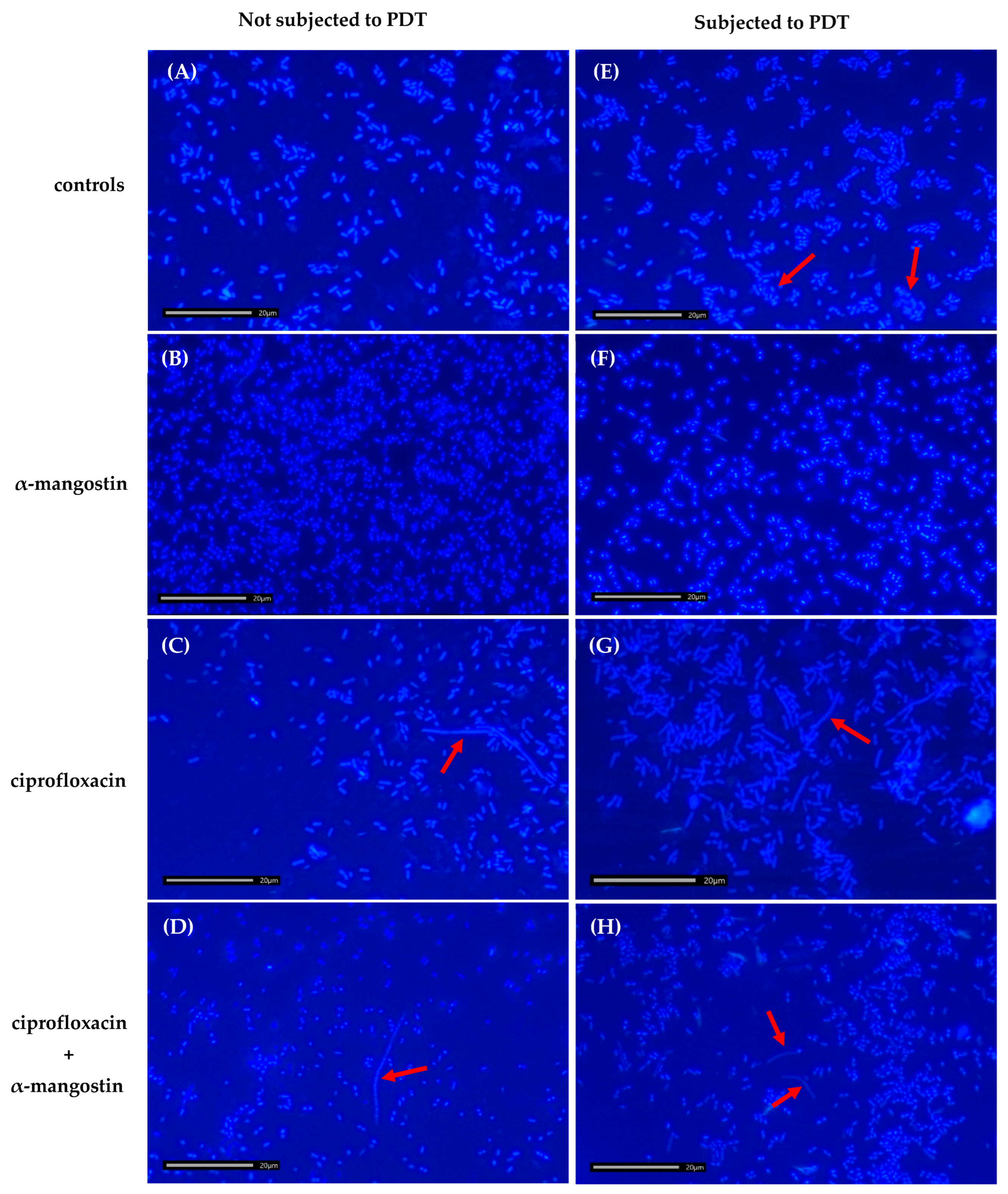
2.8. Effect of Ciprofloxacin, α-Mangostin, and PDT on the Morphology of Bacterial Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antimicrobial Agents
4.3. Photosensitizer and Light Source
4.4. Antimicrobial Susceptibility Testing
4.5. Bacterial DNA Isolation
4.6. Genome Sequencing
4.7. Identification of Antibiotic Resistance Genes
4.8. MIC Evaluation
4.9. Preparation of Bacterial Suspension
4.10. Effects of Ciprofloxacin, α-Mangostin, and Their Combination on Bacterial Survival
4.11. Estimation of the Post-Treatment Effect of Ciprofloxacin, α-Mangostin, and Their Combination
4.12. Quantitative Assessment of Photosensitizer Accumulation Inside Bacterial Cells
4.13. Experimental Conditions for the PDT
4.14. Morphological Changes of Bacterial Cells
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Lievano, A.P.; Cervantes-Flores, F.; Nava-Torres, A.; Carbajal-Morales, P.J.; Villaseñor-Garcia, L.F.; Zavala-Cerna, M.G. Fluoroquinolone resistance in Escherichia coli causing community-acquired urinary tract infections: A systematic review. Microorganisms 2024, 12, 2320. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Meesomboon, T.; Adulheem, P.; Kittiwisut, S.; Sookdee, P.; Samosornsuk, W.; Fuongfuchat, A. Physicochemical properties of alpha-mangostin loaded nanoemulsions prepared by ultrasonication technique. Heliyon 2019, 5, e02465. [Google Scholar] [CrossRef]
- Alam, M.; Rashid, S.; Fatima, K.; Adnan, M.; Shafie, A.; Akhtar, M.S.; Ganie, A.H.; Eldin, S.M.; Islam, A.; Khan, I.; et al. Biochemical features and therapeutic potential of α-mangostin: Mechanism of action, medicinal values, and health benefits. Biomed. Pharmacother. 2023, 163, 114710. [Google Scholar] [CrossRef]
- Kalick, L.S.; Khan, H.A.; Maung, E.; Baez, Y.; Atkinson, A.N.; Wallace, C.E.; Day, F.; Delgadillo, B.E.; Mondal, A.; Watanapokasin, R.; et al. Mangosteen for malignancy prevention and intervention: Current evidence, molecular mechanisms, and future perspectives. Pharmacol. Res. 2023, 188, 106630. [Google Scholar] [CrossRef]
- Al-Massarani, S.M.; El Gamal, A.A.; Al-Musayeib, N.M.; Mothana, R.A.; Basudan, O.A.; Al-Rehaily, A.J.; Farag, M.; Assaf, M.H.; El Tahir, K.H.; Maes, L. Phytochemical, antimicrobial and antiprotozoal evaluation of Garcinia mangostana pericarp and α-mangostin, its major xanthone derivative. Molecules 2013, 18, 10599–10608. [Google Scholar] [CrossRef]
- Meah, M.S.; Panichayupakaranant, P.; Sayeed, M.A.; Kim, M.G. Isolation and pharmacochemistry of α-mangostin as a chemotherapeutic agent. Pharmacogn. Mag. 2024, 20, 1–17. [Google Scholar] [CrossRef]
- Sultan, O.S.; Kantilal, H.K.; Khoo, S.P.; Davamani, A.F.; Eusufzai, S.Z.; Rashid, F.; Jamayet, N.B.; Soh, J.A.; Tan, Y.Y.; Alam, M.K. The potential of α-mangostin from Garcinia mangostana as an effective antimicrobial agent—A systematic review and meta-analysis. Antibiotics 2022, 11, 717. [Google Scholar] [CrossRef]
- Leelapornpisid, W. Efficacy of alpha-mangostin for antimicrobial activity against endodontopathogenic microorganisms in a multi-species bacterial-fungal biofilm model. Arch. Oral Biol. 2022, 133, 105304. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Ismail, Z.; Abu-Salah, K.M.; Majid, A.M.S.A. Solid dispersions of α-mangostin improve its aqueous solubility through self-assembly of nanomicelles. J. Pharm. Sci. 2012, 101, 815–825. [Google Scholar] [CrossRef]
- Koh, J.J.; Qiu, S.; Zou, H.; Lakshminarayanan, R.; Li, J.; Zhou, X.; Tang, C.; Saraswathi, P.; Verma, C.; Tan, D.T.H.; et al. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim. Biophys. Acta 2013, 1828, 834–844. [Google Scholar] [CrossRef]
- Sakagami, Y.; Iinuma, M.; Piyasena, K.G.N.P.; Dharmaratne, H.R.W. Antibacterial activity of α-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine 2005, 12, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, M.; Leskinen, K.; Aravindraja, C.; Saavalainen, P.; Pandian, S.K.; Skurnik, M.; Ravi, A.V. Deciphering the antibacterial mode of action of alpha-mangostin on Staphylococcus epidermidis RP62A through an integrated transcriptomic and proteomic approach. Front. Microbiol. 2019, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Falsetta, M.L.; Hwang, G.; Gonzalez-Begne, M.; Koo, H. α-mangostin disrupts the development of Streptococcus mutans biofilms and facilitates its mechanical removal. PLoS ONE 2014, 9, e111312. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xu, H.; Li, D.; Chen, J.; Yu, Z.; Deng, Q.; Li, P.; Zheng, J.; Zhang, H. Mechanisms of rapid bactericidal and anti-biofilm alpha-mangostin in vitro activity against Staphylococcus aureus. Pol. J. Microbiol. 2023, 72, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Rapacka-Zdonczyk, A.; Mutters, N.T.; Grinholc, M. Antimicrobials are a photodynamic inactivation adjuvant for the eradication of extensively drug-resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 229. [Google Scholar] [CrossRef]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in photodynamic antimicrobial chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Ahmad, I.; Keach, J.E.; Behl, T.; Panichayupakaranant, P. Synergistic effect of α-mangostin on antibacterial activity of tetracycline, erythromycin, and clindamycin against acne involved bacteria. Chin. Herb. Med. 2019, 11, 412–416. [Google Scholar] [CrossRef]
- Seesom, W.; Jaratrungtawee, A.; Suksamrarn, S.; Mekseepralard, C.; Ratananukul, P.; Sukhumsirichart, W. Antileptospiral activity of xanthones from Garcinia mangostana and synergy of gamma-mangostin with penicillin G. BMC Complement. Altern. Med. 2013, 13, 182. [Google Scholar] [CrossRef]
- Lai, H.W.; Tani, Y.; Sukatta, U.; Rugthaworn, P.; Thepyos, A.; Yamamoto, S.; Fukuhara, H.; Inoue, K.; Yuasa, H.; Nakamura, H.; et al. Mangostin enhances efficacy of aminolevulinic acid-photodynamic therapy against cancer through inhibition of ABCG2 activity. Photodiagn. Photodyn. Ther. 2023, 44, 103798. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- EUCAST—The European Committee on Antimicrobial Susceptibility Testing. Breakpoints Tables for Interpretation of MICs and Zone Diameters, v. 14.0.; EUCAST: Växjö, Sweden, 2024. [Google Scholar]
- Kapic, A.; Sabnis, N.; Dossou, A.S.; Chavez, J.; Ceresa, L.; Gryczynski, Z.; Fudala, R.; Dickerman, R.; Bunnell, B.A.; Lacko, A.G. Photophysical characterization and in vitro evaluation of α-mangostin-loaded HDL mimetic nano-complex in LN-229 glioblastoma spheroid model. Int. J. Mol. Sci. 2024, 25, 7378. [Google Scholar] [CrossRef] [PubMed]
- Buzalewicz, I.; Hołowacz, I.; Matczuk, A.K.; Guźniczak, M.; Skrzela, D.; Karwańska, M.; Wieliczko, A.; Kowal, K.; Ulatowska-Jarża, A. Photolon nanoporous photoactive material with antibacterial activity and label-free noncontact method for free radical detection. Int. J. Mol. Sci. 2022, 23, 279. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Zhao, H.; Tang, Q.; Chandarajoti, K.; Bai, H.; Wang, X.; Zhang, K.; Ye, W.; Han, X.; Wang, C.; et al. A novel α-mangostin derivative synergistic to antibiotics against MRSA with unique mechanisms. Microbiol. Spectr. 2024, 12, e01631-24. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Rastogi, A.; Pandey, S.; Gupta, S.; Sohal, J.S. Multidrug-resistant bacteria: Their mechanism of action and prophylaxis. Biomed. Res. Int. 2022, 2022, 5419874. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Chitchumroonchokchai, C.; Lesinski, G.B.; Suksamrarn, S.; Failla, M.L. α-Mangostin: Anti-inflammatory activity and metabolism by human cells. J. Agric. Food Chem. 2013, 61, 3891–3900. [Google Scholar] [CrossRef]
- Syam, S.; Bustamam, A.; Abdullah, R.; Sukari, M.A.; Hashim, N.M.; Mohan, S.; Looi, C.Y.; Wong, W.F.; Yahayu, M.A.; Abdelwahab, S.I. β mangostin suppress LPS-induced inflammatory response in RAW 264.7 macrophages in vitro and carrageenan-induced peritonitis in vivo. J. Ethnopharmacol. 2014, 153, 435–445. [Google Scholar] [CrossRef]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia 2007, 78, 401–408. [Google Scholar] [CrossRef]
- Azimi, H.; Fallah-Tafti, M.; Khakshur, A.A.; Abdollahi, M. A review of phytotherapy of acne vulgaris: Perspective of new pharmacological treatments. Fitoterapia 2012, 83, 1306–1317. [Google Scholar] [CrossRef]
- Rakhmawatie, M.D.; Rohmani, A.; Ahyar, F.W. In vitro effect of alfa-mangostin on multiresistant uropathogenic Escherichia coli. Sains Med. 2017, 8, 20–27. [Google Scholar] [CrossRef]
- Meah, M.S.; Panichayupakaranant, P. α-Mangostinrich extract: A potential oral antibacterial agent prepared by green extraction. IPCM 2020, 4, 000201. [Google Scholar]
- Sivaranjani, M.; Prakash, M.; Gowrishankar, S.; Rathna, J.; Pandian, S.K.; Ravi, A.V. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl. Microbiol. Biotechnol. 2017, 101, 3349–3359. [Google Scholar] [CrossRef]
- Zou, H.; Koh, J.J.; Li, J.; Qiu, S.; Aung, T.T.; Lin, H.; Lakshminarayanan, R.; Dai, X.; Tang, C.; Lim, F.H.; et al. Design and synthesis of amphiphilic xanthone-based, membrane-targeting antimicrobials with improved membrane selectivity. J. Med. Chem. 2013, 56, 2359–2373. [Google Scholar] [CrossRef]
- Wang, C.; Chen, P.; Qiao, Y.; Kang, Y.; Guo, S.; Wu, D.; Wang, J.; Hong Wu, H. Bacteria-activated chlorin e6 ionic liquid based on cation and anion dual-mode antibacterial action for enhanced photodynamic efficacy. Biomater. Sci. 2019, 7, 1399. [Google Scholar] [CrossRef]
- Le Guern, F.; Ouk, T.; Grenier, K.; Joly, N.; Lequart, V.; Sol, V. Enhancement of photobactericidal activity of chlorin-e6-cellulose nanocrystals by covalent attachment of polymyxin B. J. Mater. Chem. B 2017, 5, 6953. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Li, J.; Beuerman, R.W.; Verma, C.S. Molecular insights into the membrane affinities of model hydrophobes. ACS Omega 2018, 3, 2498–2507. [Google Scholar] [CrossRef]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 195. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Suwannapoch, N.; Phakhodee, W.; Thanuhiranlert, J.; Ratananukul, P.; Chimnoi, N.; Suksamrarn, A. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem. Pharm. Bull. 2003, 51, 857–859. [Google Scholar] [CrossRef]
- Ibrahim, N.H.S.; Taher, M.; Susanti, D.; Amiroudine, M.Z.M.; Ahmed, Q.U. Direct and indirect antimicrobial effects of α-mangostin on pathogenic microorganism. J. Coast. Life Med. 2014, 2, 70–75. [Google Scholar]
- Ding, Y.; Hao, J.; Xiao, W.; Ye, C.; Xiao, X.; Jian, C.; Tang, M.; Li, G.; Liu, J.; Zeng, Z. Role of efflux pumps, their inhibitors, and regulators in colistin resistance. Front. Microbiol. 2023, 14, 1207441. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, O.O.; Adeoye-Isijola, M.O.; Okon, V.; Adedayo, O.; Coopoosamy, R.M. In vitro pharmacological interaction of caffeine and first-line antibiotics is antagonistic against clinically important bacterial pathogens. Acta Biochem. Pol. 2017, 64, 255–263. [Google Scholar]
- Pietrowska, A.; Hołowacz, I.; Ulatowska-Jarża, A.; Guźniczak, M.; Matczuk, A.K.; Wieliczko, A.; Wolf-Baca, M.; Buzalewicz, I. The enhancement of antimicrobial photodynamic therapy of Escherichia coli by a functionalized combination of photosensitizers: In vitro examination of single cells by quantitative phase imaging. Int. J. Mol. Sci. 2022, 23, 6137. [Google Scholar] [CrossRef]
- Aebisher, D.; Czech, S.; Dynarowicz, K.; Misiołek, M.; Komosińska-Vassev, K.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic therapy: Past, current, and future. Int. J. Mol. Sci. 2024, 25, 11325. [Google Scholar] [CrossRef]
- Feng, Y.; Coradi Tonon, C.; Ashraf, S.; Hasan, T. Photodynamic and antibiotic therapy in combination against bacterial infections: Efficacy, determinants, mechanisms, and future perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113941. [Google Scholar] [CrossRef]
- Krespi, Y.P.; Kizhner, V.; Nistico, L.; Hall-Stoodley, L.; Stoodley, P. Laser disruption and killing of methicillin-resistant Staphylococcus aureus biofilms. Am. J. Otolaryngol. 2011, 32, 198–202. [Google Scholar] [CrossRef]
- Shih, M.H.; Huang, F.C. Effects of photodynamic therapy on rapidly growing nontuberculous mycobacteria keratitis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 223–229. [Google Scholar] [CrossRef]
- Ronqui, M.R.; de Aguiar, T.M.S.F.; De Freitas, L.M.; Miranda, E.T.; Fontana, C.R. Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. J. Photochem. Photobiol. B. 2016, 158, 122–129. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Donnelly, R.F.; Elborn, J.S.; Magee, N.D.; Tunney, M.M. Photodynamic Antimicrobial Chemotherapy (PACT) in combination with antibiotics for treatment of Burkholderia cepacia complex infection. J. Photochem. Photobiol. B 2012, 106, 95–100. [Google Scholar] [CrossRef]
- Javaid, S.; Qureshi, I.Z.; Khurshid, A.; Trembley, J.H.; Razak, S. Photoactive metabolite mediated photodynamic therapy of rhabdomyosarcoma cell lines using medicinal plants and doxorubicin co-treatments. BMC Complement. Med. Ther. 2024, 24, 270. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Photoactive herbal compounds: A green approach to photodynamic therapy. Molecules 2022, 27, 5084. [Google Scholar] [CrossRef] [PubMed]
- De Coster, W.; Rademakers, R. NanoPack2: Population-scale evaluation of long-read sequencing data. Bioinformatics 2023, 39, btad311. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Buzalewicz, I.; Ulatowska-Jarża, A.; Kaczorowska, A.; Gąsior-Głogowska, M.; Podbielska, H.; Karwańska, M.; Wieliczko, A.; Matczuk, A.K.; Kowal, K.; Kopaczyńska, M. Bacteria single-cell and photosensitizer interaction revealed by quantitative phase imaging. Int. J. Mol. Sci. 2021, 22, 5068. [Google Scholar] [CrossRef]
- Friebel, M.; Meinke, M. Model function to calculate the refractive index of native hemoglobin in the wavelength range of 250–1100 nm dependent on concentration. Appl. Opt. 2006, 45, 2838–2842. [Google Scholar] [CrossRef]
- Tichaczek-Goska, D.; Gleńsk, M.; Wojnicz, D. The enhancement of the photodynamic therapy and ciprofloxacin activity against uropathogenic Escherichia coli strains by Polypodium vulgare rhizome aqueous extract. Pathogens 2021, 10, 1544. [Google Scholar] [CrossRef]

| Resistance Gene | Predicted Resistance Phenotype | ResFinder Accession | Identity [%] |
|---|---|---|---|
| MRSA 101 | |||
| blaZ | Amoxicillin, Ampicillin, Penicillin, Piperacillin | CTTL01000039 | 100 |
| grlA p. S80F | Ciprofloxacin | CP026964.1 | 99.5 |
| grlB p. P585S | 99.6 | ||
| gyrA p. S84L | 99.47 | ||
| mecA | Amoxicillin, Ampicillin Amoxicillin + Clavulanic acid Ampicillin + Clavulanic acid Cefepime, Cefixime, Cefotaxime, Cefoxitin, Ceftazidime, Ertapenem, Imipenem, Meropenem, Piperacillin Piperacillin + tazobactam | NC_002951 | 99.95 |
| ermC | Lincomycin, Clindamycin, Erythromycin, Quinupristin, Pristinamycin, Virginiamycin | M13761 | 100 |
| E. coli 208 | |||
| aph (6)-Id | Streptomycin | CP000971 | 99.88 |
| aph (3″)-Ib | Streptomycin | AF321551 | 100 |
| aadA5 | Spectinomycin, Streptomycin | AF137361 | 100 |
| blaCTX-M-27 | Amoxicillin, Ampicillin, Aztreonam, Cefepime, Cefotaxime, Ceftazidime, Ceftriaxone, Piperacillin, Ticarcillin | AY156923 | 100 |
| mph (A) | Erythromycin, Azithromycin, Spiramycin, Telithromycin | D16251 | 100 |
| sul1 | Sulfamethoxazole | U12338 | 99.88 |
| sul2 | Sulfamethoxazole | AY034138 | 100 |
| tet (A) | Doxycycline, Tetracycline | AJ517790 | 99.88 |
| dfrA17 | Trimethoprim | FJ460238 | 100 |
| S. aureus ATCC 29213 | |||
| blaZ | Amoxicillin, Ampicillin, Penicillin, Piperacillin | CZWI01000159 | 99.88 |
| Ciprofloxacin | α-Mangostin | |
|---|---|---|
| MRSA 101 | 128 (r) | 512 |
| E. coli 208 | 64 (r) | 512 |
| S. aureus ATCC 29213 | 0.5 (s) | 64 |
| E. coli ATCC 25922 | 0.015 (s) | 512 |
| PTE Duration (Min) | ||||
|---|---|---|---|---|
| Treatment | S. aureus ATCC 29213 | E. coli ATCC 25922 | MRSA 101 | E. coli 208 |
| ciprofloxacin | 195 | 60 | 0 | 60 |
| α-mangostin | 195 | 180 | 0 | 0 |
| ciprofloxacin + α-mangostin | 150 | 120 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnicz, D.; Korzekwa, K.; Guźniczak, M.; Wernecki, M.; Ulatowska-Jarża, A.; Buzalewicz, I.; Tichaczek-Goska, D. Can α-Mangostin and Photodynamic Therapy Support Ciprofloxacin in the Inactivation of Uropathogenic Escherichia coli and Staphylococcus aureus Strains? Int. J. Mol. Sci. 2025, 26, 76. https://doi.org/10.3390/ijms26010076
Wojnicz D, Korzekwa K, Guźniczak M, Wernecki M, Ulatowska-Jarża A, Buzalewicz I, Tichaczek-Goska D. Can α-Mangostin and Photodynamic Therapy Support Ciprofloxacin in the Inactivation of Uropathogenic Escherichia coli and Staphylococcus aureus Strains? International Journal of Molecular Sciences. 2025; 26(1):76. https://doi.org/10.3390/ijms26010076
Chicago/Turabian StyleWojnicz, Dorota, Kamila Korzekwa, Mateusz Guźniczak, Maciej Wernecki, Agnieszka Ulatowska-Jarża, Igor Buzalewicz, and Dorota Tichaczek-Goska. 2025. "Can α-Mangostin and Photodynamic Therapy Support Ciprofloxacin in the Inactivation of Uropathogenic Escherichia coli and Staphylococcus aureus Strains?" International Journal of Molecular Sciences 26, no. 1: 76. https://doi.org/10.3390/ijms26010076
APA StyleWojnicz, D., Korzekwa, K., Guźniczak, M., Wernecki, M., Ulatowska-Jarża, A., Buzalewicz, I., & Tichaczek-Goska, D. (2025). Can α-Mangostin and Photodynamic Therapy Support Ciprofloxacin in the Inactivation of Uropathogenic Escherichia coli and Staphylococcus aureus Strains? International Journal of Molecular Sciences, 26(1), 76. https://doi.org/10.3390/ijms26010076








