Abstract
Alzheimer’s disease (AD) poses a significant worldwide health challenge, requiring novel approaches for improved models and treatment development. This comprehensive review emphasises the systematic development and improvement of a biomimetic brain environment to address the shortcomings of existing AD models and enhance the efficiency of screening potential drug treatments. We identify drawbacks in traditional models and emphasise the necessity for more physiologically accurate systems through an in-depth analysis of current literature. This review aims to study the development of an advanced AD model that accurately replicates key AD pathophysiological aspects using cutting-edge biomaterials and microenvironment design. Incorporating biomolecular elements like Tau proteins and beta-amyloid (Aβ) plaques improve the accuracy of illustrating disease mechanisms. The expected results involve creating a solid foundation for high-throughput screening with enhanced scalability, translational significance, and the possibility of speeding up drug discovery. Thus, this review fills the gaps in AD modelling and shows potential for creating precise and efficient drug treatments for AD.
1. Introduction
As Malaysia’s population ages, the number of older adults is growing rapidly, exceeding earlier predictions. By 2050, over 15% of the population is projected to be over 65. This trend suggests a higher risk of Alzheimer’s disease (AD), which affects people differently [1]. The incidence of AD is rising as the world’s population ages, highlighting the urgent need for effective treatments and preventative measures. China has seen a surge in Alzheimer’s cases, becoming the global leader [2]. Moreover, while typically associated with old age, Alzheimer’s is now affecting younger individuals in China. The disease’s duration varies greatly, ranging from 2 to 3 years to 20 years for different individuals [3]. However, Alzheimer’s disease is not limited to these regions. With the proportion of older adults rapidly increasing worldwide, more than 55 million people are currently affected by AD or other common forms of dementia, such as frontotemporal degeneration, cerebrovascular disease, hippocampal sclerosis, Lewy body disease, and even Parkinson’s disease, according to the World Health Organization. This figure is expected to triple by 2050 [4]. Notably, AD prevalence varies across regions, with higher rates observed in North America and Europe compared to Asia and Africa. These differences are likely influenced by factors such as life expectancy, lifestyle, and access to healthcare.
Therefore, the urgent need to find a cure for AD highlights the importance of creating new, scientifically based models. In the past, researchers have mainly concentrated on understanding AD by studying protein misfolding and aggregation [5,6,7,8]. It is no surprise that much research has been conducted to investigate the relationship between amyloid-beta (Aβ) plaques and tau tangles and how they contribute to the onset of AD. This link has been thoroughly investigated, and the results indicate that tau tangles and Aβ plaques play a role in developing this devastating condition [9,10,11,12,13,14]. Gradually, the buildup of Aβ aggregates initiates a number of intricate processes that affect tau proteins, interfere with glial cell and neuron function, and eventually cause dementia symptoms [15,16,17,18]. Lifestyle choices and genetics both influence the risk and mechanisms of AD. Comorbidities such as type 2 diabetes mellitus [19,20,21,22,23], stroke [24,25,26], hypercholesterolemia [27,28,29,30], heart disease [31,32,33,34], traumatic brain injury [35,36,37,38], obesity [39,40,41,42], and lifestyle or physical inactivity [43,44], as well as age-related cognitive decline [45,46], are contributors to AD pathogenesis. Pharmacological efforts aimed at targeting anti-amyloid or anti-protein misfolding strategies have, unfortunately, not produced a conclusive disease-modifying treatment for AD [47].
Over the past 25 years, numerous drugs targeting Aβ in AD trials, including five anti-Aβ antibodies (bapineuzumab [48,49], solanezumab [50,51], crenezumab [52], ponezumab [53,54], and gantenerumab [55]), have failed to demonstrate clinical effectiveness. A notable addition to this lineup was Aducanumab (Aduhelm®), introduced in June 2021 [56]. Although Aducanumab effectively reduced amyloid plaques, it did not yield improved clinical outcomes for Alzheimer’s patients [57,58,59]. One major concern surrounding Aducanumab was the high incidence of side effects, particularly amyloid-related imaging abnormalities (ARIAs). These side effects, common and dose-dependent with human monoclonal antibodies, affected about one-third of participants in Aducanumab trials. While one trial indicated that individuals receiving Aduhelm experienced less Alzheimer’s deterioration than those on a placebo, a second trial demonstrated similar worsening in both Aduhelm and placebo groups. Consequently, the European Medicines Agency (EMA) rejected Aducanumab in December 2021, highlighting the difficulties in finding effective treatments for AD [60]. As a result, a variety of molecular theories have been investigated, including metal dyshomeostatis, oxidative stress, mitochondriopathy, gliopathy, and synaptopathy. Despite these studies, a conclusive therapy breakthrough is still elusive [61].
However, the emergence of these novel hypotheses and mechanisms has often been perceived as antagonistic and even competitive. Definitive proclamations have been made, dismissing the amyloid hypothesis as invalid and advocating for its substitution. There is a debate about whether amyloid-beta is a leading player or just one part of the disease process [62,63]. However, studies on how brain cells react to Aβ, and genetic links show a strong connection between Aβ and Alzheimer’s. Rather than rejecting Aβ or any other proposed explanation, creating a new AD model that combines different ideas into one comprehensive explanation is essential. This inclusive approach is vital to finding a cure for Alzheimer’s. As a result, researchers use a range of models to explain the complex processes of AD and develop novel treatments. This review aims to address gaps in AD modelling and demonstrates the potential for developing precise and effective drug treatments.
2. Biomolecular Components Relevant to AD
The key player in AD pathogenesis derives from the intricate interaction between sirtuins and the mechanistic target of rapamycin (mTOR) [64]. A vital regulator of cellular processes, mTOR plays a crucial role in the production of Aβ peptides, the stimulation of tau hyperphosphorylation, and protein synthesis [65,66] (Figure 1). Misregulation is worsened by mTOR-triggered neuroinflammation [67]. SIRT1 and other sirtuins are involved in cellular stress responses and can impact the removal of Aβ by activating autophagy and deacetylating tau [68,69]. There is complexity in the relationship between mTOR and sirtuins since SIRT1 suppresses mTOR signalling, which influences the delicate balance between cell survival and death [70]. Furthermore, mTOR and sirtuins are essential for regulating mitochondrial activity and energy metabolism, both of which are critical to the complex nature of AD pathogenesis [71,72]. Understanding these interconnected pathways offers valuable information on potential therapeutic approaches to address Aβ deposition, tau pathology, and neurodegeneration in AD.
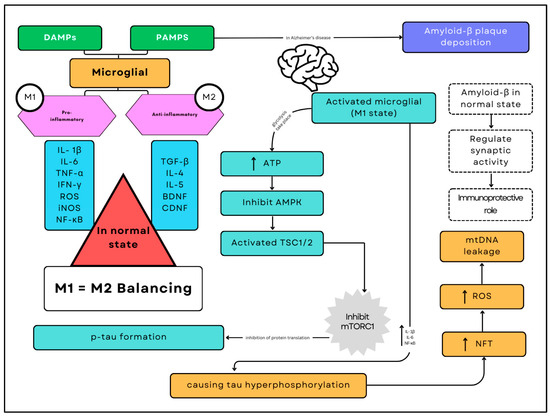
Figure 1.
Alzheimer’s disease pathogenesis. The pathogenesis of Alzheimer’s disease involves a complex interplay of immune and metabolic processes mediated by microglia, the brain’s resident immune cells. The deposition of amyloid-beta (Aβ) plaques serves as a critical trigger in this cascade. As Aβ plaques accumulate, they activate microglia through Damage-Associated Molecular Patterns (DAMPs) and Pathogen-Associated Molecular Patterns (PAMPs), shifting them predominantly into the pro-inflammatory M1 state. This activation results in increased glycolysis and higher ATP consumption, which inhibits AMP-activated protein kinase (AMPK) and activates the Tuberous Sclerosis Complex proteins (TSC1/2), ultimately leading to the inhibition of the mechanistic Target of Rapamycin Complex 1 (mTORC1).
Moreover, Aβ peptides, particularly Aβ42 and Aβ40, also play a critical role in the development of AD. Amyloid precursor protein (APP) is the source of these peptides, which are produced by the coordinated action of β- and γ-secretase [73,74]. They have a tendency to assemble and form insoluble plaque that is intricately present in the tapestry of AD-affected brains. Similar to recognisable landmarks, these plaques guide the path towards the disease’s diagnosis. Aβ peptides cause a series of disruptions to neuronal function in addition to their architectural presence [75,76]. Aβ oligomers, the intermediate characters in this unfolding drama, disrupt the delicate balance of synaptic function. This disruption impairs the release of neurotransmitters and the long-term stimulation process, which is essential for memory formation [77,78].
Furthermore, neuroinflammation contributes significantly to the complex interplay between the central nervous system and the immune system in the pathophysiology of AD [79,80]. In the immunological response of the brain, microglia and astrocytes are essential for coordinating inflammatory responses that are directly related to the development of AD [81]. Activated microglia serve as protectors of immune surveillance and break down abnormal protein aggregates like Aβ plaques through phagocytosis [82,83,84]. However, unexpected effects might occur when there is an imbalance in the intricate interactions between pro-inflammatory cytokines, chemokines, and reactive oxygen species. Chronic pro-inflammatory conditions have an adverse effect on neuronal structure where microglial persistence transitions to a more aggressive state, escalating neuroinflammation and impeding the progression of the disease [85,86].
Consequently, astrocytes, which were generally considered to have supportive roles, have been shown to have a dual nature in neuroinflammation by participating in the complex coordination of neurological processes. During chronic inflammation, these astrocytes transform into reactive states that release inflammatory mediators while withholding the crucial support necessary for neuronal survival [87,88]. This astrocytic dysfunction contributes to AD progression by breaking down the blood-brain barrier (BBB). The infiltration of peripheral immune cells significantly increases the inflammatory response when the BBB malfunctions [89,90,91]. This relentless neuroinflammatory environment plays a significant role as a main character in the progression of AD, intricately woven into its initiation and progression. This dynamic process further challenges the resilience of neurons, testing their ability to withstand the evolving complexity that characterises the landscape of AD.
Moreover, tau proteins change the AD environment since they are essential for maintaining the integrity of microtubules in the nervous system [92]. The transformed tau proteins undergo abnormal phosphorylation, leading to the development of neurofibrillary tangles (NFTs). Tau, typically a stabilising force for microtubules, loses its grip due to genetic predispositions and environmental influences, thus forming insoluble NFTs [93]. NFTs cast a shadow and have a negative impact on the neuronal landscape by disrupting microtubules and hindering intracellular transport [94]. The essential movement of cellular components along neuronal processes falters, affecting the distribution of nutrients and signalling molecules. Synaptic function, an integral chapter in the story of cognitive decline, faces adversity [95,96]. Tau aggregates establish a cascade reaction that triggers off pathways leading to oxidative stress, inflammation, and cell death [97]. The unfolding of tau proteins across interconnected brain regions represents a tragic propagation of neurodegeneration, contributing to the gradual and widespread demise of neurons in the Alzheimer’s narrative [98].
Furthermore, mitochondria have emerged as a key player in the pathophysiology of AD [99]. Their primary function, elucidated as the cellular powerhouse, undergoes a transformation, contributing substantively to the slow death of nerve cells [99]. Mitochondrial dysfunction emerges as a subtle subplot, triggering a decline in adenosine triphosphate (ATP) generation-a vital energy source for neurons [100]. This intricate interplay within the cellular milieu underscores the significance of mitochondrial dysfunction as a pivotal aspect in understanding AD progression. Moreover, in mitochondrial dysfunction, oxidative stress emerges as a pivotal antagonist, playing a role in the cascading events of AD development [100]. Impaired electron transport elevates reactive oxygen species (ROS), unleashing oxidative harm upon the neuronal landscape. The interaction between mitochondrial dysfunction and oxidative stress becomes a self-perpetuating cycle that hastens the deterioration of neuronal function [101]. Once heralded as protectors, the mitochondria become unwitting contributors to the unfolding tragedy of AD.
Alzheimer’s narrative is characterised by an early chapter on synaptic dysfunction, which contributes significantly to the cognitive decline witnessed by individuals with the condition [102]. Synapses, the communication points between neurons, face an early demise, reflecting the progression of cognitive decline [96]. Neurotransmitter release encounters interference and synaptic plasticity, a dynamic element in the cognitive storyline. Aβ aggregates, tau pathology, and the inflammatory subplot collaboratively disrupt the normal process of synapses [96]. Aβ oligomers, the elusive characters in this synaptic drama, interfere with synaptic transmission, binding to receptors and disrupting signalling pathways [77]. Tau pathology becomes a formidable force, disrupting microtubules and hindering the transport of synaptic vesicles.
When synaptic dysfunction occurs in AD, the BBB acts as a protector, controlling the flow of substances between the blood and the brain, thereby forming an environment for the unfolding narrative [103]. The breakdown of the BBB becomes a pivotal plot point, allowing the infiltration of detrimental substances into the brain’s sacred territory-from inflammatory molecules to toxins. The compromised barrier integrity escalates the neuroinflammatory environment within the central nervous system [104]. Immune cells from the periphery become unwitting characters in this unfolding drama, intensifying the inflammatory reaction [104]. The understanding of BBB alterations offers a glimpse into the early chapters of AD, unravelling potential therapeutic targets and opening new avenues for intervention in the ongoing narrative of AD.
A balance between M1 (pro-inflammatory) and M2 (anti-inflammatory) microglial states is essential for maintaining normal brain function in a healthy brain. However, in Alzheimer’s disease, the predominance of the M1 state disrupts this balance. The sustained activation of M1 microglia promotes hyperphosphorylation of tau protein, forming neurofibrillary tangles (NFTs) and increasing the release of pro-inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and nuclear factor kappa B (NF-κB). This inflammatory environment exacerbates neuronal damage, generating reactive oxygen species (ROS) and causing leakage of mitochondrial DNA (mtDNA). These pathological processes accelerate neurodegeneration and cognitive decline, characterising the progression of Alzheimer’s disease.
3. Existing Animal Models for AD
Many models have appeared in this context, each providing distinct perspectives on AD pathogenesis. The models vary from cellular and molecular systems to animal models, each created to mimic particular aspects of AD pathology and aid in investigating underlying mechanisms. The complex interplay of genetic, molecular, and environmental factors that impact AD onset and course may be better understood by researchers using these models. Existing models for AD encompass a wide range of approaches, from transgenic mouse models to computational and data-driven models. Understanding β-amyloid toxicity and the involvement of various β-amyloid species has been a key focus of studies on AD pathophysiology utilising transgenic mouse models including 5xFAD, APP3, PDAPP, Tg2576, P301S, and 3xTg-AD rodent models [105,106,107]. These models have provided a significant understanding of the pathogenic pathways of AD, particularly related to β-amyloid oligomers.
3.1. PDAPP
The first AD model that displayed the deposit of Aβ was the PDAPP mouse model. The Indiana mutation (V717F) in the human amyloid precursor protein (APP) gene is expressed in PDAPP mice [108]. This gene is regulated by the platelet-derived growth factor (PDGF)-β promoter [109]. Age-related increases in gliosis correspond to the formation of Aβ in the brain’s cortex, which starts to occur between the ages of six and nine months old. Moreover, at eight months of age, the hippocampus dentate gyrus molecular layer’s synaptic and dendritic densities start to decline. Cognitive deficits, particularly memory impairments, become evident in 3 months and worsen with age.
3.2. 5xFAD
Since the 5xFAD transgenic mouse model of AD consists of five mutations linked to familial AD, it is often used for studying the late stages of AD diseases [110]. They involve two mutations in the presenilin-1 (PS1) gene (L386V and M14L) and three in the APP gene (Swedish K670N/M671L, Florida I716V, and London V717I). According to Oakley et al. [111], developing APP/PS1 double transgenic mice that co-express 5xFAD causes an increase in Aβ42 synthesis and deposition, which causes amyloid plaques to rapidly and significantly accumulate in the brain. Another study found that β-amyloid plaque was also accumulated in the spinal cord of a 5xFAD mouse model in both the grey and white matter [112]. Girard et al. [113] found that 5xFAD mice have the highest corticolimbic Aβ compared to another model. Cognitive deficiencies in AD, which are characterised by the presence of gliosis and amyloid plaque, were linked to the presence of Aβ in 5xFAD mice, particularly in the frontal cortex of the brain. This frontal cortex has a crucial role in learning abilities, which could be an early indicator of AD-related cognitive decline [113]. Poon et al. [114] explore the relationship between cognitive impairment, neuronal loss, and amyloid deposition in the 5xFAD transgenic mouse. The hippocampus and entorhinal regions of female 5xFAD mice show significantly higher levels of amyloid deposition than those of male mice, highlighting the sex-related differences in AD. Keszycki, Rodriguez, Dunn, Locci, Orellana, Haupfear, Dominguez, Fisher, and Dong [105] indicated that 5xFAD mice exhibit apathy-like behaviours that worsen as they age. Amyloid plaques and soluble Aβ42 levels in the hippocampus and prefrontal cortex were linked to this apathy-like behaviour.
Additionally, the rise in β-site APP-cleaving enzyme 1 (BACE1) was associated with the overproduction of Aβ in AD [115]. The BACE1 causes the accumulation of β-cleaved C-terminal fragment C99 and full-length amyloid precursor protein (fl-APP) in the mitochondria, leading to mitochondrial dysfunction and cognitive impairment [116]. The pathology of neurodegeneration in AD is also related to alterations in astrocyte and oligodendrocyte gap junction expression in the 5xFAD mouse spinal cord [117]. Meanwhile, Hüttenrauch displayed that neprilysin (NEP) deficiency in the 5xFAD mouse modifies AD’s neuropathological and behavioural phenotype. NEP is recognised as an enzyme that breaks down Aβ in AD progression. The decrease in NEP level in 5xFAD contributed to increased Aβ accumulation. The effect of short-term and long-term exposure to volatile anaesthetics on neuropathological and behavioural systems was also observed in 5xFAD mice [118]. Prolonged exposure to a volatile anaesthetic significantly enhanced Aβ deposition in the hippocampal CA1 and CA2 regions and activated glial cells in the amygdala.
Meanwhile, Giesers and Wirths [119] demonstrated that, in 5xFAD mice, a notable reduction in calretinin-positive interneurons was observed in the hippocampal CA1 and CA2/3, while parvalbumin-positive interneurons were reduced throughout the hippocampal, particularly in the dentate gyrus. These interneurons may be lost in the 5xFAD mice model due to the presence of extracellular amyloid plaques. The administration of orphan nuclear receptor Nurr1 (NR4A2) in a 5xFAD mouse model regulates the AD pathophysiology by reducing Aβ plaque deposition, neuronal loss, microgliosis, and impairment of adult hippocampus neurogenesis [120].
3.3. APP23
The APP23 mouse model encodes the APP with the Swedish mutation (KM670/671NL), which contributes to the increased production of Aβ peptides [121]. Spatial memory deficit in the AD APP23 transgenic mouse model occurs before the formation of plaques and the subsequent increase in plaque-associated amyloid-β1-42 peptides [122]. The Aβ deposition plaques were observed in the cerebral cortex, thalamus, olfactory nucleus, hippocampus, and putamen of the APP23 mouse model [123]. In addition, there was selective neuronal death in the brain regions of APP23 mice [124]. Lefterov et al. [125] showed that ABCa1 is necessary for memory impairment in old APP23 mice, which are probably affected by the quantity of accumulated Aβ oligomers in the hippocampus. Along with the formation of amyloid plaque, the APP23 transgenic mouse model also showed loss of pyramidal neurons in the hippocampus CA1 area, with the activated microglia linked to amyloid plaques and hyperphosphorylation of tau protein [121].
Boncristiano et al. [126] displayed that there was a cholinergic modification in AD. The cholinergic deficit in the cortex of the APP23 mice is induced by amyloid accumulation and impairment of cholinergic basal forebrain neurons. However, AD is not facilitated by disturbance of the basal cholinergic forebrain system. Amyloid plaque formation and amyloid-associated microglial activation in the brain of APP23 transgenic mice are stimulated by neuron-derived beta precursor protein (βPP) [127]. Aβ causes some commissural neuron types to gradually degenerate, primarily affecting the most complicated dendritic structure. This degeneration begins with Aβ deposits appearing and worsens as Aβ deposits spread to other brain regions [128]. In another study, Yue et al. [129] demonstrated that oestrogen -deficient APP23 mice exhibited increased Aβ deposition and impaired Aβ degradation by microglia, indicating that oestrogen reduction in the brain may significantly influence the progression of AD neuropathology. However, Aβ deposits were decreased in the absence of enzyme tissue transglutaminase (TG2) [106]. This TG2 was found abundantly in the human brain. It is crucial for post-translation modification in Aβ, producing covalently linked, stable, and toxic Aβ aggregates.
3.4. Tg2576
A transgene with the Swedish mutation (KM670/671NL) in the APP gene is present in Tg2576 mice. In Tg2576 transgenic mice, the increased Aβ may be associated with synaptic dysfunction even without synapse loss [130]. Meanwhile, Kalback et al. [131] demonstrated that the increase in soluble Aβ levels with age is accompanied by relatively minimal vascular amyloid deposition. King and Arendash [132] evaluated the role of synaptophysin immunoreactivity (SYN-IR) in the hippocampus and neocortex regions of Tg2576 transgenic mice. This SYN-IR is known as a marker for synaptic terminals. King and Arendash [132] displayed that maintained SYN-IR during ageing is related to impaired synaptic function, contributing to cognitive deficits in AD. Another study, which utilised Tg2576 transgenic mice to explore β-amyloid-mediated inflammation in AD, discovered that the neocortex and hippocampus of Tg2576 mice models had diffuse and senile β-amyloid plaques [133]. The early β-amyloid exposure induces both pro- and anti-inflammatory mechanisms by upregulating TGF-β, IL-1β, and IL-10 in adjacent reactive astrocytes. Senile plaques disrupt cortical cytoarchitecture, leading to a gradual loss of antioxidant capacity [134].
3.5. PS01S
The PS01S mouse model integrates APP and presenilin-1 (PS-1) gene mutation related to familial AD. Tau aggregation initially occurs in the PS01S transgenic mouse model’s cerebral cortex and hippocampus [135], followed by the appearance of neurofibrillary tangles (NFTs) and hyperphosphorylated tau in the hippocampus and frontal cortex [136]. This tau formation increases with age. In another study, they found that the accumulation of NFTs occurs progressively in the amygdala, hippocampus, brainstem, neocortex, and spinal cord [137]. However, NFTs progressed synapse loss, and decreased synaptic function emerged, with microglial activation preceding the formation of tau tangles. Pre-tangles, characterised by argyrophilic tangle-like inclusions, are displayed in the cortex and hippocampal regions, even in the absence of amyloid pathology [138].
3.6. 3xTg-AD
The 3xTg-AD mice model may be beneficial to investigate the emphasis on late-onset AD 3xTg-AD [107]. This mouse model consists of three mutations: APP, PS1, and Tau. Aβ deposition in the 3xTg-AD mouse model begins in the cerebral cortex and subsequently extends to the hippocampus [139]. The administration of RGFP-966, a selective inhibitor of HDAC3, reduces the formation of Aβ1-42 and decreases both tau acetylation and phosphorylation [140]. However, at an intermediate phase of AD, Orta-Salazar et al. [141] showed that the primary motor cortex (M1) region of the 3xTg-AD female mice model had a hyperphosphorylated tau protein and accumulation of Aβ. The development of AD is connected to motor function, which is impacted by the damage of the M1 cell. Changes in synaptic excitability, particularly in hippocampal, can be observed in the 3xTg-AD mice model [142]. These mice models also exhibit episodic memory deficits at 3-6 months of age, which might be caused by the development of an aberrant hyper-excitable state condition in the hippocampal formation rather than a loss of synaptic connectivity. Compared to the female mouse model, male mice exhibit reduced plaque production and no plaques outside the subiculum [107]. In addition, Aβ40 and Aβ42 were additionally elevated in the soluble fractions of the cortical and hippocampal regions of female 3xTg-AD mice, possibly due to enhanced Thy-1 mini-gene. The summary of the mice experimental models of AD is shown in Table 1.
Although existing models have advanced our understanding of human neurodegenerative disease, biomimetic models based on recent technological innovations could further enhance the characterisation of pathological mechanisms [143]. This also makes them more suitable for high-throughput drug screening [144]. Looking ahead, we hope that technological advancements will bring the prospect of personalised medicine closer to reality, even though it is not yet economically feasible.

Table 1.
Experimental animal models of Alzheimer’s disease.
Table 1.
Experimental animal models of Alzheimer’s disease.
| Transgenic Mouse Model | Neuropathologies |
|---|---|
| PDAPP |
|
| 5xFAD |
|
| APP23 |
|
| Tg2576 |
|
| P301S |
|
| 3xTg-AD |
|
4. Biomimetic Approaches in Neurodegenerative Disease Modelling
Developing advanced biomimetic brain environments has become crucial in biomedical research to find effective treatments for AD. Due to AD’s complexity, innovative models that accurately mimic the brain’s intricate microenvironment are needed. These models facilitate better drug screening and the development of targeted therapeutic approaches. Recent scientific efforts have focused on creating biomimetic models designed to replicate the precise complexities of the brain microenvironment. This marks a significant shift in disease modelling and drug screening methodologies.
Computational models, such as the AlzheimerNet model proposed by Shamrat et al. [149], leverage deep learning to classify AD stages based on functional brain alterations in magnetic resonance imaging. This model demonstrates the possibility of artificial intelligence in AD diagnosis and staging by evaluating the performance of models such as VGG16, InceptionV3, AlexNet, MobileNetV2, and ResNet50, with InceptionV3 achieving the most remarkable test accuracy of 96.31% [150,151,152]. Furthermore, data-driven models, such as those developed by Bayraktar et al. [153], utilise molecular communication and artificial neural network models to analyse AD from biomedical and socio-economic perspectives. These models offer innovative approaches to understanding AD progression and diagnosis. Additionally, Gnanadesigan et al. [154] introduced a novel approach that uses intelligent-based deep learning models and network topology measurement to find potential genes for AD. Their study demonstrated the effectiveness of the suggested network topology model for an artificial neural network (ANN) classifier compared to existing models [154].
One notable advancement in this realm is developing a rapid three-dimensional (3D) bioprinting method, which holds immense promise for constructing biomimetic tissue models. Initially applied in replicating glioblastoma microenvironments [155,156,157], this cutting-edge technique exhibits adaptability to the unique challenges posed by AD modelling [158,159]. This introduction sets the stage for exploring the significance of biomimetic brain environments, the latest strides in biomimetic model development, and the potential of 3D bioprinting as a transformative tool for advancing our understanding and treatment options for AD. A 3D-bioprinted vascularised glioblastoma-on-a-chip was suggested to investigate the effects of simulated microgravity, showing promise in developing biomimetic brain microenvironments for disease modelling [160,161]. The study investigated the potential of using camouflaging nanoparticles loaded with brain malignant cancer cell membranes to cross the BBB for imaging and treating brain tumours. This research suggests the possibility of creating targeted drug delivery systems for AD treatment [162,163,164,165].
Recent studies have stressed the significance of incorporating a biomimetic mechanical microenvironment into in vitro models to simulate the tumour microenvironment accurately, which could also be applied to AD modelling [166]. The significance of the microenvironment in AD was emphasised by Qiu et al. [167], who highlighted the molecular and clinical relevance of the brain microenvironment and glycolysis in AD, offering valuable information on potential therapeutic targets. A microfluidic platform is one tool for creating in vitro models that closely mimic the cellular microenvironment and physiological conditions of AD [144]. In AD research, microfluidic platforms are used to investigate various AD mechanisms, including Aβ transmission, Aβ neurotoxicity, Aβ aggregation and clearance, microglial activation, and Tau pathology.
Su et al. [168] proposed that the study on using a nanotheranostic system coated with erythrocyte membrane for targeted immune suppression during AD therapy presents a promising approach for influencing the brain microenvironment in treating AD. Apart from that, astrocytes from individuals with sporadic Alzheimer’s disease (SAD)-linked APOE4 mutations and familial Alzheimer’s disease (FAD)-linked PSEN1M146L mutations showed reduced morphological complexity and changes in the localisation of marker proteins. These findings suggest that FAD and SAD mutations have similar effects on astrocytes [169]. In AD, the multifunctional lipoprotein-biomimetic nanostructure RAP-RL has effectively regulated the cerebral vasculature and re-established the neurovascular unit [170]. RAP-RL consists of an antagonist peptide that binds to the receptor for advanced glycation end-products (RAGE), facilitating the clearance of perivascular Aβ and restoring structure and function within the neurovascular unit. In a study by Ye et al. [171], targeted AD treatment was improved through the synthesis of a macrophage membrane-encapsulated, nitrogen-doped carbon quantum dot (CDQs) nanosystem, which effectively captures excess Cu2+ and inhibits rapid Aβ aggregations. Another study utilised red blood cell membranes as templates for the in situ growth of cerium oxide nanocrystals, later encapsulating them with carbon quantum dots to form CDQ-Ce-RBC nanocomposites [172]. When combined with photothermal therapy, this nanocomposite effectively impacts multiple pathways in AD progression The advantage of this approach could be observed through antioxidant protection from CeO2 nanocrystals, the inhibition of Aβ aggregation, and the disruption of Aβ fibres.
Innate immune cells called microglia are found in the brain, carrying out diverse, supportive functions throughout the brain’s development, adulthood, and ageing processes [173]. These functions include synaptic pruning, cellular debris clearance, and neuroinflammation regulation. Microglia generated from healthy patient-derived cells exhibit capabilities such as synaptic pruning, phagocytosis, and Aβ uptake accompanied by the secretion of various cytokines [174]. Exposure to exogenous Aβ triggers alterations in gene expression within these microglia. Conversely, microglia generated from SAD patients created pluripotent stem cells (iPSCs) that exhibit variations in phagocytosis and the increased production of specific cytokines in reaction to lipopolysaccharide treatment [173,174,175]. In addition, biomimetic remodelling of microglial riboflavin metabolism was discovered to alleviate cognitive impairment in AD [176]. This approach involves the design of microglial nanoparticles encapsulating flavin mononucleotides (FMN) for efficient brain delivery [176]. One important enzyme in riboflavin metabolism, riboflavin kinase (RK), is inhibited by the FMN. This inhibition subsequently attenuates the pro-inflammatory TNFR1/NF-Kβ pathway, reducing neuroinflammation (Figure 2).
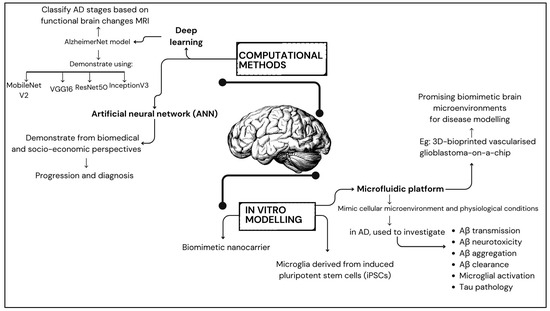
Figure 2.
Summary of disease modelling in developing biomimetic brain microenvironment.
Another strategy to enhance drug delivery and improve BBB penetration in AD treatments involves the use of biomimetic nanocarriers [177,178]. One example is lipid nanocomposites (APLN), designed to mimic the function and structure of high-density lipoprotein, essential for facilitating microglia-targeted delivery and BBB crossing [177]. These lipid nanocomposites are loaded into methylene blue (MB) to produce APLN/MB, simultaneously targeting both Aβ and Tau pathways. This dual action promotes Aβ clearance by microglia and inhibits Tau phosphorylation, potentially alleviating AD symptoms and improving cognitive functions. In another study, neutrophil membrane-coated metal-organic framework (MOF) nanoenzymes (Neu-MOF/Fla) were used to target inflammatory sites and deliver therapeutic agents [178]. Neu-MOF/Fla is biomimetically engineered to exploit the innate ability of neutrophils to cross the BBB and target inflammatory signals in AD. Solid lipid nanoparticles (SLNs) also show promise as a biomimetic strategy for control nervous system (CNS) drug delivery, enhancing BBB penetration and improving drug bioavailability [179].
5. Challenges and Limitations in AD Modelling
Since AD is a complex and complicated disease, modelling it in preclinical research is very challenging. While many current models primarily focus on Aβ and tau pathologies, hallmark features of AD, they often fail to fully capture the condition’s intricacies. One key limitation is the inability to replicate the complex relationship between genetic, environmental, and ageing factors leading to AD pathology [180]. Moreover, neuroinflammation, synaptic dysfunction, and microglial dysregulation, critical components of AD, are frequently overlooked or not accurately represented in existing models. Consequently, these models may not accurately represent the wide range of cellular and molecular alterations seen in AD. Achieving a more comprehensive and accurate representation of AD pathology in preclinical models is vital for advancing our understanding of the disease and creating effective treatment strategies.
Achieving translational relevance in AD modelling poses a formidable challenge for researchers [181]. While animal models provide an essential understanding of the fundamental causes of disease, it can be difficult to apply these discoveries in human clinical trials. The response of animal models to therapeutic interventions often differs from that of humans, creating significant translational barriers [182]. The human brain’s intricate molecular and cellular processes, which are key to disease progression, may be challenging to replicate in animal models accurately. This difficulty hampers the successful transition from promising preclinical results to effective therapeutic strategies for human patients. Therefore, bridging this gap in translational relevance is of utmost importance to ensure that advancements in preclinical AD research can translate into meaningful clinical outcomes [183,184].
The financial burden associated with AD modelling, encompassing the development and maintenance of animal models and conducting extensive preclinical trials, poses a significant challenge. The costs involved in long-term studies, especially those aimed at capturing the gradual progression of AD, can be prohibitive [185,186]. Additionally, the high attrition rates of potential therapies during clinical trials further escalate costs. Despite these challenges, balancing the need for comprehensive and accurate modelling with the economic constraints of research resources remains critical in advancing our understanding and treatment of AD [187].
Scalability is a concern in AD modelling, particularly as research endeavours aim to accommodate the increasing demand for more advanced and high-throughput screening approaches [188,189,190]. Traditional models may need help scaling up efficiently to meet the growing requirements of large-scale studies and drug screening initiatives. Therefore, developing robust and scalable models that include diverse genetic backgrounds and environmental factors is essential to enhancing the efficiency and productivity of AD research. Overcoming scalability challenges is pivotal for the field to accommodate the expanding breadth of investigations needed for a comprehensive understanding of AD pathogenesis and treatment development.
6. Conclusions
AD presents a significant global health challenge, requiring innovative approaches for advancing models and treatments. This comprehensive review highlights the systematic enhancement of biomimetic brain environments to address limitations in current AD models and enhance the effectiveness of drug screening. Integrating biomolecular components such as Aβ plaques and tau proteins would improve the dependability in representing disease mechanisms. Expected outcomes include establishing a robust platform for high-throughput screening with enhanced scalability and translational relevance, potentially accelerating drug discovery. This study addresses gaps in AD modelling and demonstrates the potential for refining precise and effective drug treatments.
Author Contributions
Conceptualization, N.M.M.; data curation, N.M.M.; writing—original draft preparation, N.M.M.; writing—review and editing, S.M. and N.F.N.M.S.; visualisation, N.M.M.; supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Malaysia Ministry of Higher Education Fundamental Research Grant Scheme, FRGS/1/2024/SKK10/UKM/01/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ministry of Finance Malaysia. Economic Performance and Outlook. Available online: https://belanjawan.mof.gov.my/pdf/belanjawan2023/economy-fiscal/section1.pdf (accessed on 25 May 2024).
- Lobanov-Rostovsky, S.; He, Q.; Chen, Y.; Liu, Y.; Wu, Y.; Liu, Y.; Venkatraman, T.; French, E.; Curry, N.; Hemmings, N.; et al. Growing old in China in socioeconomic and epidemiological context: Systematic review of social care policy for older people. BMC Public Health 2023, 23, 1272. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, S.; Liu, Z.; Hu, L.; Lin, J.; Tan, S.; Mai, Y.; Peng, W.; Mai, H.; Hou, Q.; et al. The Prevalence of Alzheimer’s Disease in China: A Systematic Review and Meta-analysis. Iran. J. Public Health 2018, 47, 1615–1626. [Google Scholar] [PubMed]
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimers Dement. Available online: https://www.alz.org/alzheimers-dementia/facts-figures (accessed on 25 May 2024).
- Stefani, M.; Dobson, C.M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003, 81, 678–699. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, T.N.; Athar, T.; Parveen, R.; Fatima, S. A review on protein misfolding, aggregation and strategies to prevent related ailments. Int. J. Biol. Macromol. 2017, 105, 993–1000. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Rahman, M.A.; Behl, T.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Emerging proof of protein misfolding and interactions in multifactorial Alzheimer’s disease. Curr. Top. Med. Chem. 2020, 20, 2380–2390. [Google Scholar] [CrossRef]
- Louros, N.; Schymkowitz, J.; Rousseau, F. Mechanisms and pathology of protein misfolding and aggregation. Nat. Rev. Mol. Cell Biol. 2023, 24, 912–933. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Sajjad, R.; Arif, R.; Shah, A.; Manzoor, I.; Mustafa, G. Pathogenesis of Alzheimer’s disease: Role of amyloid-beta and hyperphosphorylated tau protein. Indian J. Pharm. Sci. 2018, 80, 581–591. [Google Scholar] [CrossRef]
- Gallardo, G.; Holtzman, D.M. Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease. In Tau Biology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 187–203. [Google Scholar]
- Volicer, L. Physiological and pathological functions of beta-amyloid in the brain and Alzheimer’s disease: A review. J. Physiol. Investig. 2020, 63, 95–100. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol. Scand. 1996, 94, 3–12. [Google Scholar] [CrossRef]
- Van Zeller, M.; Dias, D.; Sebastiao, A.M.; Valente, C.A. NLRP3 inflammasome: A starring role in amyloid-β-and tau-driven pathological events in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 83, 939–961. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Y.; Rajput, A.; Maleysm, S.; Pareek, A.; Jain, V.; Pareek, A.; Kaur, R.; Singh, G. An Insight into Cellular and Molecular Mechanisms Underlying the Pathogenesis of Neurodegeneration in Alzheimer’s Disease. Biomedicines 2023, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Preeti, K.; Sood, A.; Fernandes, V. Metabolic regulation of glia and their neuroinflammatory role in Alzheimer’s disease. Cell. Mol. Neurobiol. 2022, 42, 2527–2551. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.W. Pathogenesis of Dementia. Int. J. Mol. Sci. 2022, 24, 543. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hölscher, C. Common pathological processes in Alzheimer disease and type 2 diabetes: A review. Brain Res. Rev. 2007, 56, 384–402. [Google Scholar] [CrossRef]
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clin. Interv. Aging 2015, 10, 549–560. [Google Scholar] [CrossRef]
- Jayaraman, A.; Pike, C.J. Alzheimer’s disease and type 2 diabetes: Multiple mechanisms contribute to interactions. Curr. Diabetes Rep. 2014, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mudher, A. Alzheimer’s disease and type 2 diabetes: A critical assessment of the shared pathological traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Type 2 diabetes mellitus and Alzheimer’s disease. World J. Diabetes 2014, 5, 889. [Google Scholar] [CrossRef]
- Vijayan, M.; Reddy, P.H. Stroke, vascular dementia, and Alzheimer’s disease: Molecular links. J. Alzheimer’s Dis. 2016, 54, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.-X.; Stern, Y.; Shea, S.; Mayeux, R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 2001, 154, 635–641. [Google Scholar] [CrossRef]
- Honig, L.S.; Tang, M.-X.; Albert, S.; Costa, R.; Luchsinger, J.; Manly, J.; Stern, Y.; Mayeux, R. Stroke and the risk of Alzheimer disease. Arch. Neurol. 2003, 60, 1707–1712. [Google Scholar] [CrossRef]
- Zambón, D.; Quintana, M.; Mata, P.; Alonso, R.; Benavent, J.; Cruz-Sánchez, F.; Gich, J.; Pocoví, M.; Civeira, F.; Capurro, S. Higher incidence of mild cognitive impairment in familial hypercholesterolemia. Am. J. Med. 2010, 123, 267–274. [Google Scholar] [CrossRef]
- Wu, M.; Zhai, Y.; Liang, X.; Chen, W.; Lin, R.; Ma, L.; Huang, Y.; Zhao, D.; Liang, Y.; Zhao, W. Connecting the dots between Hypercholesterolemia and Alzheimer’s disease: A potential mechanism based on 27-hydroxycholesterol. Front. Neurosci. 2022, 16, 842814. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.-X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010, 67, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.; Bryant-Thomas, T.; Herbert, D.; Pacheco, J.; Fabra Garcia, M.; Manjon, M.; Girones, X.; Henry, T.; Matsubara, E.; Zambon, D. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 2003, 61, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Fitzpatrick, A.L.; Lopez, O.; Jackson, S.; Lyketsos, C.; Jagust, W.; Ives, D.; DeKosky, S.T.; Kuller, L.H. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J. Am. Geriatr. Soc. 2005, 53, 1101–1107. [Google Scholar] [CrossRef]
- Cermakova, P.; Eriksdotter, M.; Lund, L.; Winblad, B.; Religa, P.; Religa, D. Heart failure and Alzheimer′s disease. J. Intern. Med. 2015, 277, 406–425. [Google Scholar] [CrossRef]
- Qiu, C.; Winblad, B.; Marengoni, A.; Klarin, I.; Fastbom, J.; Fratiglioni, L. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch. Intern. Med. 2006, 166, 1003–1008. [Google Scholar] [CrossRef]
- Sun, W.; Zhuo, S.; Wu, H.; Cai, X. Association between Coronary Heart Disease, Heart Failure, and Risk of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Ann. Indian Acad. Neurol. 2023, 26, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.C.; Sturm, V.E.; Peterson, M.J.; Pieper, C.F.; Bullock, T.; Boeve, B.F.; Miller, B.L.; Guskiewicz, K.M.; Berger, M.S.; Kramer, J.H. Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. J. Neurosurg. 2016, 124, 511–526. [Google Scholar] [CrossRef]
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I.; Loane, D.J. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics 2015, 12, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Havlik, R.; Steffens, D.; Helms, M.; Newman, T.; Drosdick, D.; Phillips, C.; Gau, B.; Welsh–Bohmer, K.; Burke, J. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000, 55, 1158–1166. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.-L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef]
- Keller, L.; Xu, W.; Wang, H.-X.; Winblad, B.; Fratiglioni, L.; Graff, C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: A prospective cohort study. J. Alzheimer’s Dis. 2011, 23, 461–469. [Google Scholar] [CrossRef]
- Flores-Cordero, J.A.; Pérez-Pérez, A.; Jiménez-Cortegana, C.; Alba, G.; Flores-Barragán, A.; Sánchez-Margalet, V. Obesity as a risk factor for dementia and Alzheimer’s disease: The role of leptin. Int. J. Mol. Sci. 2022, 23, 5202. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Quindós-Rubial, M.; Fiuza-Luces, C.; Cristi-Montero, C.; Emanuele, E.; Garatachea, N.; Lucia, A. Physical activity and Alzheimer disease: A protective association. Mayo Clin. Proc. 2016, 91, 999–1020. [Google Scholar] [CrossRef]
- Scarmeas, N.; Luchsinger, J.A.; Brickman, A.M.; Cosentino, S.; Schupf, N.; Xin-Tang, M.; Gu, Y.; Stern, Y. Physical activity and Alzheimer disease course. Am. J. Geriatr. Psychiatry 2011, 19, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, F.R.; D’Anca, M.; Tartaglia, G.M.; Del Fabbro, M.; Scarpini, E.; Galimberti, D. Treatment of Alzheimer’s Disease: Beyond Symptomatic Therapies. Int. J. Mol. Sci. 2023, 24, 13900. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Elmaraezy, A.; Aglan, A.; Salama, R.; Fouda, S.; Fouda, R.; AlSafadi, A.M. Bapineuzumab for mild to moderate Alzheimer’s disease: A meta-analysis of randomized controlled trials. BMC Neurol. 2017, 17, 66. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Sperling, R.A.; Donohue, M.C.; Raman, R.; Rafii, M.S.; Johnson, K.; Masters, C.L.; van Dyck, C.H.; Iwatsubo, T.; Marshall, G.A.; Yaari, R. Trial of solanezumab in preclinical Alzheimer’s disease. N. Engl. J. Med. 2023, 389, 1096–1107. [Google Scholar] [CrossRef]
- Cummings, J.L.; Cohen, S.; van Dyck, C.H.; Brody, M.; Curtis, C.; Cho, W.; Ward, M.; Friesenhahn, M.; Rabe, C.; Brunstein, F. ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 2018, 90, e1889–e1897. [Google Scholar] [CrossRef]
- Landen, J.W.; Zhao, Q.; Cohen, S.; Borrie, M.; Woodward, M.; Billing Jr, C.B.; Bales, K.; Alvey, C.; McCush, F.; Yang, J. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: A phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin. Neuropharmacol. 2013, 36, 14–23. [Google Scholar] [CrossRef]
- Landen, J.W.; Andreasen, N.; Cronenberger, C.L.; Schwartz, P.F.; Börjesson-Hanson, A.; Östlund, H.; Sattler, C.A.; Binneman, B.; Bednar, M.M. Ponezumab in mild-to-moderate Alzheimer’s disease: Randomized phase II PET-PIB study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 393–401. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Heidebrink, J.L.; Paulson, H.L. Lessons Learned from Approval of Aducanumab for Alzheimer’s Disease. Annu. Rev. Med. 2024, 75, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-K.; Kuan, Y.-C.; Lin, H.-W.; Hu, C.-J. Clinical trials of new drugs for Alzheimer disease: A 2020–2023 update. J. Biomed. Sci. 2023, 30, 83. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer’s disease: A focus on aducanumab and lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Villain, N.; Planche, V.; Levy, R. High-clearance anti-amyloid immunotherapies in Alzheimer’s disease. Part 1: Meta-analysis and review of efficacy and safety data, and medico-economical aspects. Rev. Neurol. 2022, 178, 1011–1030. [Google Scholar] [CrossRef]
- Weaver, D.F. Alzheimer’s disease as an innate autoimmune disease (AD2): A new molecular paradigm. Alzheimer’s Dement. 2023, 19, 1086–1098. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Chakrabarti, M.; Govindaraju, T. Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease. Chem. Commun. 2015, 51, 13434–13450. [Google Scholar] [CrossRef]
- Di Carlo, M. Beta amyloid peptide: From different aggregation forms to the activation of different biochemical pathways. Eur. Biophys. J. 2010, 39, 877–888. [Google Scholar] [CrossRef]
- Abdullah, A.; Mohd Murshid, N.; Makpol, S. Antioxidant modulation of mTOR and sirtuin pathways in age-related neurodegenerative diseases. Mol. Neurobiol. 2020, 57, 5193–5207. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Oliver, D.M. Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer’s disease. Cells 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Mamun, A.A.; Labu, Z.K.; Hidalgo-Lanussa, O.; Barreto, G.E.; Ashraf, G.M. Autophagic dysfunction in Alzheimer’s disease: Cellular and molecular mechanistic approaches to halt Alzheimer’s pathogenesis. J. Cell. Physiol. 2019, 234, 8094–8112. [Google Scholar] [CrossRef] [PubMed]
- Padilha, C.S.; Kushkestani, M.; Baptista, L.P.; Krüger, K.; Lira, F.S. Autophagy of naïve CD4+ T cells in aging–the role of body adiposity and physical fitness. Expert Rev. Mol. Med. 2023, 25, e9. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Gong, Z. The beneficial roles of SIRT1 in neuroinflammation-related diseases. Oxidative Med. Cell. Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef]
- Donmez, G.; Outeiro, T.F. SIRT1 and SIRT2: Emerging targets in neurodegeneration. EMBO Mol. Med. 2013, 5, 344–352. [Google Scholar] [CrossRef]
- Cetrullo, S.; D’Adamo, S.; Tantini, B.; Borzi, R.M.; Flamigni, F. mTOR, AMPK, and Sirt1: Key players in metabolic stress management. Crit. Rev.™ Eukaryot. Gene Expr. 2015, 25, 59–75. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.-H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Sadria, M.; Layton, A.T. Interactions among mTORC, AMPK and SIRT: A computational model for cell energy balance and metabolism. Cell Commun. Signal. 2021, 19, 57. [Google Scholar] [CrossRef]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Ronisz, A.; Tousseyn, T.; Rijal Upadhaya, A.; Balakrishnan, K.; Vandenberghe, R.; Vandenbulcke, M.; von Arnim, C.A.F.; Otto, M.; Beach, T.G.; et al. Different aspects of Alzheimer’s disease-related amyloid β-peptide pathology and their relationship to amyloid positron emission tomography imaging and dementia. Acta Neuropathol. Commun. 2019, 7, 178. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Gyngard, F.; Guillermier, C.; Patterson, B.W.; Elbert, D.; Mawuenyega, K.G.; Schneider, T.; Green, K.; Roth, R.; Schmidt, R.E.; et al. Amyloid-β Plaques in Clinical Alzheimer’s Disease Brain Incorporate Stable Isotope Tracer In Vivo and Exhibit Nanoscale Heterogeneity. Front. Neurol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, X.; Ma, L.; Wei, W.; Li, Z.; Chang, S.; Wen, J.; Sun, J.; Li, H. Role of Aβ in Alzheimer’s-related synaptic dysfunction. Front. Cell Dev. Biol. 2022, 10, 964075. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.; Alifragis, P. Synaptic dysfunction in Alzheimer’s disease: The effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen. Res. 2018, 13, 616–623. [Google Scholar]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P., Jr.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Hu, H.; Zhao, M.; Sun, L. Microglia in Alzheimer’s Disease: A Target for Therapeutic Intervention. Front. Cell. Neurosci. 2021, 15, 749587. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of neuropathology-associated reactive astrocytes: A systematic review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Olude, M.A.; Mouihate, A.; Mustapha, O.A.; Farina, C.; Quintana, F.J.; Olopade, J.O. Astrocytes and Microglia in Stress-Induced Neuroinflammation: The African Perspective. Front. Immunol. 2022, 13, 795089. [Google Scholar] [CrossRef]
- Preininger, M.K.; Kaufer, D. Blood-Brain Barrier Dysfunction and Astrocyte Senescence as Reciprocal Drivers of Neuropathology in Aging. Int. J. Mol. Sci. 2022, 23, 6217. [Google Scholar] [CrossRef]
- Cruz, J.V.R.; Batista, C.; Diniz, L.P.; Mendes, F.A. The Role of Astrocytes and Blood–Brain Barrier Disruption in Alzheimer’s Disease. Neuroglia 2023, 4, 209–221. [Google Scholar] [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood–Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, 24, 17146. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; De Silva, R.; Di Giovanni, G.; et al. Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; ElIdrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of Tau Associates with Changes in Its Function Beyond Microtubule Stability. Front. Cell. Neurosci. 2018, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Meftah, S.; Gan, J. Alzheimer’s disease as a synaptopathy: Evidence for dysfunction of synapses during disease progression. Front. Synaptic Neurosci. 2023, 15, 1129036. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Grant, S.G.N. Synapse pathology in Alzheimer’s disease. Semin. Cell Dev. Biol. 2023, 139, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Subramanian, J.; Savage, J.C.; Tremblay, M. Synaptic Loss in Alzheimer’s Disease: Mechanistic Insights Provided by Two-Photon in vivo Imaging of Transgenic Mouse Models. Front. Cell Neurosci. 2020, 14, 592607. [Google Scholar] [CrossRef]
- Reiss, A.B.; Gulkarov, S.; Jacob, B.; Srivastava, A.; Pinkhasov, A.; Gomolin, I.H.; Stecker, M.M.; Wisniewski, T.; De Leon, J. Mitochondria in Alzheimer’s Disease Pathogenesis. Life 2024, 14, 196. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef]
- Jaroudi, W.; Garami, J.; Garrido, S.; Hornberger, M.; Keri, S.; Moustafa, A.A. Factors underlying cognitive decline in old age and Alzheimer’s disease: The role of the hippocampus. Rev. Neurosci. 2017, 28, 705–714. [Google Scholar] [CrossRef]
- Storck, S.E.; Hartz, A.M.S.; Pietrzik, C.U. The Blood-Brain Barrier in Alzheimer’s Disease. Handb. Exp. Pharmacol. 2022, 273, 247–266. [Google Scholar] [CrossRef]
- Skaper, S.D. Impact of Inflammation on the Blood-Neural Barrier and Blood-Nerve Interface: From Review to Therapeutic Preview. Int. Rev. Neurobiol. 2017, 137, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Keszycki, R.; Rodriguez, G.; Dunn, J.T.; Locci, A.; Orellana, H.; Haupfear, I.; Dominguez, S.; Fisher, D.W.; Dong, H. Characterization of apathy-like behaviors in the 5xFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2023, 126, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmus, M.M.M.; Chouchane, O.; Loos, M.; Jongenelen, C.A.M.; Brevé, J.J.P.; Jonker, A.; Bol, J.G.J.M.; Smit, A.B.; Drukarch, B. Absence of tissue transglutaminase reduces amyloid-beta pathology in APP23 mice. Neuropathol. Appl. Neurobiol. 2022, 48, e12796. [Google Scholar] [CrossRef]
- Javonillo, D.I.; Tran, K.M.; Phan, J.; Hingco, E.; Kramár, E.A.; da Cunha, C.; Forner, S.; Kawauchi, S.; Milinkeviciute, G.; Gomez-Arboledas, A.; et al. Systematic Phenotyping and Characterization of the 3xTg-AD Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2022, 15, 785276. [Google Scholar] [CrossRef]
- Games, D.; Adams, D.; Alessandrini, R.; Barbour, R.; Berthelette, P.; Blackwell, C.; Carr, T.; Clemens, J.; Donaldson, T.; Gillespie, F.; et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 1995, 373, 523–527. [Google Scholar] [CrossRef]
- Yokoyama, M.; Kobayashi, H.; Tatsumi, L.; Tomita, T. Mouse Models of Alzheimer’s Disease. Front. Mol. Neurosci. 2022, 15, 912995. [Google Scholar] [CrossRef] [PubMed]
- Oblak, A.L.; Lin, P.B.; Kotredes, K.P.; Pandey, R.S.; Garceau, D.; Williams, H.M.; Uyar, A.; O’Rourke, R.; O’Rourke, S.; Ingraham, C.; et al. Comprehensive Evaluation of the 5XFAD Mouse Model for Preclinical Testing Applications: A MODEL-AD Study. Front. Aging Neurosci. 2021, 13, 713726. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Chu, T.H.; Cummins, K.; Sparling, J.S.; Tsutsui, S.; Brideau, C.; Nilsson, K.P.R.; Joseph, J.T.; Stys, P.K. Axonal and myelinic pathology in 5xFAD Alzheimer’s mouse spinal cord. PLoS ONE 2017, 12, e0188218. [Google Scholar] [CrossRef]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S. Evidence for Early Cognitive Impairment Related to Frontal Cortex in the 5XFAD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Poon, C.H.; Wong, S.T.N.; Roy, J.; Wang, Y.; Chan, H.W.H.; Steinbusch, H.; Blokland, A.; Temel, Y.; Aquili, L.; Lim, L.W. Sex Differences between Neuronal Loss and the Early Onset of Amyloid Deposits and Behavioral Consequences in 5xFAD Transgenic Mouse as a Model for Alzheimer’s Disease. Cells 2023, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Cai, Y.; Xiong, K.; Cai, H.; Luo, X.G.; Feng, J.C.; Clough, R.W.; Struble, R.G.; Patrylo, P.R.; Yan, X.X. β-Secretase-1 elevation in transgenic mouse models of Alzheimer’s disease is associated with synaptic/axonal pathology and amyloidogenesis: Implications for neuritic plaque development. Eur. J. Neurosci. 2009, 30, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. Mitochondrial dysfunction and accumulation of the β-secretase-cleaved C-terminal fragment of APP in Alzheimer’s disease transgenic mice. Neurobiol. Dis. 2012, 45, 417–424. [Google Scholar] [CrossRef]
- Pechlivanidou, M.; Kousiappa, I.; Angeli, S.; Sargiannidou, I.; Koupparis, A.M.; Papacostas, S.S.; Kleopa, K.A. Glial Gap Junction Pathology in the Spinal Cord of the 5xFAD Mouse Model of Early-Onset Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 15597. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhao, J.; Zhao, G. Prolonged Volatile Anesthetic Exposure Exacerbates Cognitive Impairment and Neuropathology in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 84, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Giesers, N.K.; Wirths, O. Loss of Hippocampal Calretinin and Parvalbumin Interneurons in the 5XFAD Mouse Model of Alzheimer’s Disease. ASN Neuro 2020, 12, 1759091420925356. [Google Scholar] [CrossRef]
- Moon, M.; Jung, E.S.; Jeon, S.G.; Cha, M.Y.; Jang, Y.; Kim, W.; Lopes, C.; Mook-Jung, I.; Kim, K.S. Nurr1 (NR4A2) regulates Alzheimer’s disease-related pathogenesis and cognitive function in the 5XFAD mouse model. Aging Cell 2019, 18, e12866. [Google Scholar] [CrossRef]
- Kelly, P.; Bondolfi, L.; Hunziker, D.; Schlecht, H.-P.; Carver, K.; Maguire, E.; Abramowski, D.; Wiederhold, K.-H.; Sturchler-Pierrat, C.; Jucker, M. Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol. Aging 2003, 24, 365–378. [Google Scholar] [CrossRef]
- Van Dam, D.; d’Hooge, R.; Staufenbiel, M.; Van Ginneken, C.; Van Meir, F.; De Deyn, P.P. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur. J. Neurosci. 2003, 17, 388–396. [Google Scholar] [CrossRef]
- Sturchler-Pierrat, C.; Abramowski, D.; Duke, M.; Wiederhold, K.H.; Mistl, C.; Rothacher, S.; Ledermann, B.; Bürki, K.; Frey, P.; Paganetti, P.A.; et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 1997, 94, 13287–13292. [Google Scholar] [CrossRef]
- Calhoun, M.E.; Wiederhold, K.-H.; Abramowski, D.; Phinney, A.L.; Probst, A.; Sturchler-Pierrat, C.; Staufenbiel, M.; Sommer, B.; Jucker, M. Neuron loss in APP transgenic mice. Nature 1998, 395, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Lefterov, I.; Fitz, N.F.; Cronican, A.; Lefterov, P.; Staufenbiel, M.; Koldamova, R. Memory Deficits in APP23/Abca1+/− Mice Correlate with the Level of Aβ Oligomers. ASN Neuro 2009, 1, AN20090015. [Google Scholar] [CrossRef]
- Boncristiano, S.; Calhoun, M.E.; Kelly, P.H.; Pfeifer, M.; Bondolfi, L.; Stalder, M.; Phinney, A.L.; Abramowski, D.; Sturchler-Pierrat, C.; Enz, A. Cholinergic changes in the APP23 transgenic mouse model of cerebral amyloidosis. J. Neurosci. 2002, 22, 3234–3243. [Google Scholar] [CrossRef]
- Stalder, M.; Phinney, A.; Probst, A.; Sommer, B.; Staufenbiel, M.; Jucker, M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am. J. Pathol. 1999, 154, 1673–1684. [Google Scholar] [CrossRef]
- Capetillo-Zarate, E.; Staufenbiel, M.; Abramowski, D.; Haass, C.; Escher, A.; Stadelmann, C.; Yamaguchi, H.; Wiestler, O.D.; Thal, D.R. Selective vulnerability of different types of commissural neurons for amyloid β-protein-induced neurodegeneration in APP23 mice correlates with dendritic tree morphology. Brain 2006, 129, 2992–3005. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Lu, M.; Lancaster, T.; Cao, P.; Honda, S.-I.; Staufenbiel, M.; Harada, N.; Zhong, Z.; Shen, Y.; Li, R. Brain estrogen deficiency accelerates Aβ plaque formation in an Alzheimer’s disease animal model. Proc. Natl. Acad. Sci. USA 2005, 102, 19198–19203. [Google Scholar]
- Chapman, P.F.; White, G.L.; Jones, M.W.; Cooper-Blacketer, D.; Marshall, V.J.; Irizarry, M.; Younkin, L.; Good, M.A.; Bliss, T.V.; Hyman, B.T.; et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999, 2, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kalback, W.; Watson, M.D.; Kokjohn, T.A.; Kuo, Y.-M.; Weiss, N.; Luehrs, D.C.; Lopez, J.; Brune, D.; Sisodia, S.S.; Staufenbiel, M.; et al. APP Transgenic Mice Tg2576 Accumulate Aβ Peptides That Are Distinct from the Chemically Modified and Insoluble Peptides Deposited in Alzheimer’s Disease Senile Plaques. Biochemistry 2002, 41, 922–928. [Google Scholar] [CrossRef]
- King, D.L.; Arendash, G.W. Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging: Correlations with cognitive impairment. Brain Res. 2002, 926, 58–68. [Google Scholar] [CrossRef]
- Apelt, J.; Schliebs, R. β-Amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001, 894, 21–30. [Google Scholar] [CrossRef]
- Porcellotti, S.; Fanelli, F.; Fracassi, A.; Sepe, S.; Cecconi, F.; Bernardi, C.; Cimini, A.; Cerù, M.P.; Moreno, S. Oxidative Stress during the Progression of β-Amyloid Pathology in the Neocortex of the Tg2576 Mouse Model of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 967203. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeugd, A.; Ahmed, T.; Burnouf, S.; Belarbi, K.; Hamdame, M.; Grosjean, M.E.; Humez, S.; Balschun, D.; Blum, D.; Buée, L.; et al. Hippocampal tauopathy in tau transgenic mice coincides with impaired hippocampus-dependent learning and memory, and attenuated late-phase long-term depression of synaptic transmission. Neurobiol. Learn. Mem. 2011, 95, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Billings, L.M.; Oddo, S.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 2005, 45, 675–688. [Google Scholar] [CrossRef]
- Janczura, K.J.; Volmar, C.-H.; Sartor, G.C.; Rao, S.J.; Ricciardi, N.R.; Lambert, G.; Brothers, S.P.; Wahlestedt, C. Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc. Natl. Acad. Sci. USA 2018, 115, E11148–E11157. [Google Scholar] [CrossRef]
- Orta-Salazar, E.; Feria-Velasco, A.I.; Díaz-Cintra, S. Primary motor cortex alterations in Alzheimer disease: A study in the 3xTg-AD model. Neurol. (Engl. Ed.) 2019, 34, 429–436. [Google Scholar] [CrossRef]
- Davis, K.E.; Fox, S.; Gigg, J. Increased Hippocampal Excitability in the 3xTgAD Mouse Model for Alzheimer’s Disease In Vivo. PLoS ONE 2014, 9, e91203. [Google Scholar] [CrossRef]
- Velikic, G.; Maric, D.M.; Maric, D.L.; Supic, G.; Puletic, M.; Dulic, O.; Vojvodic, D. Harnessing the Stem Cell Niche in Regenerative Medicine: Innovative Avenue to Combat Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 993. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Zhao, P.; Nandakumar, K.; Wang, L.; Song, Y. Microfluidics-Based Systems in Diagnosis of Alzheimer’s Disease and Biomimetic Modeling. Micromachines 2020, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Youmans, K.L.; Tai, L.M.; Kanekiyo, T.; Stine Jr, W.B.; Michon, S.-C.; Nwabuisi-Heath, E.; Manelli, A.M.; Fu, Y.; Riordan, S.; Eimer, W.A. Intraneuronal Aβ detection in 5xFAD mice by a new Aβ-specific antibody. Mol. Neurodegener. 2012, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Hüttenrauch, M.; Baches, S.; Gerth, J.; Bayer, T.A.; Weggen, S.; Wirths, O. Neprilysin Deficiency Alters the Neuropathological and Behavioral Phenotype in the 5XFAD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 44, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Locci, A.; Orellana, H.; Rodriguez, G.; Gottliebson, M.; McClarty, B.; Dominguez, S.; Keszycki, R.; Dong, H. Comparison of memory, affective behavior, and neuropathology in APPNLGF knock-in mice to 5xFAD and APP/PS1 mice. Behav. Brain Res. 2021, 404, 113192. [Google Scholar] [CrossRef]
- Pratap, A.A.; Holsinger, R.M.D. Altered Brain Adiponectin Receptor Expression in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2020, 13, 150. [Google Scholar] [CrossRef]
- Shamrat, F.J.M.; Akter, S.; Azam, S.; Karim, A.; Ghosh, P.; Tasnim, Z.; Hasib, K.M.; De Boer, F.; Ahmed, K. AlzheimerNet: An effective deep learning based proposition for alzheimer’s disease stages classification from functional brain changes in magnetic resonance images. IEEE Access 2023, 11, 16376–16395. [Google Scholar] [CrossRef]
- Savaş, S. Detecting the stages of Alzheimer’s disease with pre-trained deep learning architectures. Arab. J. Sci. Eng. 2022, 47, 2201–2218. [Google Scholar] [CrossRef]
- Shad, H.A.; Rahman, Q.A.; Asad, N.B.; Bakshi, A.Z.; Mursalin, S.F.; Reza, M.T.; Parvez, M.Z. Exploring Alzheimer’s disease prediction with XAI in various neural network models. In Proceedings of the TENCON 2021—2021 IEEE Region 10 Conference (TENCON), Auckland, New Zealand, 7–10 December 2021; pp. 720–725. [Google Scholar]
- Rana, M.M.; Islam, M.M.; Talukder, M.A.; Uddin, M.A.; Aryal, S.; Alotaibi, N.; Alyami, S.A.; Hasan, K.F.; Moni, M.A. A robust and clinically applicable deep learning model for early detection of Alzheimer’s. IET Image Process. 2023, 17, 3959–3975. [Google Scholar] [CrossRef]
- Bayraktar, Y.; Isik, E.; Isik, I.; Ozyilmaz, A.; Toprak, M.; Kahraman Guloglu, F.; Aydin, S. Analyzing of Alzheimer’s Disease Based on Biomedical and Socio-Economic Approach Using Molecular Communication, Artificial Neural Network, and Random Forest Models. Sustainability 2022, 14, 7901. [Google Scholar] [CrossRef]
- Gnanadesigan, N.S.; Dhanasegar, N.; Ramasamy, M.D.; Loganathan, A.K.; Muthusamy, S.; Panchal, H.; Thangaraj, K.; Ravindaran, A.K. A Novel Method for Identification of Candidate Genes for Alzheimer’s Disease Using Network Topology Measure and Intelligent Based Deep Learning Models; Research Square: Durham, NC, USA, 2022. [Google Scholar] [CrossRef]
- Krieger, T.G.; Tirier, S.M.; Park, J.; Jechow, K.; Eisemann, T.; Peterziel, H.; Angel, P.; Eils, R.; Conrad, C. Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro-Oncology 2020, 22, 1138–1149. [Google Scholar] [CrossRef]
- Azzarelli, R.; Ori, M.; Philpott, A.; Simons, B.D. Three-dimensional model of glioblastoma by co-culturing tumor stem cells with human brain organoids. Biol. Open 2021, 10, bio056416. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, A.; Goranci-Buzhala, G.; Ricci-Vitiani, L.; Pallini, R.; Gopalakrishnan, J. Trends and challenges in modeling glioma using 3D human brain organoids. Cell Death Differ. 2021, 28, 15–23. [Google Scholar] [CrossRef]
- Kang, Y.J.; Cho, H. Human brain organoids in Alzheimer’s disease. Organoid 2021, 1, e5. [Google Scholar] [CrossRef]
- Jorfi, M.; D’Avanzo, C.; Kim, D.Y.; Irimia, D. Three-dimensional models of the human brain development and diseases. Adv. Healthc. Mater. 2018, 7, 1700723. [Google Scholar] [CrossRef]
- Ahmed, T. Biomaterial-based in vitro 3D modeling of glioblastoma multiforme. Cancer Pathog. Ther. 2023, 1, 177–194. [Google Scholar] [CrossRef]
- Amiri, E.; Sanjarnia, P.; Sadri, B.; Jafarkhani, S.; Khakbiz, M. Recent advances and future directions of 3D to 6D printing in brain cancer treatment and neural tissue engineering. Biomed. Mater. 2023, 18, 052005. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Y.; Zhang, C.; Bi, R.; Qiu, Y.; Li, Y.; Hu, B. Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases. Pharmaceutics 2023, 15, 621. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Tang, Q.; Xiao, M.; Tong, X.; Yang, T.; Yin, D.; Lei, L.; Li, S. Cell membrane-coated nanomaterials for cancer therapy. Mater. Today Bio 2023, 20, 100633. [Google Scholar] [CrossRef]
- Allami, P.; Heidari, A.; Rezaei, N. The role of cell membrane-coated nanoparticles as a novel treatment approach in glioblastoma. Front. Mol. Biosci. 2022, 9, 1083645. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- He, C.; Lu, F.; Liu, Y.; Lei, Y.; Wang, X.; Tang, N. Emergent trends in organ-on-a-chip applications for investigating metastasis within tumor microenvironment: A comprehensive bibliometric analysis. Heliyon 2024, 10, e23504. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Bai, X.; Ji, X.; Wang, X.; Han, X.; Wang, D.; Jiang, F.; An, Y. The significance of glycolysis index and its correlations with immune infiltrates in Alzheimer’s disease. Front. Immunol. 2022, 13, 960906. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, Y.; Kou, Q.; Lu, L.; Jiang, H.; Li, X.; Gui, R.; Huang, R.; Huang, X.; Ma, J. Study on the Role of an Erythrocyte Membrane-Coated Nanotheranostic System in Targeted Immune Regulation of Alzheimer’s Disease. Adv. Sci. 2023, 10, 2301361. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.C.; Atkinson-Dell, R.; Verkhratsky, A.; Mohamet, L. Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis. 2017, 8, e2696. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Q.; Gu, X.; Zheng, M.; Wang, A.; Jiang, G.; Huang, M.; Chen, H.; Qiu, Y.; Bo, B.; et al. Multifunctional Nanostructure RAP-RL Rescues Alzheimer’s Cognitive Deficits through Remodeling the Neurovascular Unit. Adv. Sci. 2021, 8, 2001918. [Google Scholar] [CrossRef]
- Ye, P.; Li, L.; Qi, X.; Chi, M.; Liu, J.; Xie, M. Macrophage membrane-encapsulated nitrogen-doped carbon quantum dot nanosystem for targeted treatment of Alzheimer’s disease: Regulating metal ion homeostasis and photothermal removal of β-amyloid. J. Colloid Interface Sci. 2023, 650, 1749–1761. [Google Scholar] [CrossRef]
- Chi, M.; Liu, J.; Li, L.; Zhang, Y.; Xie, M. In-situ growth of CeO2 on biofilms: Innovative nanoparticles for photothermal therapy & multi-pronged attack on Alzheimer’s disease. Colloids Surf. B Biointerfaces 2024, 238, 113887. [Google Scholar] [CrossRef]
- Salter, M.W.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 2017, 94, 278–293.e9. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, L.; Liu, G.; Jiang, N.; Zhou, W.; Zhang, Y. Pathological changes in Alzheimer’s disease analyzed using induced pluripotent stem cell-derived human microglia-like cells. J. Alzheimer’s Dis. 2019, 67, 357–368. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Zhang, W.; Liu, Y.; Ding, L.; Gong, J.; Ma, R.; Zheng, S.; Zhang, Y. Biomimetic remodeling of microglial riboflavin metabolism ameliorates cognitive impairment by modulating neuroinflammation. Adv. Sci. 2023, 10, 2300180. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Bai, K.; Yang, X.; Sun, C.; Ji, Y.; Zhou, J.; Zhang, H.; Ding, Y. “Drug-Carrier” Synergy Therapy for Amyloid-β Clearance and Inhibition of Tau Phosphorylation via Biomimetic Lipid Nanocomposite Assembly. Adv. Sci. 2022, 9, e2106072. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Zhang, H.; Zhao, C.; Du, X.; Ren, J.; Qu, X. Biomimetic engineering of a neuroinflammation-targeted MOF nanozyme scaffolded with photo-trigger released CO for the treatment of Alzheimer’s disease. Chem. Sci. 2024, 15, 13201–13208. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Arber, C.; Lovejoy, C.; Wray, S. Stem cell models of Alzheimer’s disease: Progress and challenges. Alzheimer’s Res. Ther. 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Polis, B.; Samson, A.O. Addressing the Discrepancies Between Animal Models and Human Alzheimer’s Disease Pathology: Implications for Translational Research. J. Alzheimer’s Dis. 2024, 98, 1199–1218. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Hernández-Ávalos, I.; Martínez-Burnes, J.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef]
- Marshall, L.J.; Bailey, J.; Cassotta, M.; Herrmann, K.; Pistollato, F. Poor Translatability of Biomedical Research Using Animals—A Narrative Review. Altern. Lab. Anim. 2023, 51, 102–135. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Cummings, J.; Reiber, C.; Kumar, P. The price of progress: Funding and financing Alzheimer’s disease drug development. Alzheimers Dement. 2018, 4, 330–343. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Yang, W.; Zhong, M.; Lin, Z.-X.; Gray, N.E.; Xian, Y.-F. Animal models of Alzheimer’s disease: Preclinical insights and challenges. Acta Mater. Medica 2023, 2, 192–215. [Google Scholar] [CrossRef]
- Kearney, A.; Rosala-Hallas, A.; Bacon, N.; Daykin, A.; Shaw, A.R.G.; Lane, A.J.; Blazeby, J.M.; Clarke, M.; Williamson, P.R.; Gamble, C. Reducing attrition within clinical trials: The communication of retention and withdrawal within patient information leaflets. PLoS ONE 2018, 13, e0204886. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.; Jaenisch, R.; Mooney, D.J. Engineered tissues and strategies to overcome challenges in drug development. Adv. Drug Deliv. Rev. 2020, 158, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Milat, A.J.; Bauman, A.; Redman, S. Narrative review of models and success factors for scaling up public health interventions. Implement. Sci. 2015, 10, 113. [Google Scholar] [CrossRef]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future. Org. Process Res. Dev. 2019, 23, 1213–1242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).