Anti-Inflammatory 8-Shogaol Mediates Apoptosis by Inducing Oxidative Stress and Sensitizes Radioresistance in Gastric Cancer
Abstract
1. Introduction
2. Results
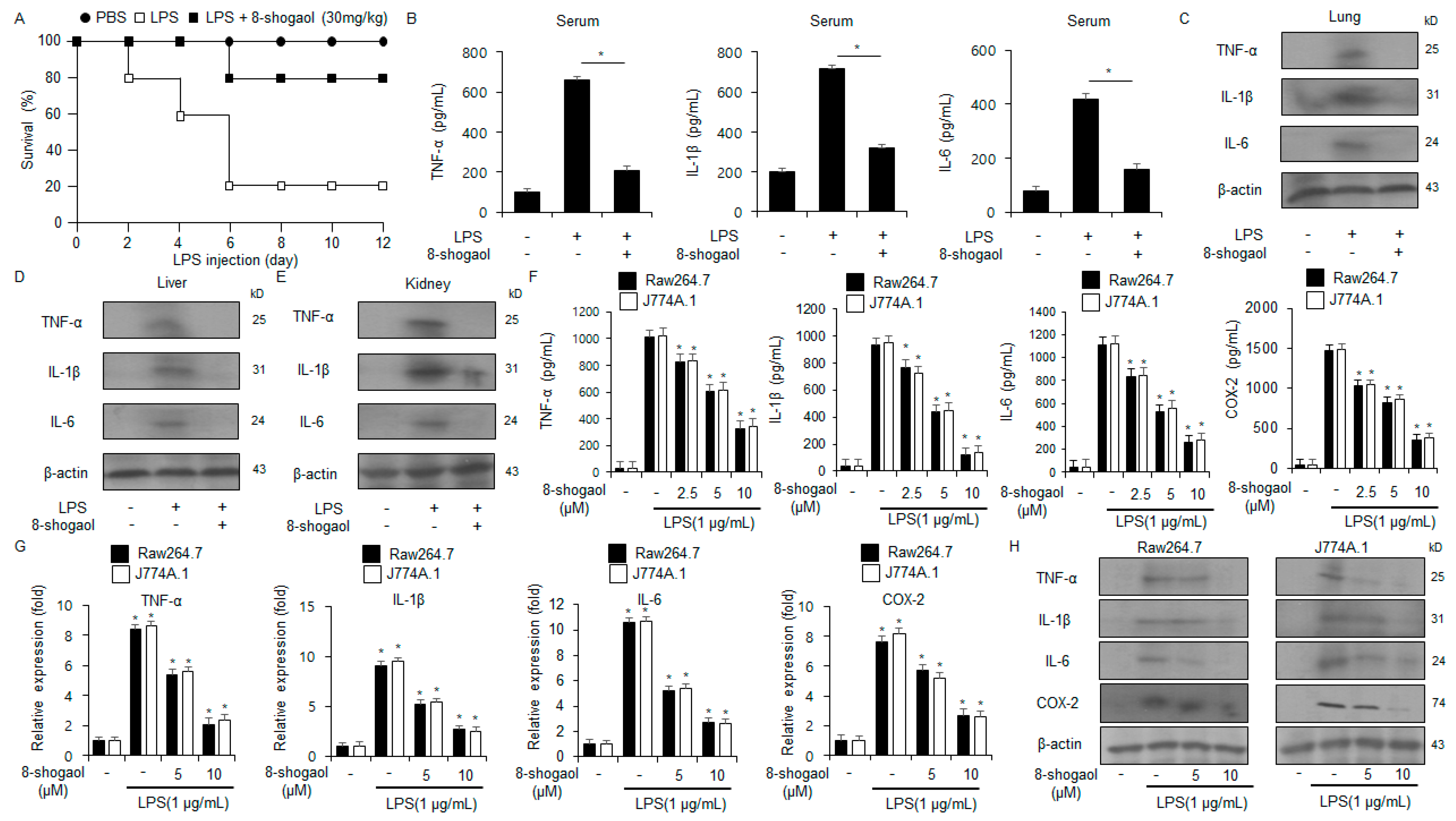
2.1. 8-Shogaol Inhibited Inflammation in LPS-Treated Macrophages and a Sepsis Mouse Model
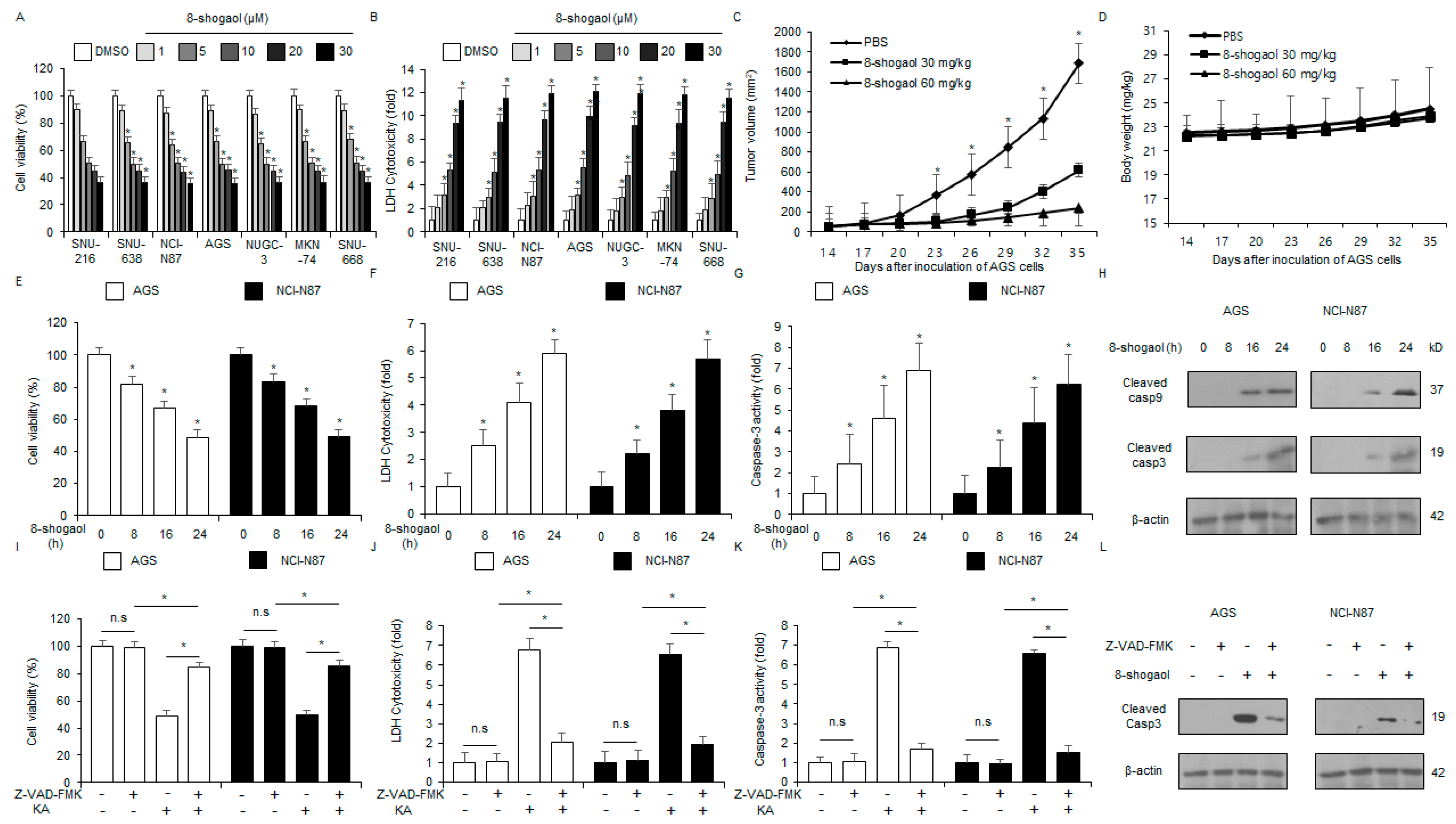
2.2. 8-Shogaol Induced Caspase-Dependent Apoptosis in Gastric Cancer Cells
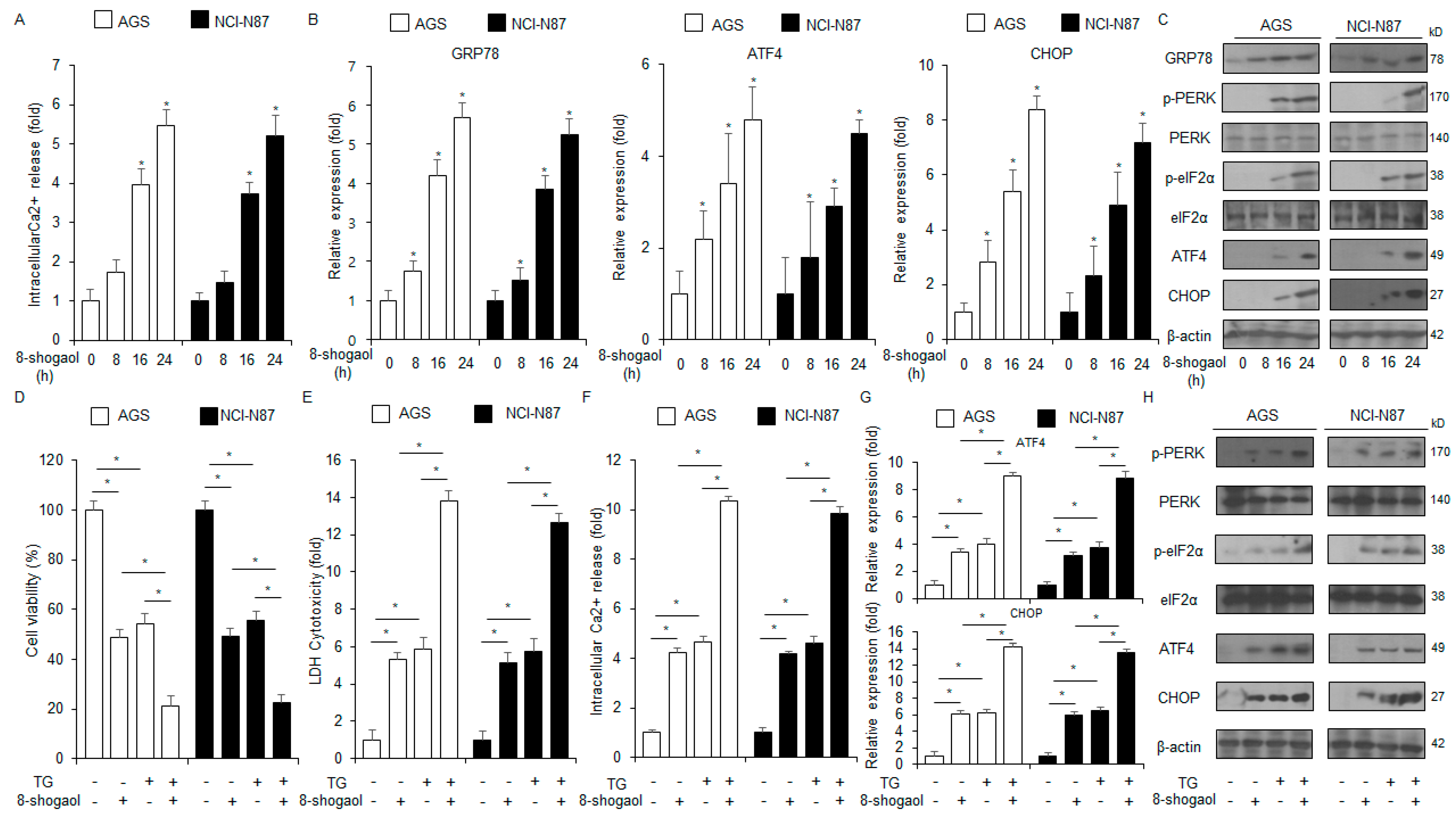
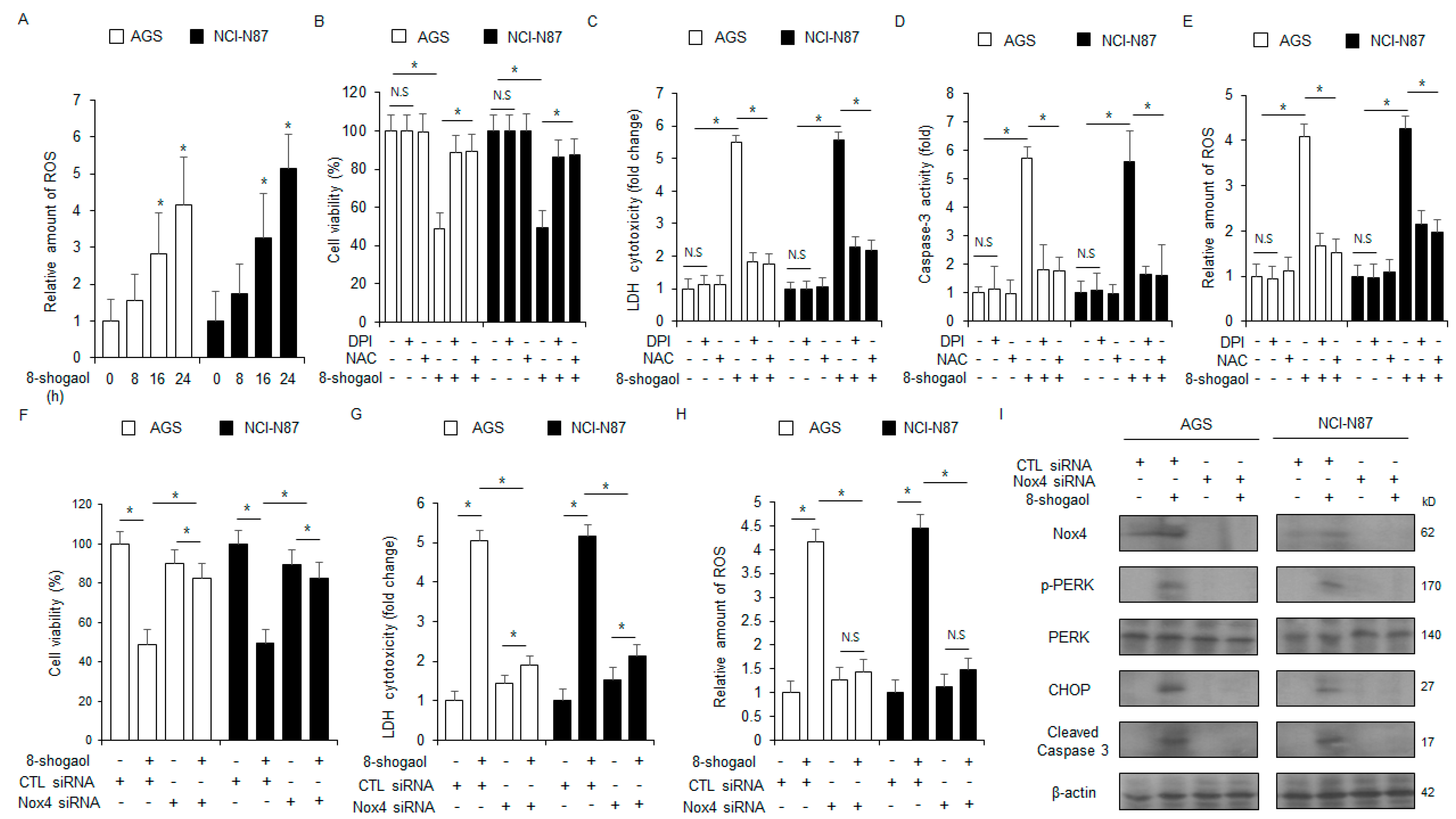
2.3. 8-Shogaol-Induced ER Stress Mediated Apoptosis in Gastric Cancer Cells
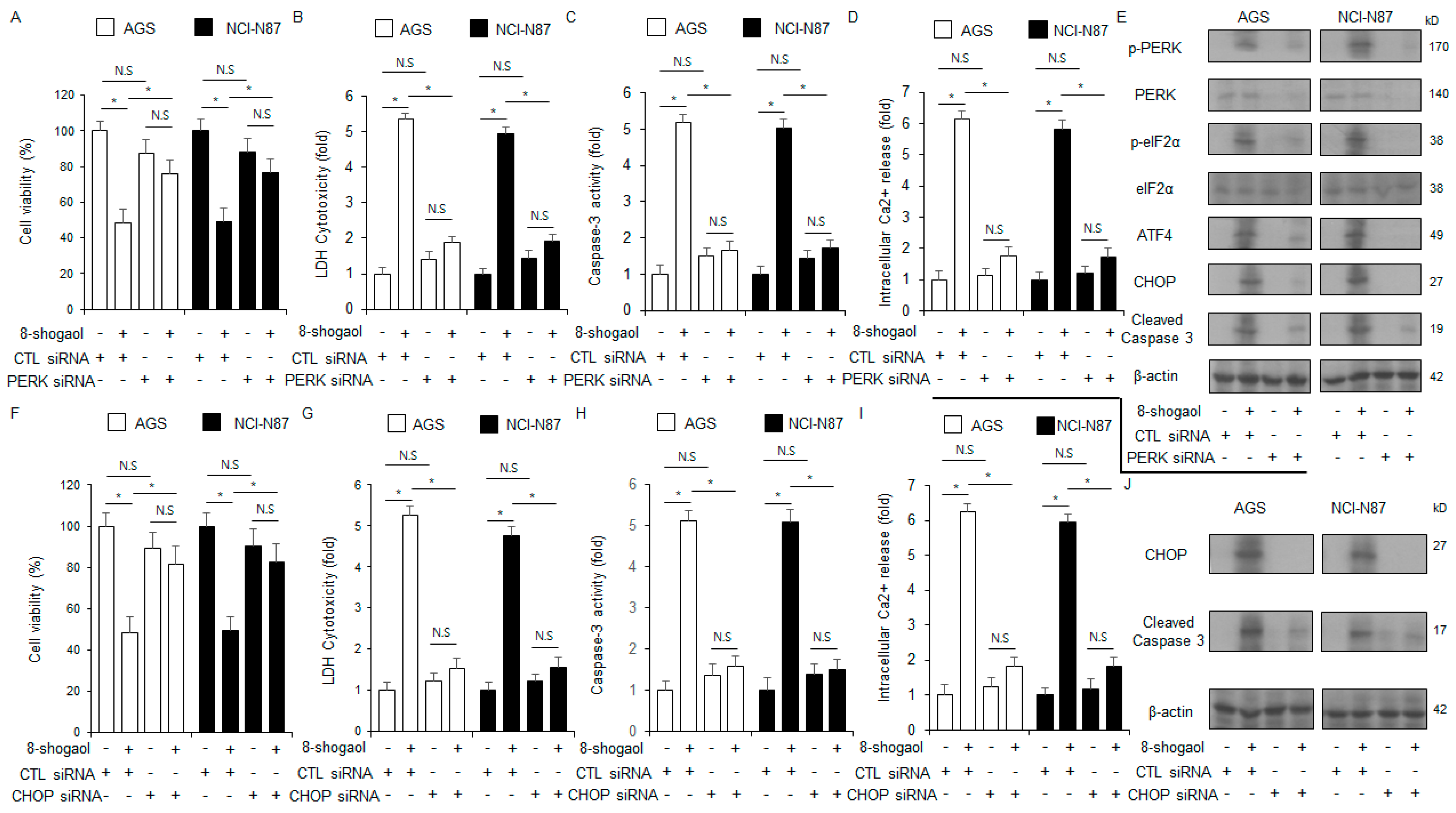
2.4. PERK or CHOP Silencing Inhibited 8-Shogaol-Induced Apoptosis in Gastric Cancer Cells
2.5. Nox4 Silencing Inhibited 8-Shogaol-Induced Apoptosis in Gastric Cancer Cells
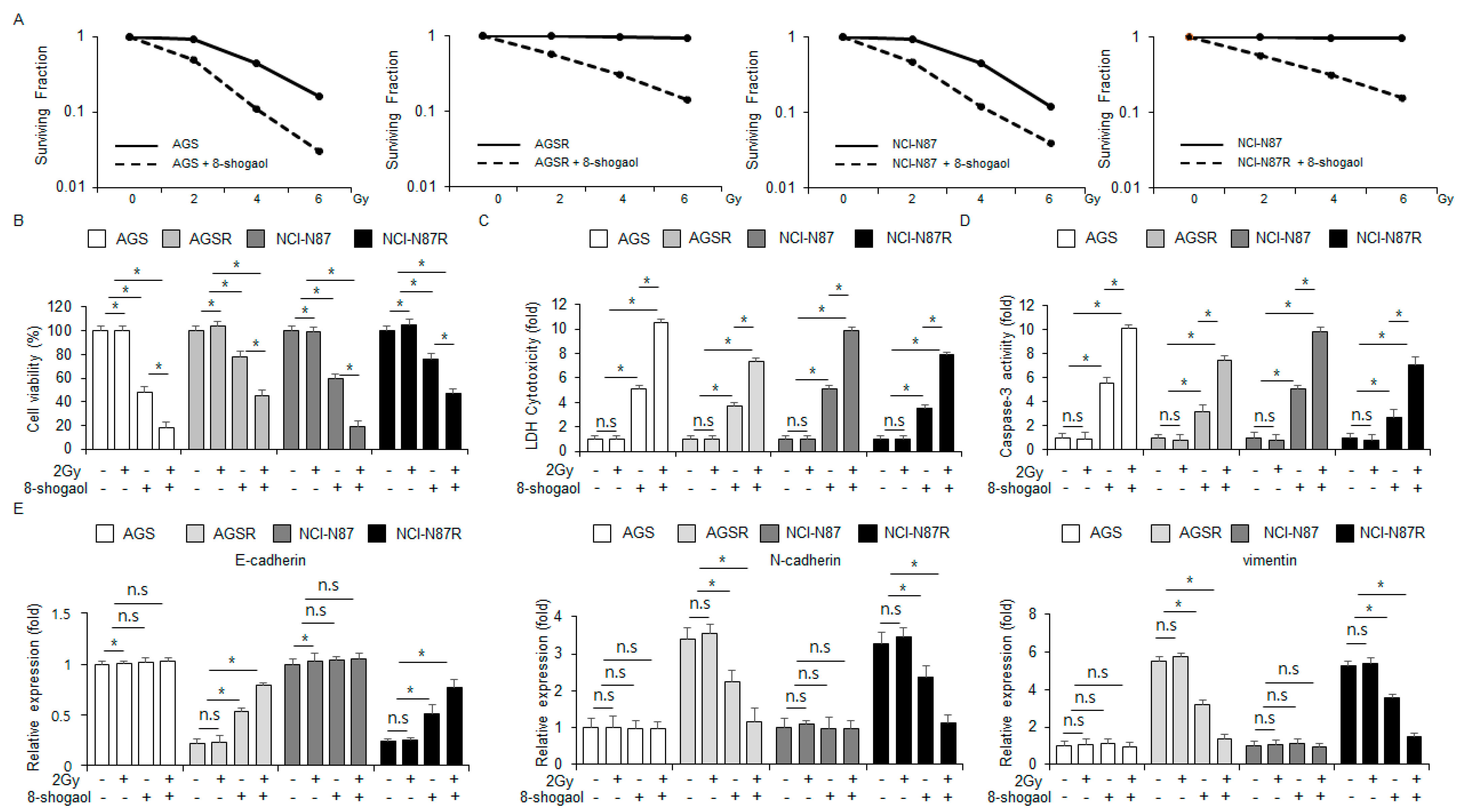
2.6. 8-Shogaol Sensitized Radio-Resistant Gastric Cancer Cells to Radiotherapy by Modulating EMT Markers
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Viability and Proliferation Assays
4.4. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
4.5. Colorimetric Caspase-3 Activity Assay
4.6. Intracellular Ca2+ Assay
4.7. Intracellular ROS Assay
4.8. Radio-Resistant NCI-N87R and AGSR Cell Lines
4.9. Radiotherapy
4.10. Clonogenic Cell Assays
4.11. Transfection Assay
4.12. Protein and RNA Purification
4.13. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT PCR) and Western Blot Analyses
4.14. Tumor and Inflammation Mouse Models
4.15. Cytokine Levels
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thrift, A.P.; Wenker, T.N.; El-Serag, H.B. Global burden of gastric cancer: Epidemiological treands, risk factors, screening and prevention. Nat. Rev. Clin. Oncol. 2023, 20, 338–349. [Google Scholar] [CrossRef]
- Guan, W.L.; He, Y.; Xu, R.H. Gastric cancer treatment: Recent progress and future perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef]
- Chong, I.Y.; Chau, I. Is there still a place for radiotherapy in gastric cancer? Curr. Opin. Pharmacol. 2023, 68, 102325. [Google Scholar] [CrossRef]
- Baskar, P.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Pagliari, F.; Jansen, J.; Knoll, J.; Hanley, R.; Seco, J.; Tirinato, L. Cancer radioresistance is characterized by a differential lipid droplet content along the cell cycle. Cell Div. 2024, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013, 4, e875. [Google Scholar] [CrossRef] [PubMed]
- Komorowska, D.; Radzik, T.; Kalenik, S.; Rodacka, A. Natural radiosensitizers in radiotherapy: Cancer treatment by combining ionizing radiation with resveratrol. Int. J. Mol. Sci. 2022, 23, 10627. [Google Scholar] [CrossRef]
- Cheng, X.L.; Ruan, Y.L.; Dai, J.Y.; Fan, H.Z.; Ling, J.Y.; Chen, J.; Lu, W.G.; Gao, X.J.; Cao, P. 8-shogaol derived from dietary ginger alleviated acute and inflammatory pain by targeting TRPV1. Phytomedicine 2024, 128, 1555000. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; Labib, R.M.; Sobeh, M.; Farag, M.A. Gingerols and shogaols: A multi-faceted review of their extraction, formulation, and analysis in drugs and biofluids to maximize their nutraceutical and pharmaceutical applications. Food Chem. X 2023, 20, 100947. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Noh, E.M.; Kim, S.Y. Anti-inflammatory effect and signaling mechanism of 8-shogaoland 10-shogaol in a dextran sodium sulfate-induced colitis mouse model. Heliyon 2023, 9, e12778. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.C.; Chen, Y.O.; Kuo, D.H.; Chen, F.A.; Tsai, M.L.; Chang, I.S.; Wu, H.; Sang, S.; Ho, C.T.; Pan, M.H. Induction of apoptosis by 8-shogaol via reactive oxygen species generation, glutathione depletion, and caspase activation in human leukemia cells. J. Agric. Food Chem. 2010, 58, 3847–3854. [Google Scholar] [CrossRef]
- Groenendyk, J.; Michalak, M. Interplay between calcium and endoplasmic reticulum stress. Cell Calcium 2023, 113, 102753. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, A.; Appathurai, S.; Plumb, R.; Mariappan, M. Dynamic changes in complexes of IRE1α, PERK, ATF6α during endoplasmic reticulum stress. Mol. Biol. Cell 2018, 29, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Spencer, B.G.; Finnie, J.W. The role of endoplasmic reticulum stress in cell survival and death. J. Comp. Pathol. 2020, 181, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Santos, C.X.; Nabeebaccus, A.A.; Shah, A.M.; Camargo, L.L.; Filho, S.V.; Lopes, L.R. Endoplasmic reticulum stress and nox-mediated reactive oxygen species signaling in the peripheral vasculature: Potential role in hypertension. Antioxid. Redox Signal 2014, 20, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O. Calcium’s role in orchestrating cancer apoptosis: Mitochondrial-centric perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004, 11, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, K.; Fan, X.; Wang, J.; Jin, Y.; Zheng, Z.J. Access to radiotherapy in improving gastric cancer care quality and equality. Commun Med. 2024, 4, 225. [Google Scholar] [CrossRef]
- Nan, Y.; Su, H.; Zhou, B.; Liu, S. The function of natural compounds in important anticancer mechanisms. Front. Oncol. 2023, 12, 1049888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.F. Natural compounds as anticancer agents: Experimental evidence. World J. Exp. Med. 2012, 2, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Az-Zahra, F.; Wongso, H.; Setyawati, L.U.; Novitasari, D.; Ikram, E.H.K. Molecular mechanism of natural food antioxidants to regulate ROS in treating cancer: A review. Antioxidants 2024, 13, 207. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 12, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Hong, J.; Wu, H.; Liu, J.; Yang, C.S.; Pan, M.H.; Badmaev, V.; Ho, C.T. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009, 57, 10645–10650. [Google Scholar] [CrossRef] [PubMed]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczyńska, H.; Alan Diehl, J.; Majsterek, I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, M.; Pucci, G.; Musso, R.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Forte, G.I.; Minafra, L. Nutraceutical compounds as sensitizers for cancer treatment in radiation therapy. Int. J. Mol. Sci. 2019, 20, 5267. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Ghahremani, F.; Safavi, A.; Hajiaghababa, A.; Moshtaghian, J. C-phycocyanin: A natural product with radiosensitizing property for enhancement of colon cancer radiation therapy efficacy through inhibition of COX-2 expression. Sci. Rep. 2019, 9, 19161. [Google Scholar] [CrossRef]
- Xiong, H.; Nie, X.; Zou, Y.; Gong, C.; Li, Y.; Wu, H.; Qiu, H.; Yang, L.; Zhuang, L.; Zhang, P.; et al. Twist1 enhances hypoxia induced radioresistance in cervical cancer cells by promoting nuclear EGFR localization. J. Cancer 2017, 8, 345–353. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, C.; Li, C.; Ye, S.; Zhao, J.; Zhu, C.; Wang, X.; Ma, Q.; Li, B. Natural compound alternol actives multiple endoplasmic reticulum stress-responding pathways contributing to cell death. Front. Pharmacol. 2024, 15, 1397116. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Ediriweera, M.K.; Ryu, J.Y.; Kim, H.Y.; Kim Cho, S. Catechol enhances chemo- and radio-sensitivity by targeting AMPK-Hippo signaling in pancreatic cancer cells. Oncol. Rep. 2021, 45, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shin, J.A.; Lee, Y.G.; Jin, B.H.; Lee, W.W.; Lee, Y.S.; Choi, S.J.; Han, J.M.; Ahn, M.H.; Kim, J.H.; et al. Zingiber officinale promotes autophagy and apoptosis in human oral cancer through the C/EBP homologous protein. Cancer Sci. 2024, 115, 2701–2717. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, C.; Jiang, X.; Zhang, Z. ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction underlie apoptosis inducedby resveratrol and arsenic trioxide in A549 cells. Chem. Biol. Interact. 2016, 245, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, E.; Jo, E.; Kim, Z.H.; Yim, K.J.; Woo, S.H.; Choi, Y.S.; Jang, H.J. Gossypol induces apoptosis of human pancreatic cancer cells via CHOP/endoplasmic reticulum stress signaling pathway. J. Microbiol. Biotechnol. 2022, 32, 645–656. [Google Scholar] [CrossRef]
- Tuy, K.; Rickenbacker, L.; Hjelmeland, A.B. Reactive oxygen species produced by altered tumor metabolism impacts cancer stem cell maintenance. Redox Biol. 2021, 44, 101953. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide radicals in the execution of cell death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Seo, S.U.; Kim, T.H.; Kim, D.E.; Min, K.J.; Kwon, T.K. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment thioridazine and curcumin. Redox Biol. 2017, 13, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Yao, P.; Shen, W.; Wu, Y.; Ye, Z.; Zhao, K.; Chen, H.; Cao, J.; Xing, C. B7-H3 increases the radioresistance of gastric cancer cells through regulating baseline levels of cell autophagy. Am. J. Transl. Res. 2019, 11, 4438–4449. [Google Scholar] [PubMed]
- Poon, D.J.J.; Tay, L.M.; Ho, D.; Chua, M.L.K.; Chow, E.K.H.; Yeo, E.L.L. Improving the therapeutic ratio of radiotherapy against radioresistant cancers: Leveraging on novel artificial intelligence-based approaches for drug combination discovery. Cancer Lett. 2021, 511, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Theys, J.; Jutten, B.; Habets, R.; Paesmans, K.; Groot, A.J.; Lambin, P.; Wouters, B.G.; Lammering, G.; Vooijs, M. E-cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother. Oncol. 2011, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, H.G. 6-Shogaol overcomes gefitinib resistance via ER stress in ovarian cancer cells. Int. J. Mol. Sci. 2023, 24, 2639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.W.; Lee, H.G. Anti-Inflammatory 8-Shogaol Mediates Apoptosis by Inducing Oxidative Stress and Sensitizes Radioresistance in Gastric Cancer. Int. J. Mol. Sci. 2025, 26, 173. https://doi.org/10.3390/ijms26010173
Kim TW, Lee HG. Anti-Inflammatory 8-Shogaol Mediates Apoptosis by Inducing Oxidative Stress and Sensitizes Radioresistance in Gastric Cancer. International Journal of Molecular Sciences. 2025; 26(1):173. https://doi.org/10.3390/ijms26010173
Chicago/Turabian StyleKim, Tae Woo, and Hee Gu Lee. 2025. "Anti-Inflammatory 8-Shogaol Mediates Apoptosis by Inducing Oxidative Stress and Sensitizes Radioresistance in Gastric Cancer" International Journal of Molecular Sciences 26, no. 1: 173. https://doi.org/10.3390/ijms26010173
APA StyleKim, T. W., & Lee, H. G. (2025). Anti-Inflammatory 8-Shogaol Mediates Apoptosis by Inducing Oxidative Stress and Sensitizes Radioresistance in Gastric Cancer. International Journal of Molecular Sciences, 26(1), 173. https://doi.org/10.3390/ijms26010173




