Prostaglandins Differentially Regulate the Constitutive and Mechanosensitive Release of Soluble Nucleotidases in the Urinary Bladder Mucosa
Abstract
1. Introduction
2. Results
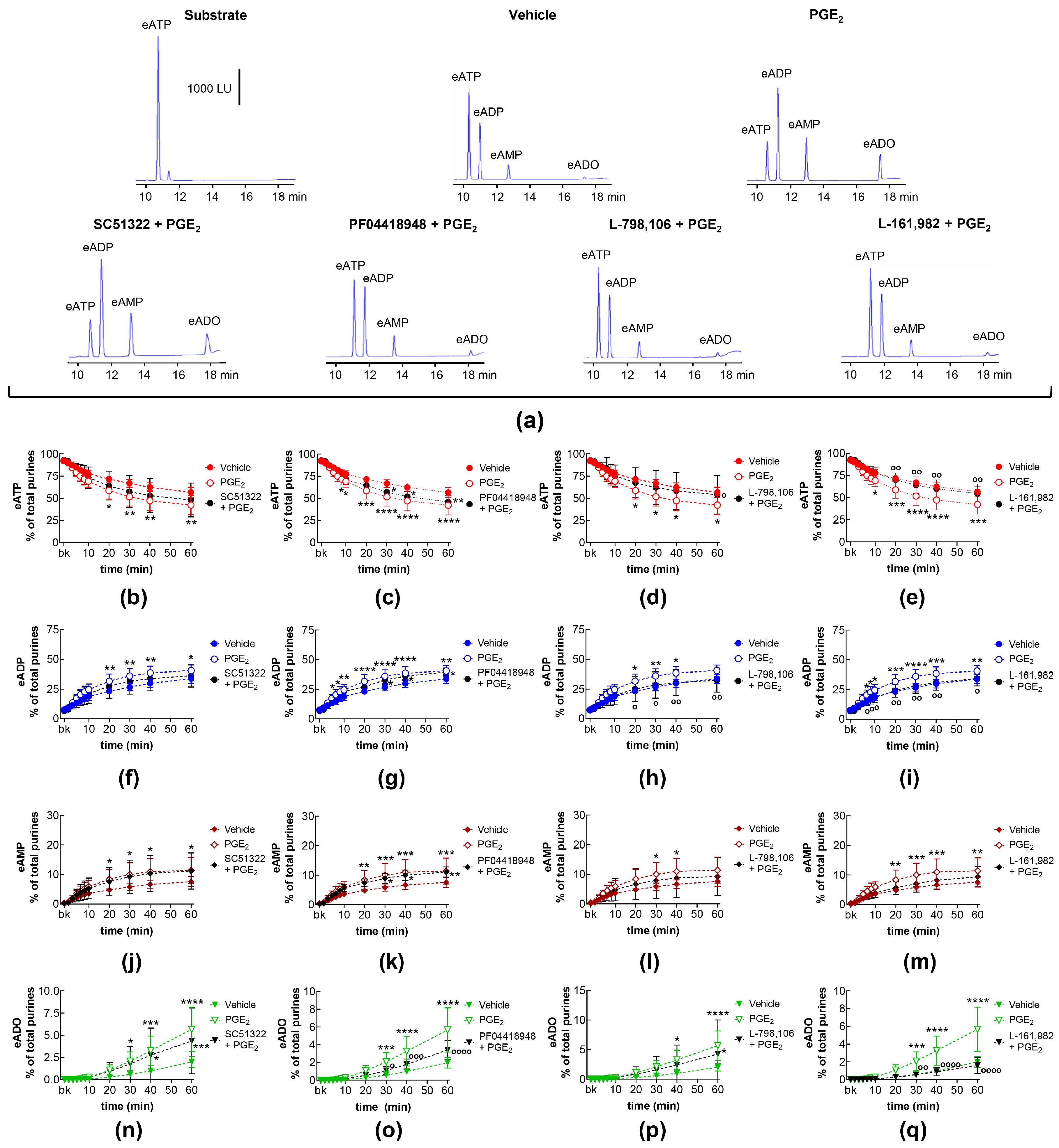
2.1. Influences of Endogenous Activators of EP Prostanoid Receptors on Spontaneous Release of s-NTDs
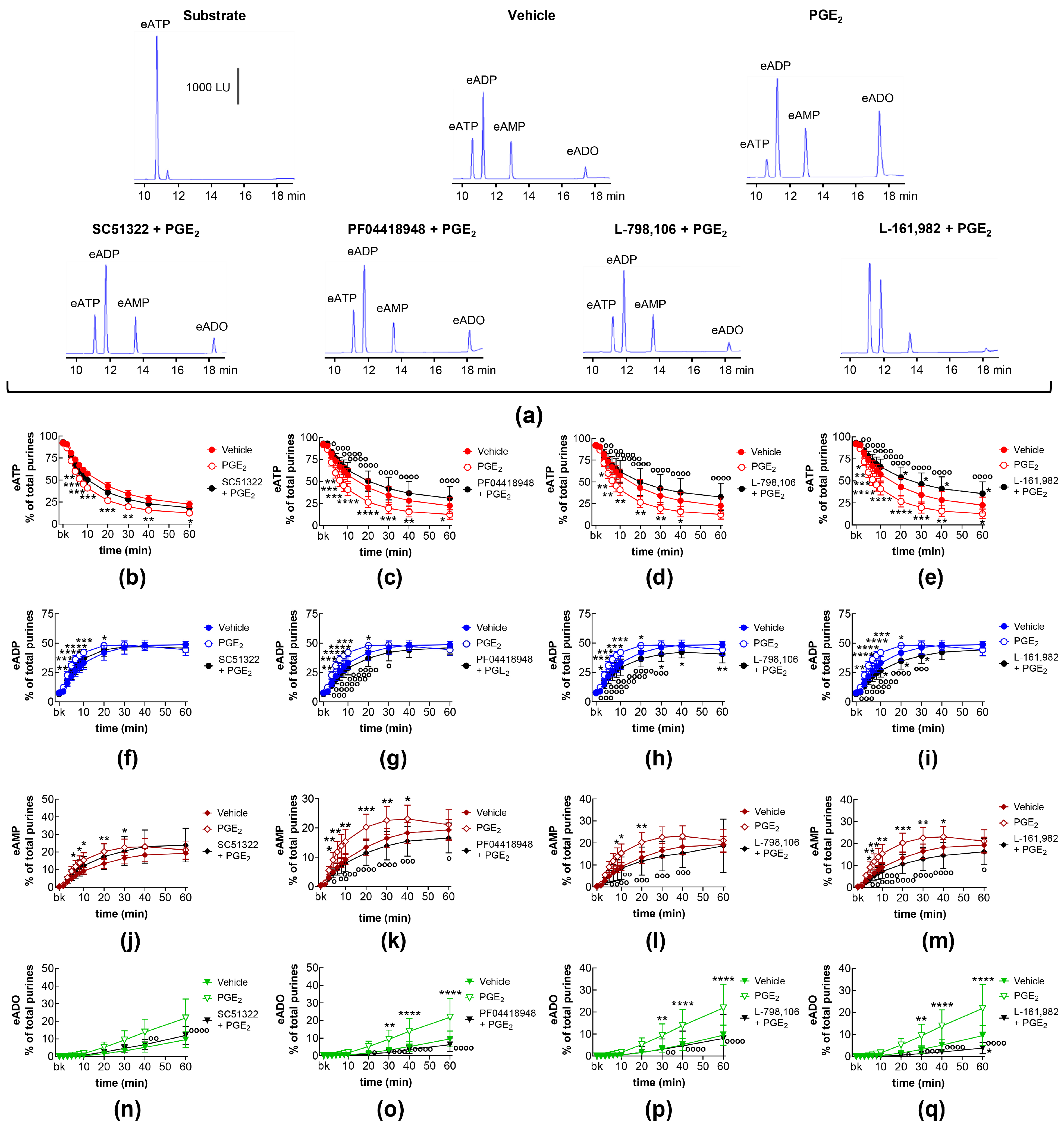
2.2. Influences of Endogenous Activators of EP Prostanoid Receptors on Distention-Induced Release of s-NTDs
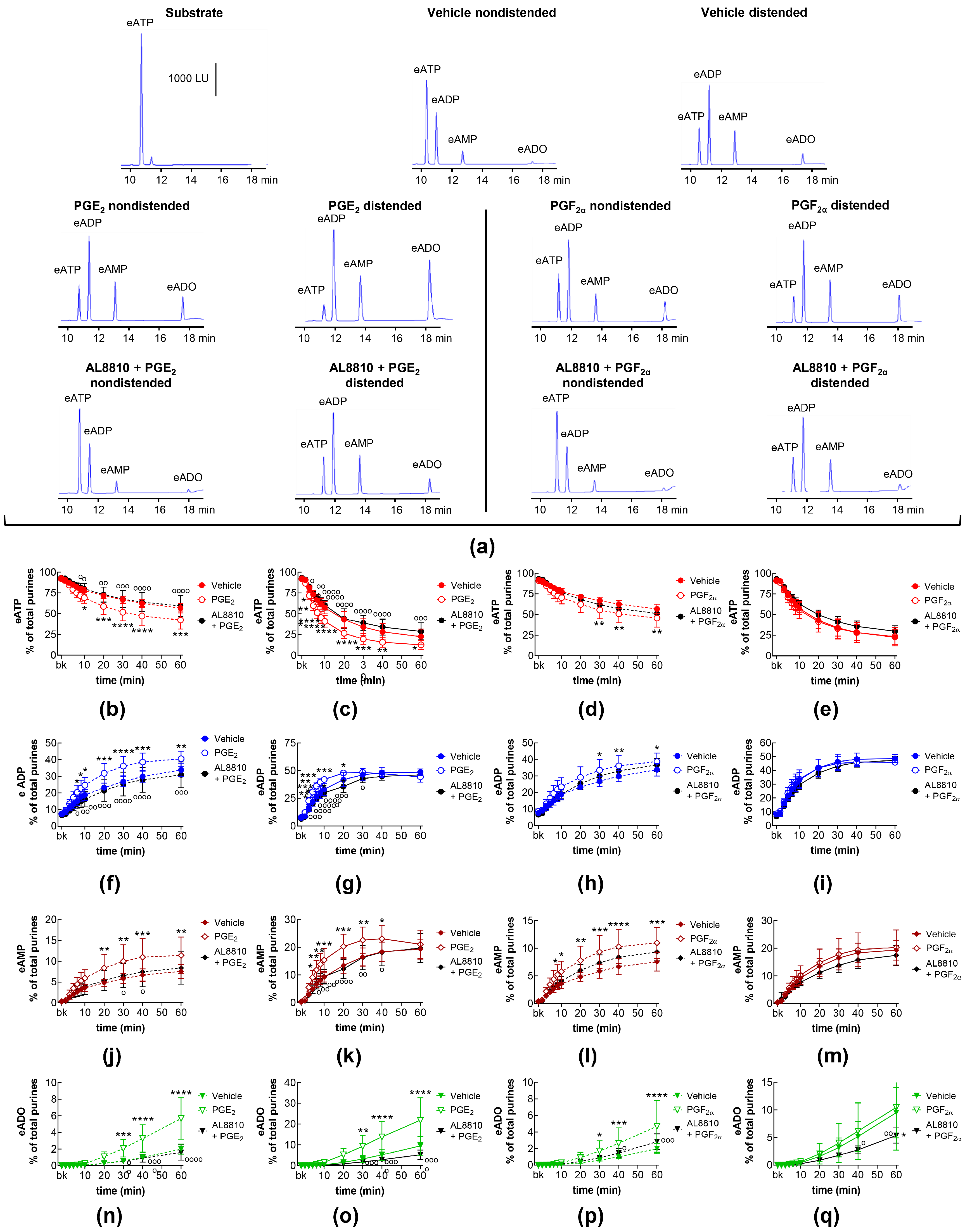
2.3. Influences of Endogenous Activators of Prostanoid DP and FP Receptors on Spontaneous and Distention-Induced Release of s-NTDs
2.4. Influences of Endogenous Activators of Prostanoid IP Receptors on Spontaneous and Distention-Induced Release of s-NTDs
2.5. Effects of COX Inhibition on Spontaneous and Distention-Induced Release of s-NTDs
2.6. Effects of Exogenous PGI2 and TXA2 Analog on the Spontaneous and Distention-Induced Release of s-NTDs
2.7. Effects of Exogenous PGE2 on the Spontaneous Release of s-NTDs and Involvement of EP Prostanoid Receptors
2.8. Effects of Exogenous PGE2 on Distention-Induced Release of s-NTDs and Involvement of EP Prostanoid Receptors
2.9. Effects of FP Receptor Blockade on the Effects of Exogenous PGE2 and PGF2α on Spontaneous and Distention-Induced Release of s-NTDs
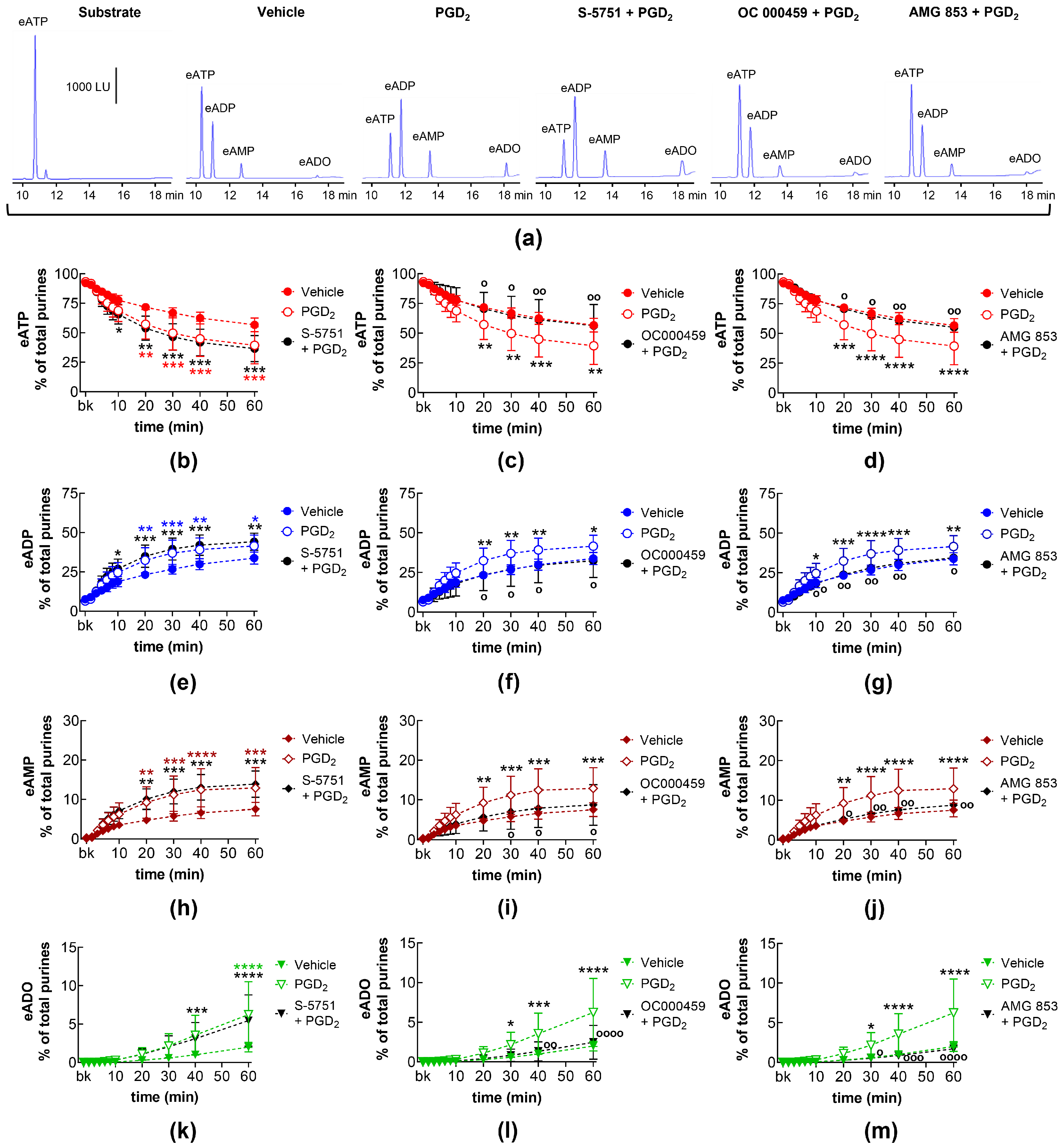
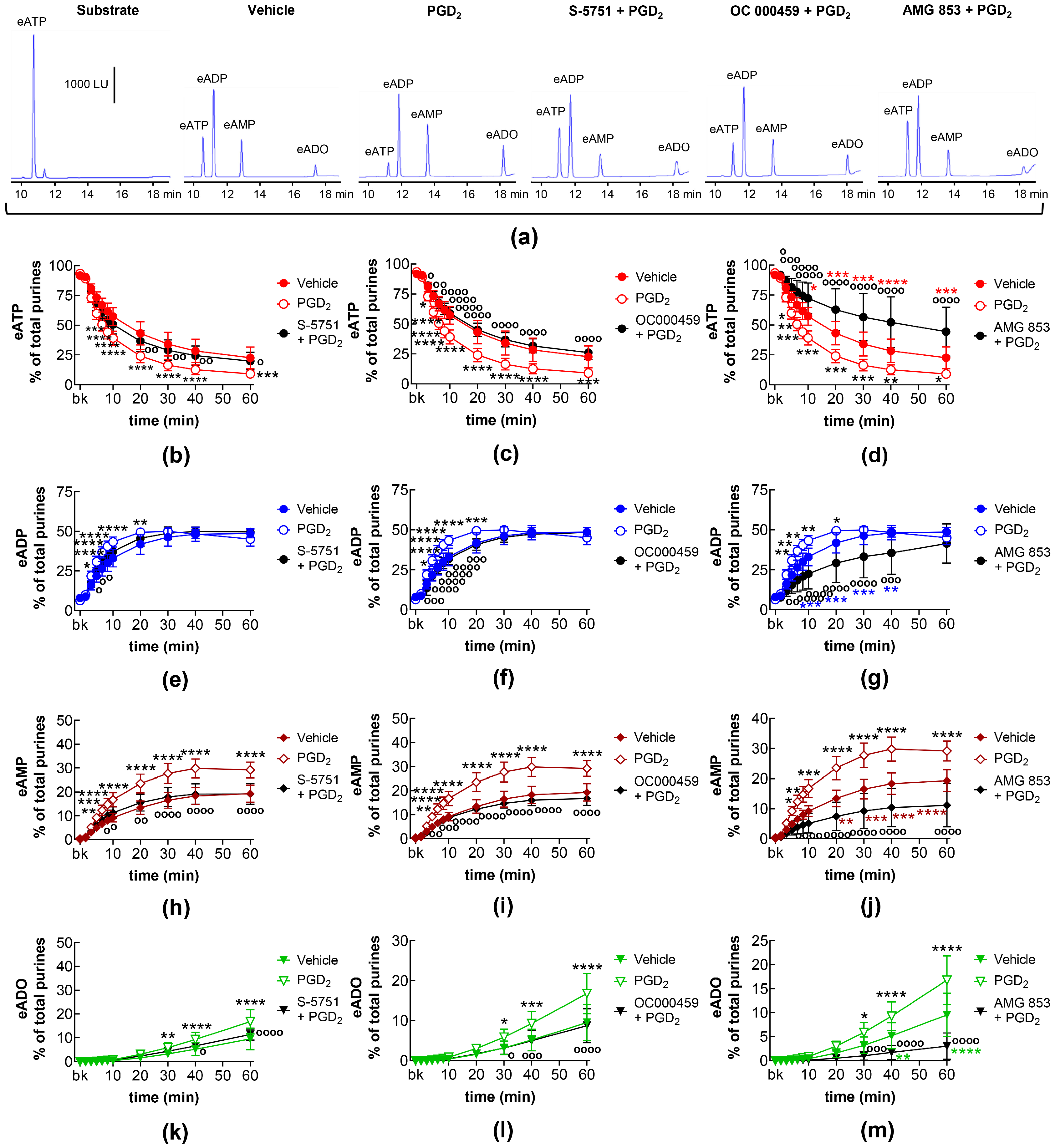
2.10. Effects of Exogenous PGD2 on Spontaneous Release of s-NTDs and Involvement of DP Prostanoid Receptors
2.11. Effects of Exogenous PGD2 on Distention-Induced Release of s-NTDs and Involvement of DP Prostanoid Receptors
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.1.1. Euthanasia and Tissue Collection
4.1.2. Ethical Approval
4.2. Ex Vivo Detrusor-Free Bladder Preparation
4.3. Soluble Nucleotidase (s-NTD) Activity in Concentrated Extraluminal Solutions
4.3.1. Collection of Extraluminal Solutions Containing s-NTDs
4.3.2. Concentration of Extraluminal Solutions Containing s-NTDs
4.3.3. Time Course of Extracellular eATP Degradation by s-NTDs in cELS of Nondistended and Distended LP
4.4. HPLC Analysis of 1,N6-Etheno-Purines
4.5. Drugs
4.6. Statistical Analysis of Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winder, M.; Tobin, G.; Zupancic, D.; Romih, R. Signalling molecules in the urothelium. Biomed. Res. Int. 2014, 2014, 297295. [Google Scholar] [CrossRef] [PubMed]
- Merrill, L.; Gonzalez, E.J.; Girard, B.M.; Vizzard, M.A. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat. Rev. Urol. 2016, 13, 193–204. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Yin, P.; Liu, L.; Chen, Y. Mechanotransduction in the urothelium: ATP signalling and mechanoreceptors. Heliyon 2023, 9, e19427. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, N.J.; Vane, J.R. Hormones released into the circulation when the urinary bladder of the anaesthetized dog is distended. Clin. Sci. 1971, 41, 69–83. [Google Scholar] [CrossRef]
- Khan, M.A.; Thompson, C.S.; Mumtaz, F.H.; Jeremy, J.Y.; Morgan, R.J.; Mikhailidis, D.P. Role of prostaglandins in the urinary bladder: An update. Prostaglandins Leukot. Essent. Fat. Acids 1998, 59, 415–422. [Google Scholar] [CrossRef]
- Tanaka, I.; Nagase, K.; Tanase, K.; Aoki, Y.; Akino, H.; Yokoyama, O. Modulation of stretch evoked adenosine triphosphate release from bladder epithelium by prostaglandin E2. J. Urol. 2011, 185, 341–346. [Google Scholar] [CrossRef]
- Rahnama’i, M.S.; van Kerrebroeck, P.E.; de Wachter, S.G.; van Koeveringe, G.A. The role of prostanoids in urinary bladder physiology. Nat. Rev. Urol. 2012, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Zha, X.; Ito, H.; Aoki, Y.; Akino, H.; Kobayashi, M.; Yokoyama, O. Underlying mechanisms of urine storage dysfunction in rats with salt-loading hypertension. Life Sci. 2015, 141, 8–12. [Google Scholar] [CrossRef]
- Kasakov, L.N.; Vlaskovska, M.V. Profile of prostaglandins generated in the detrusor muscle of rat urinary bladder: Effects of adenosine triphosphate and adenosine. Eur. J. Pharmacol. 1985, 113, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Wein, A.J. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol. Rev. 2004, 56, 581–631. [Google Scholar] [CrossRef] [PubMed]
- Downie, J.W.; Karmazyn, M. Mechanical trauma to bladder epithelium liberates prostanoids which modulate neurotransmission in rabbit detrusor muscle. J. Pharmacol. Exp. Ther. 1984, 230, 445–449. [Google Scholar] [PubMed]
- Lecci, A.; Birder, L.A.; Meini, S.; Catalioto, R.M.; Tramontana, M.; Giuliani, S.; Criscuoli, M.; Maggi, C.A. Pharmacological evaluation of the role of cyclooxygenase isoenzymes on the micturition reflex following experimental cystitis in rats. Br. J. Pharmacol. 2000, 130, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Dobrek, Ł.; Thor, P.J. The role of prostanoids in the urinary bladder function and a potential use of prostanoid-targeting pharmacological agents in bladder overactivity treatment. Acta Pol. Pharm. 2015, 72, 13–19. [Google Scholar] [PubMed]
- Andersson, K.E. Prostanoid receptor subtypes: New targets for OAB drugs? J. Urol. 2009, 182, 2099–2100. [Google Scholar] [CrossRef]
- Rahnama’i, M.S.; van Koeveringe, G.A.; Essers, P.B.; de Wachter, S.G.; de Vente, J.; van Kerrebroeck, P.E.; Gillespie, J.I. Prostaglandin receptor EP1 and EP2 site in guinea pig bladder urothelium and lamina propria. J. Urol. 2010, 183, 1241–1247. [Google Scholar] [CrossRef]
- Norel, X.; Sugimoto, Y.; Ozen, G.; Abdelazeem, H.; Amgoud, Y.; Bouhadoun, A.; Bassiouni, W.; Goepp, M.; Mani, S.; Manikpurage, H.D.; et al. International Union of Basic and Clinical Pharmacology. CIX. Differences and Similarities between Human and Rodent Prostaglandin E(2) Receptors (EP1-4) and Prostacyclin Receptor (IP): Specific Roles in Pathophysiologic Conditions. Pharmacol. Rev. 2020, 72, 910–968. [Google Scholar] [CrossRef]
- Hou, R.; Yu, Y.; Jiang, J. PGE2 receptors in detrusor muscle: Drugging the undruggable for urgency. Biochem. Pharmacol. 2021, 184, 114363. [Google Scholar] [CrossRef]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.F.; Jones, R.L.; Narumiya, S. International Union of Basic and Clinical Pharmacology. LXXXIII: Classification of prostanoid receptors, updating 15 years of progress. Pharmacol. Rev. 2011, 63, 471–538. [Google Scholar] [CrossRef]
- Andersson, K.E.; Fry, C.; Panicker, J.; Rademakers, K. Which molecular targets do we need to focus on to improve lower urinary tract dysfunction? ICI-RS 2017. Neurourol. Urodyn 2018, 37, S117–S126. [Google Scholar] [CrossRef]
- Heppner, T.J.; Fallon, H.J.; Rengo, J.L.; Beaulieu, E.M.; Hennig, G.W.; Nelson, M.T.; Herrera, G.M. Urothelium-derived prostanoids enhance contractility of urinary bladder smooth muscle and stimulate bladder afferent nerve activity in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 327, R97–R108. [Google Scholar] [CrossRef]
- Ishizuka, O.; Mattiasson, A.; Andersson, K.E. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: Involvement of tachykinins? J. Urol. 1995, 153, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Stromberga, Z.; Chess-Williams, R.; Moro, C. The five primary prostaglandins stimulate contractions and phasic activity of the urinary bladder urothelium, lamina propria and detrusor. BMC Urol. 2020, 20, 48. [Google Scholar] [CrossRef]
- Stromberga, Z.; Chess-Williams, R.; Moro, C. Prostaglandin E2 and F2alpha Modulate Urinary Bladder Urothelium, Lamina Propria and Detrusor Contractility via the FP Receptor. Front. Physiol. 2020, 11, 705. [Google Scholar] [CrossRef]
- Ferguson, D.R.; Kennedy, I.; Burton, T.J. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J. Physiol. 1997, 505, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Beckel, J.M.; Daugherty, S.L.; Tyagi, P.; Wolf-Johnston, A.S.; Birder, L.A.; Mitchell, C.H.; de Groat, W.C. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J. Physiol. 2015, 593, 1857–1871. [Google Scholar] [CrossRef]
- Durnin, L.; Hayoz, S.; Corrigan, R.D.; Yanez, A.; Koh, S.D.; Mutafova-Yambolieva, V.N. Urothelial purine release during filling of murine and primate bladders. Am. J. Physiol. Ren. Physiol. 2016, 311, F708–F716. [Google Scholar] [CrossRef] [PubMed]
- Mutafova-Yambolieva, V.N. Mechanosensitive release of ATP in the urinary bladder mucosa. Purinergic Signal. 2024. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C.; Yoshimura, N. Afferent nerve regulation of bladder function in health and disease. Handb. Exp. Pharmacol. 2009, 194, 91–138. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 2014, 10, 103–155. [Google Scholar]
- Sun, Y.; Keay, S.; De Deyne, P.G.; Chai, T.C. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J. Urol. 2001, 166, 1951–1956. [Google Scholar] [CrossRef]
- Mansfield, K.J.; Hughes, J.R. Effect of inflammatory mediators on ATP release of human urothelial RT4 cells. Biomed. Res. Int. 2014, 2014, 182862. [Google Scholar] [CrossRef]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Dayton, J.; Perrino, B.A.; Mutafova-Yambolieva, V.N. Mechanosensitive Hydrolysis of ATP and ADP in Lamina Propria of the Murine Bladder by Membrane-Bound and Soluble Nucleotidases. Front. Physiol. 2022, 13, 918100. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Cruz, A.; Aresta Branco, M.S.L.; Perrino, B.A.; Sanders, K.M.; Mutafova-Yambolieva, V.N. Urinary ATP Levels Are Controlled by Nucleotidases Released from the Urothelium in a Regulated Manner. Metabolites 2022, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Jia, Z.; Kong, X.; Pi, G.; Ma, S.; Yang, J. Effects of PGE2 EP3/EP4 receptors on bladder dysfunction in mice with experimental autoimmune encephalomyelitis. Am. J. Physiol. Ren. Physiol. 2013, 305, F1656–F1662. [Google Scholar] [CrossRef] [PubMed]
- Abramovitz, M.; Adam, M.; Boie, Y.; Carrière, M.; Denis, D.; Godbout, C.; Lamontagne, S.; Rochette, C.; Sawyer, N.; Tremblay, N.M.; et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta 2000, 1483, 285–293. [Google Scholar] [CrossRef]
- af Forselles, K.J.; Root, J.; Clarke, T.; Davey, D.; Aughton, K.; Dack, K.; Pullen, N. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP2 receptor antagonist. Br. J. Pharmacol. 2011, 164, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Juteau, H.; Gareau, Y.; Labelle, M.; Sturino, C.F.; Sawyer, N.; Tremblay, N.; Lamontagne, S.; Carrière, M.C.; Denis, D.; Metters, K.M. Structure-activity relationship of cinnamic acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg Med. Chem. 2001, 9, 1977–1984. [Google Scholar] [CrossRef]
- Machwate, M.; Harada, S.; Leu, C.T.; Seedor, G.; Labelle, M.; Gallant, M.; Hutchins, S.; Lachance, N.; Sawyer, N.; Slipetz, D.; et al. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2). Mol. Pharmacol. 2001, 60, 36–41. [Google Scholar] [CrossRef]
- Guan, N.N.; Svennersten, K.; de Verdier, P.J.; Wiklund, N.P.; Gustafsson, L.E. Prostaglandin D(2) effects and DP(1)/DP(2) receptor distribution in guinea pig urinary bladder out-flow region. J. Cell Mol. Med. 2017, 21, 234–243. [Google Scholar] [CrossRef]
- Guan, N.N.; Nilsson, K.F.; Wiklund, P.N.; Gustafsson, L.E. Release and inhibitory effects of prostaglandin D2 in guinea pig urinary bladder and the role of urothelium. Biochim. Biophys. Acta 2014, 1840, 3443–3451. [Google Scholar] [CrossRef]
- Shichijo, M.; Arimura, A.; Hirano, Y.; Yasui, K.; Suzuki, N.; Deguchi, M.; Abraham, W.M. A prostaglandin D2 receptor antagonist modifies experimental asthma in sheep. Clin. Exp. Allergy 2009, 39, 1404–1414. [Google Scholar] [CrossRef]
- Pettipher, R.; Vinall, S.L.; Xue, L.; Speight, G.; Townsend, E.R.; Gazi, L.; Whelan, C.J.; Armer, R.E.; Payton, M.A.; Hunter, M.G. Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. J. Pharmacol. Exp. Ther. 2012, 340, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, A.R.; Wang, Y.; Johnson, M.G.; Su, Y.; Shen, W.; Wang, X.; Lively, S.; Brown, M.; Lai, S.; et al. Discovery of AMG 853, a CRTH2 and DP Dual Antagonist. ACS Med. Chem. Lett. 2011, 2, 326–330. [Google Scholar] [CrossRef]
- Sharif, N.A.; Klimko, P.G. Prostaglandin FP receptor antagonists: Discovery, pharmacological characterization and therapeutic utility. Br. J. Pharmacol. 2019, 176, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- Bley, K.R.; Bhattacharya, A.; Daniels, D.V.; Gever, J.; Jahangir, A.; O’Yang, C.; Smith, S.; Srinivasan, D.; Ford, A.P.; Jett, M.F. RO1138452 and RO3244794: Characterization of structurally distinct, potent and selective IP (prostacyclin) receptor antagonists. Br. J. Pharmacol. 2006, 147, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Fiddler, G.I.; Lumley, P. Preliminary clinical studies with thromboxane synthase inhibitors and thromboxane receptor blockers. A review. Circulation 1990, 81, I69–I78, discussion I79–I80. [Google Scholar]
- Birder, L.; Andersson, K.E. Urothelial signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef] [PubMed]
- Sellers, D.; Chess-Williams, R.; Michel, M.C. Modulation of lower urinary tract smooth muscle contraction and relaxation by the urothelium. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 675–694. [Google Scholar] [CrossRef]
- Lips, K.S.; Wunsch, J.; Zarghooni, S.; Bschleipfer, T.; Schukowski, K.; Weidner, W.; Wessler, I.; Schwantes, U.; Koepsell, H.; Kummer, W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur. Urol. 2007, 51, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Beckel, J.M.; Barbadora, S.; Kanai, A.J.; de Groat, W.C.; Birder, L.A. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007, 80, 2298–2302. [Google Scholar] [CrossRef]
- Birder, L.A.; Nealen, M.L.; Kiss, S.; de Groat, W.C.; Caterina, M.J.; Wang, E.; Apodaca, G.; Kanai, A.J. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J. Neurosci. 2002, 22, 8063–8070. [Google Scholar] [CrossRef] [PubMed]
- Arms, L.; Vizzard, M.A. Neuropeptides in lower urinary tract function. Handb. Exp. Pharmacol. 2011, 2011, 395–423. [Google Scholar]
- Saban, R.; Franz, J.; Bjorling, D.E. Spontaneously released substance P and bradykinin from isolated guinea-pig bladder. Br. J. Urol. 1997, 79, 516–524. [Google Scholar] [CrossRef]
- Kim, J.C.; Park, E.Y.; Hong, S.H.; Seo, S.I.; Park, Y.H.; Hwang, T.K. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int. J. Urol. 2005, 12, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ochodnický, P.; Michel, M.B.; Butter, J.J.; Seth, J.; Panicker, J.N.; Michel, M.C. Bradykinin modulates spontaneous nerve growth factor production and stretch-induced ATP release in human urothelium. Pharmacol. Res. 2013, 70, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Stenqvist, J.; Aronsson, P.; Carlsson, T.; Winder, M.; Tobin, G. In vivo paracrine effects of ATP-induced urothelial acetylcholine in the rat urinary bladder. Auton. Neurosci. 2020, 227, 102689. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Strater, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Borhani Peikani, M.; Mutafova-Yambolieva, V.N. Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 7322. [Google Scholar] [CrossRef]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Peri, L.E.; Mutafova-Yambolieva, V.N. The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 9964. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Cruz, A.; Aresta Branco, M.S.L.; Borhani Peikani, M.; Mutafova-Yambolieva, V.N. Differential Influences of Endogenous and Exogenous Sensory Neuropeptides on the ATP Metabolism by Soluble Ectonucleotidases in the Murine Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 15650. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998, 30, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Vemulakonda, V.M.; Kiss, S.; Boone, T.B.; Somogyi, G.T. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: Effect of botulinum toxin A. Neurochem. Int. 2005, 47, 291–297. [Google Scholar] [CrossRef]
- Nile, C.J.; de Vente, J.; Gillespie, J.I. Stretch independent regulation of prostaglandin E(2) production within the isolated guinea-pig lamina propria. BJU Int. 2010, 105, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Durnin, L.; Kwok, B.; Kukadia, P.; McAvera, R.; Corrigan, R.D.; Ward, S.M.; Zhang, Y.; Chen, Q.; Koh, S.D.; Sanders, K.M.; et al. An ex vivo bladder model with detrusor smooth muscle removed to analyse biologically active mediators released from the suburothelium. J. Physiol. 2019, 597, 1467–1485. [Google Scholar] [CrossRef]
- Jackson, E.K.; Gillespie, D.G.; Cheng, D.; Mi, Z.; Menshikova, E.V. Characterization of the N(6)-etheno-bridge method to assess extracellular metabolism of adenine nucleotides: Detection of a possible role for purine nucleoside phosphorylase in adenosine metabolism. Purinergic Signal. 2020, 16, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Morita, Y.; Sakai, E.; Yatomi, Y.; Kurano, M. Modulations of urinary lipid mediators in acute bladder cystitis. Prostaglandins Other Lipid Mediat. 2023, 164, 106690. [Google Scholar] [CrossRef] [PubMed]
- Kuga, N.; Tanioka, A.; Hagihara, K.; Kawai, T. Modulation of afferent nerve activity by prostaglandin E2 upon urinary bladder distension in rats. Exp. Physiol. 2016, 101, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Hirata, T.; Narumiya, S. Prostanoid receptors. Chem. Rev. 2011, 111, 6209–6230. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, J.Y.; Mikhailidis, D.P.; Dandona, P. The rat urinary bladder produces prostacyclin as well as other prostaglandins. Prostaglandins Leukot. Med. 1984, 16, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Kuo, H.C. Usefulness of Urinary Biomarkers for Assessing Bladder Condition and Histopathology in Patients with Interstitial Cystitis/Bladder Pain Syndrome. Int. J. Mol. Sci. 2022, 23, 1244. [Google Scholar] [CrossRef] [PubMed]
- Odom, M.R.; Hughes, F.M., Jr.; Pope, N.; Jin, H.; Purves, J.T. Female Type 1 Diabetic Akita Mice Demonstrate Increased Bladder Contractility via FP Receptor Activation due to NLRP3-Mediated Inflammation. Front. Biosci. Landmark 2024, 29, 154. [Google Scholar] [CrossRef] [PubMed]
- Gilman, K.E.; Limesand, K.H. The complex role of prostaglandin E(2)-EP receptor signaling in wound healing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R287–R296. [Google Scholar] [CrossRef] [PubMed]
- Abadir, P.M.; Siragy, H.M. Angiotensin type 1 receptor mediates renal production and conversion of prostaglandins E2 to F2α in conscious diabetic rats. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Bradding, P.; Porsbjerg, C.; Côté, A.; Dahlén, S.E.; Hallstrand, T.S.; Brightling, C.E. Airway hyperresponsiveness in asthma: The role of the epithelium. J. Allergy Clin. Immunol. 2024, 153, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Marone, G.; Galli, S.J.; Varricchi, G. Mast cells: A novel therapeutic avenue for cardiovascular diseases? Cardiovasc. Res. 2024, 120, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, J.M.; Ulven, T.; Martini, L.; Gerlach, L.O.; Heinemann, A.; Kostenis, E. Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol. Pharmacol. 2005, 68, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, S.F.; Lemberg, M.K.; Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 2018, 37, e99456. [Google Scholar] [CrossRef] [PubMed]
- Pratico, D.; Lawson, J.A.; Rokach, J.; FitzGerald, G.A. The isoprostanes in biology and medicine. Trends Endocrinol. Metab. 2001, 12, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, M.; Hogger, P. Oxidative stress and 8-iso-prostaglandin F2a induce ectodomain shedding of CD163 and release of tumor necrosis factor-a from human monocytes. Free Radic. Biol. Med. 2005, 39, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Durnin, L.; Corrigan, R.D.; Sanders, K.M.; Mutafova-Yambolieva, V.N. A Decentralized (Ex Vivo) Murine Bladder Model with the Detrusor Muscle Removed for Direct Access to the Suburothelium during Bladder Filling. J. Vis. Exp. 2019, 153, e60344. [Google Scholar] [CrossRef]
- Bobalova, J.; Bobal, P.; Mutafova-Yambolieva, V.N. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Anal. Biochem. 2002, 305, 269–276. [Google Scholar] [CrossRef]
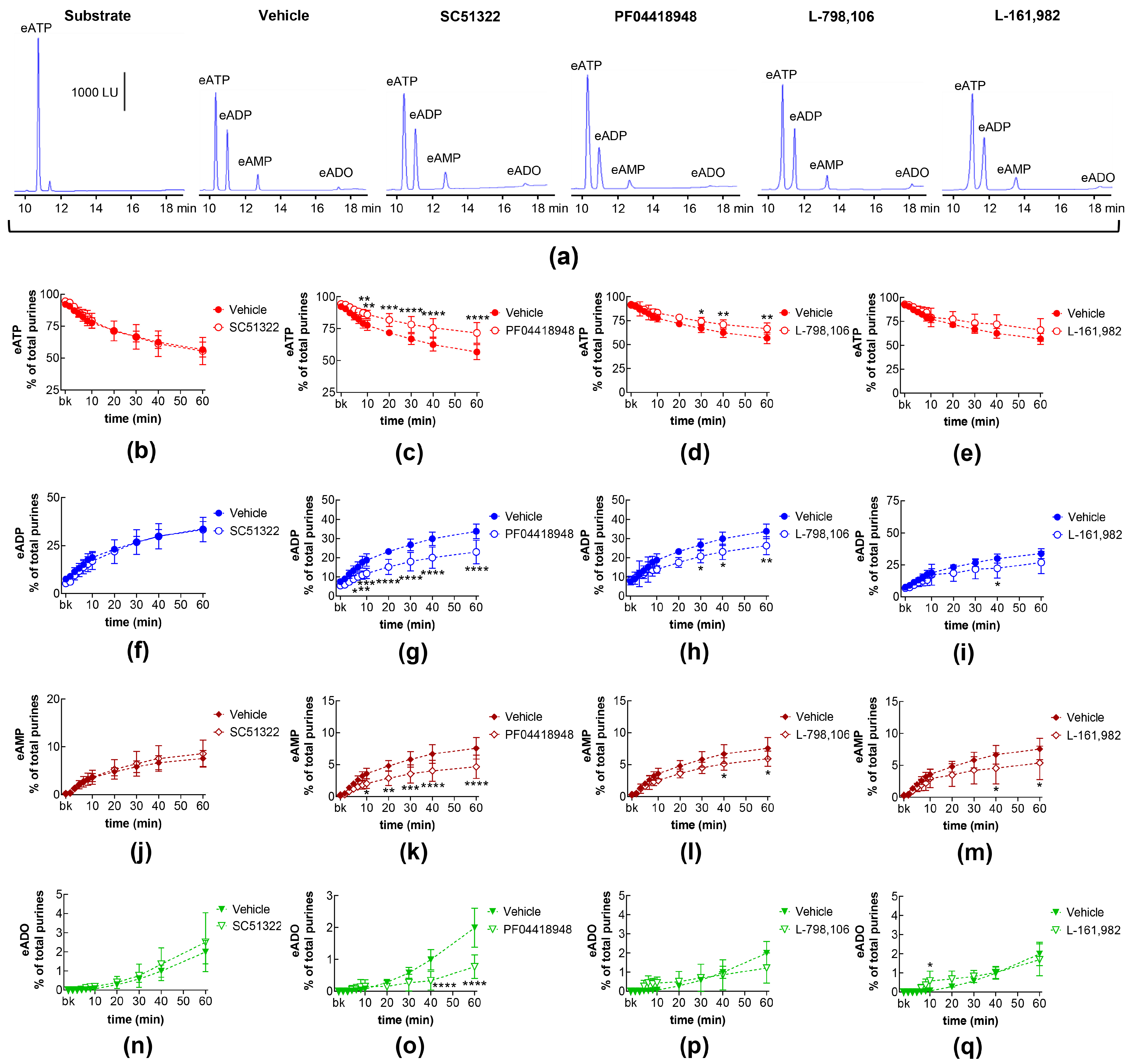
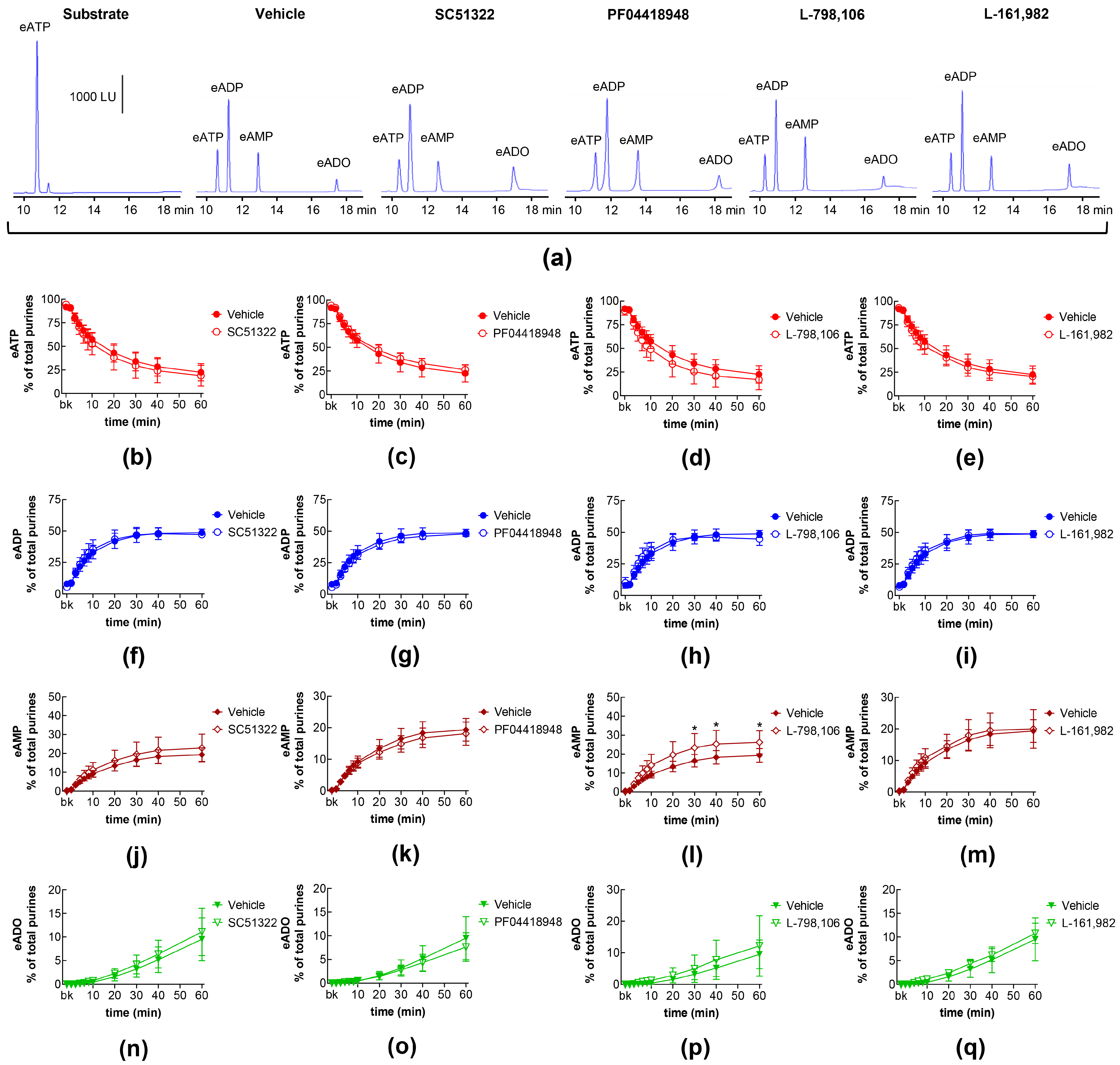
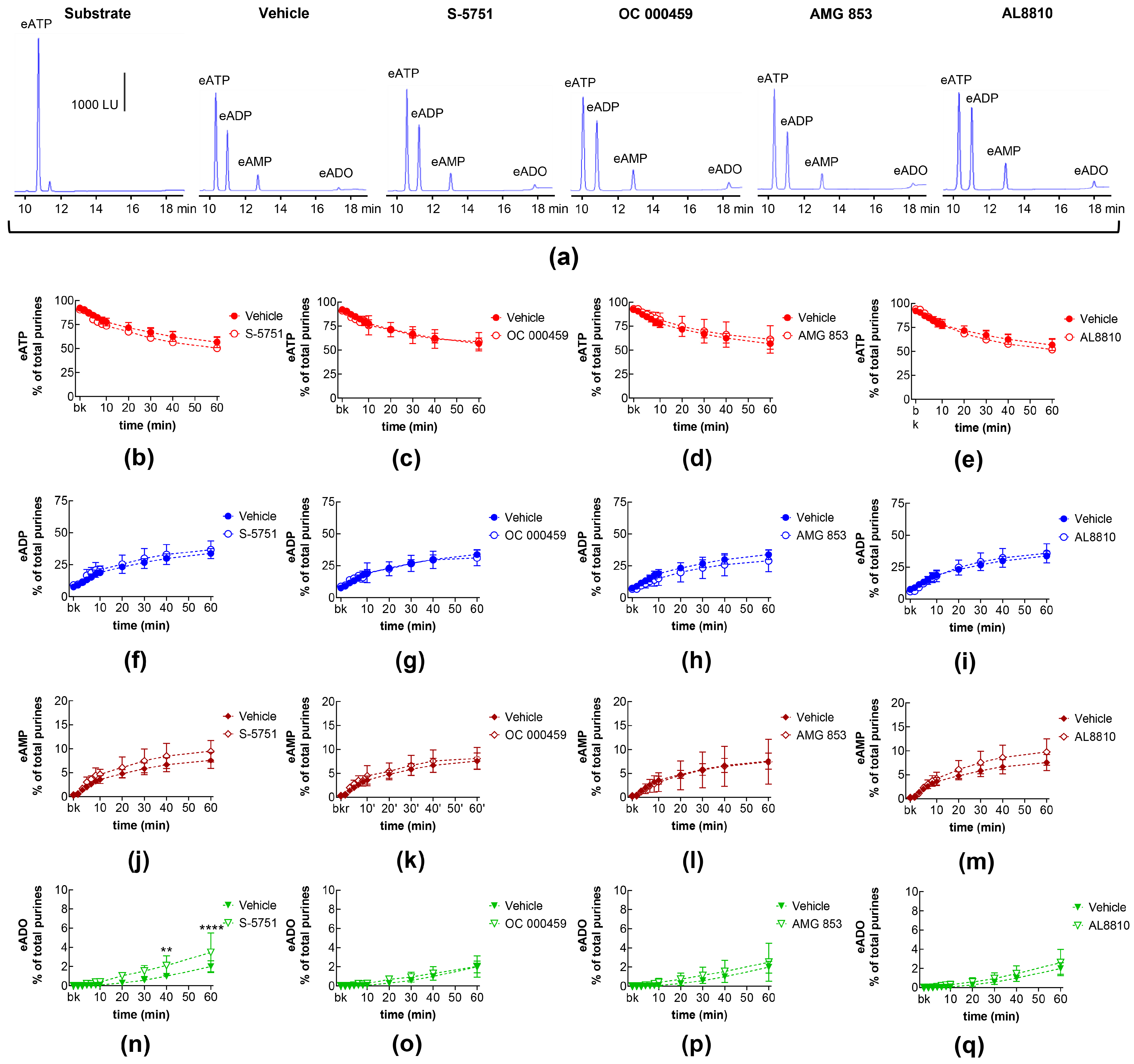
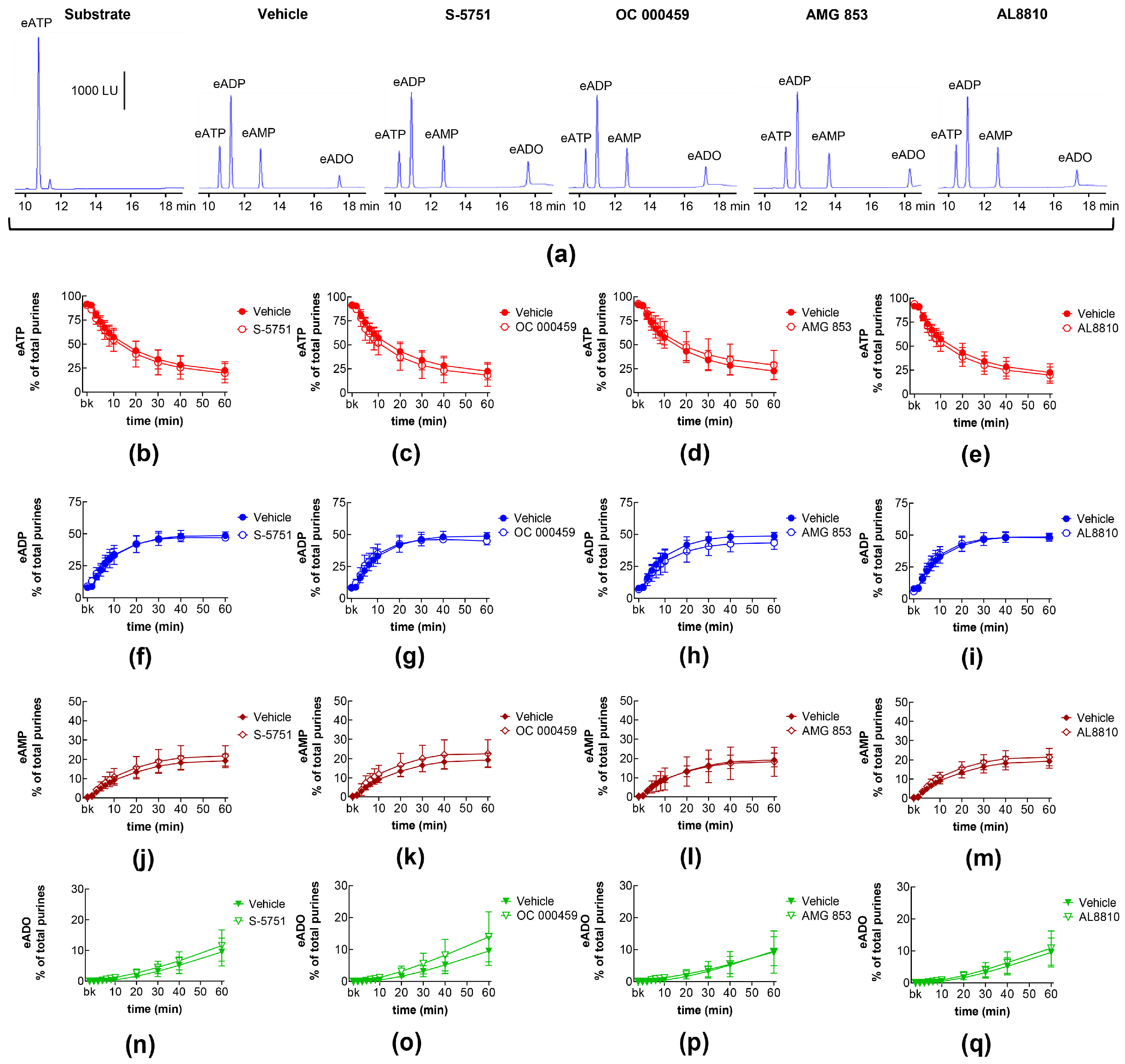
| Name | Type | Concentration (μM) | Vehicle |
|---|---|---|---|
| PGE2 | EP1-4 receptor agonist | 10 | DMSO |
| PGD2 | DP1, DP2 receptor agonist | 10 | DMSO |
| PGF2α | FP receptor agonist | 10 | DMSO |
| PGI2 | IP receptor agonist | 10 | DMSO |
| U46619 | TP (TXA2) receptor agonist | 10 | DMSO |
| SC51322 | EP1 receptor antagonist | 1 | DMSO |
| PF04418948 | EP2 receptor antagonist | 1 | DMSO |
| L-798,106 | EP3 receptor antagonist | 0.25 | DMSO |
| L-161,982 | EP4 receptor antagonist | 1 | DMSO |
| S-5751 | DP1 receptor antagonist | 1 | DMSO |
| OC000459 | DP2 receptor antagonist | 10 | DMSO |
| AMG 853 | DP1/DP2 receptor antagonist | 1 | DMSO |
| AL8810 | FP receptor antagonist | 10 | DMSO |
| RO1138452 | IP receptor antagonist | 10 | DMSO |
| Indomethacin | COX1/COX2 inhibitor | 10 | DMSO |
| Vehicle (n = 6) | PGI2 (n = 4) | RO1138452 (n = 6) | RO1138452 + PGI2 (n = 4) | U46619 (n = 4) | Indomethacin (n = 4) | |
|---|---|---|---|---|---|---|
| eATP | 56.67 ± 5.8 | 59.69 ± 8.16 | 54.39 ± 16.58 | 55.79 ± 10.63 | 53.63 ± 10.26 | 57.52 ± 11.21 |
| eADP | 33.79 ± 3.79 | 31.35 ± 5.08 | 34.71 ± 10.33 | 33.78 ± 7.12 | 35.24 ± 5.47 | 32.40 ± 7.79 |
| eAMP | 7.55 ± 1.69 | 6.39 ± 0.92 | 8.12 ± 3.62 | 7.94 ± 2.64 | 8.40 ± 2.18 | 9.00 ± 2.66 |
| eADO | 1.99 ± 0.62 | 2.57 ± 2.17 | 2.77 ± 2.83 | 2.49 ± 1.02 | 2.74 ± 2.78 | 1.08 ± 1.00 **** |
|
Vehicle (n = 6) |
PGI2 (n = 4) |
RO1138452 (n = 6) |
RO1138452 + PGI2 (n = 4) |
U46619 (n = 4) |
Indomethacin (n = 4) | |
|---|---|---|---|---|---|---|
| eATP | 22.53 ± 9.09 | 27.53 ± 11.58 | 13.53 ± 9.38 | 23.32 ± 12.80 | 19.42 ± 8.00 | 30.70 ± 13.90 |
| eADP | 48.63 ± 2.83 | 47.41 ± 2.70 | 45.86 ± 3.37 | 46.77 ± 2.42 | 47.46 ± 2.16 | 46.37 ± 5.84 |
| eAMP | 19.33 ± 3.60 | 15.53 ± 2.17 | 21.14 ± 5.78 | 20.25 ± 8.58 | 21.56 ± 2.98 | 18.32 ± 4.99 |
| eADO | 9.50 ± 4.55 | 9.23 ± 7.80 | 19.47 ± 12.32 *** | 9.66 ± 5.70 | 11.56 ± 8.64 | 4.61 ± 3.33 **** |
| PGE2 | PGF2α | PGD2 | PGI2 | |
|---|---|---|---|---|
| Nondistended LP | ↑ EP2, ↑ EP3 | → | → | → |
| Distended LP | → | → | → | ↓ IP |
| PGE2 | PGF2α | PGD2 | PGI2 | TXA2 | |
|---|---|---|---|---|---|
| Nondistended LP | ↑ EP3, ↑ EP4, ↑ FP | ↑ FP | ↑ DP2 | → | → |
| Distended LP | ↑ EP1, ↑ EP2, ↑ EP3, ↑ EP4, ↑ FP | → | ↑ DP1, ↑ DP2, ↓“X” | → | → |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez Cruz, A.; Borhani Peikani, M.; Beaulac, T.D.; Mutafova-Yambolieva, V.N. Prostaglandins Differentially Regulate the Constitutive and Mechanosensitive Release of Soluble Nucleotidases in the Urinary Bladder Mucosa. Int. J. Mol. Sci. 2025, 26, 131. https://doi.org/10.3390/ijms26010131
Gutierrez Cruz A, Borhani Peikani M, Beaulac TD, Mutafova-Yambolieva VN. Prostaglandins Differentially Regulate the Constitutive and Mechanosensitive Release of Soluble Nucleotidases in the Urinary Bladder Mucosa. International Journal of Molecular Sciences. 2025; 26(1):131. https://doi.org/10.3390/ijms26010131
Chicago/Turabian StyleGutierrez Cruz, Alejandro, Mahsa Borhani Peikani, Tori D. Beaulac, and Violeta N. Mutafova-Yambolieva. 2025. "Prostaglandins Differentially Regulate the Constitutive and Mechanosensitive Release of Soluble Nucleotidases in the Urinary Bladder Mucosa" International Journal of Molecular Sciences 26, no. 1: 131. https://doi.org/10.3390/ijms26010131
APA StyleGutierrez Cruz, A., Borhani Peikani, M., Beaulac, T. D., & Mutafova-Yambolieva, V. N. (2025). Prostaglandins Differentially Regulate the Constitutive and Mechanosensitive Release of Soluble Nucleotidases in the Urinary Bladder Mucosa. International Journal of Molecular Sciences, 26(1), 131. https://doi.org/10.3390/ijms26010131






