The SOS Response Activation and the Risk of Antibiotic Resistance Enhancement in Proteus spp. Strains Exposed to Subinhibitory Concentrations of Ciprofloxacin
Abstract
1. Introduction
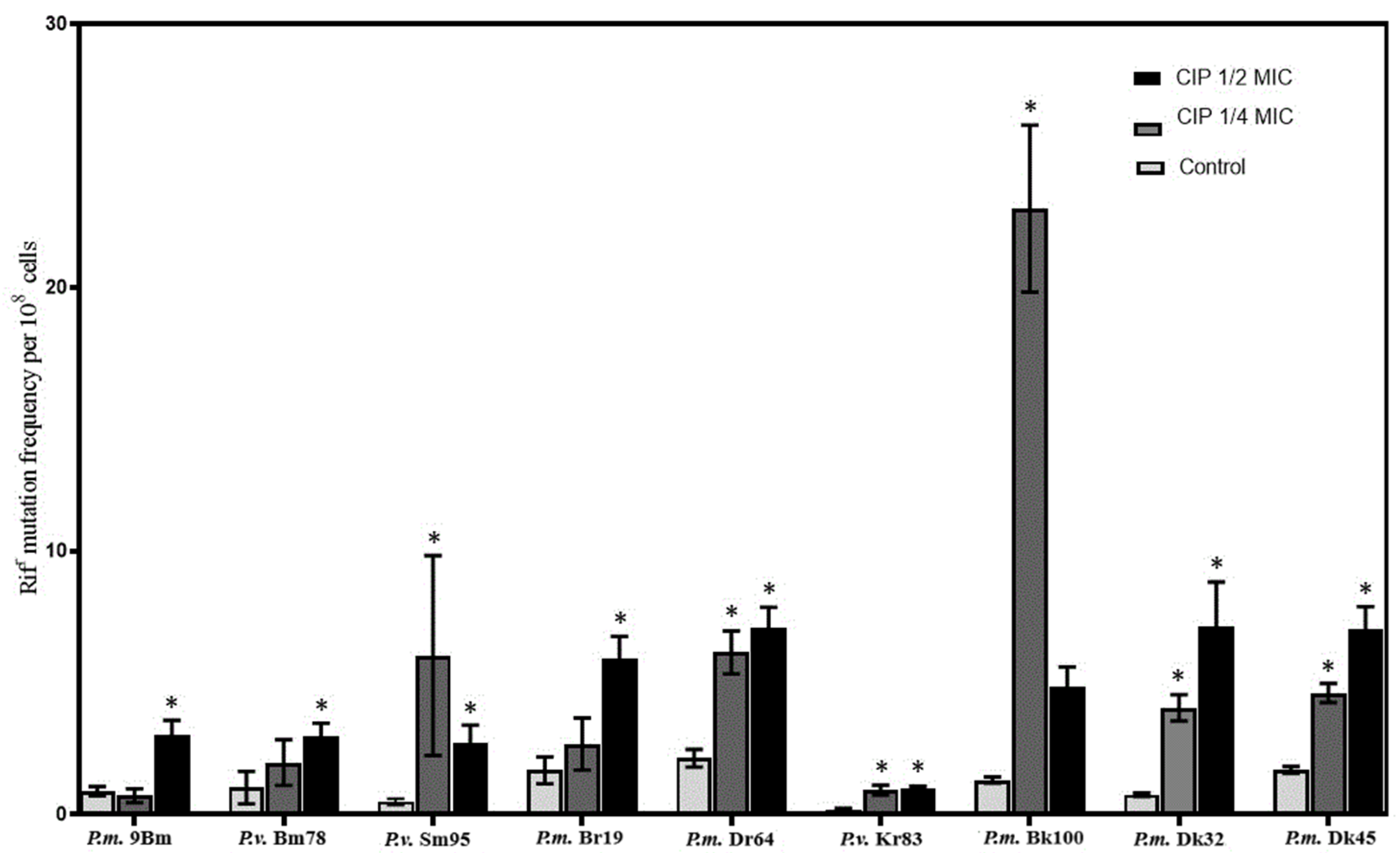
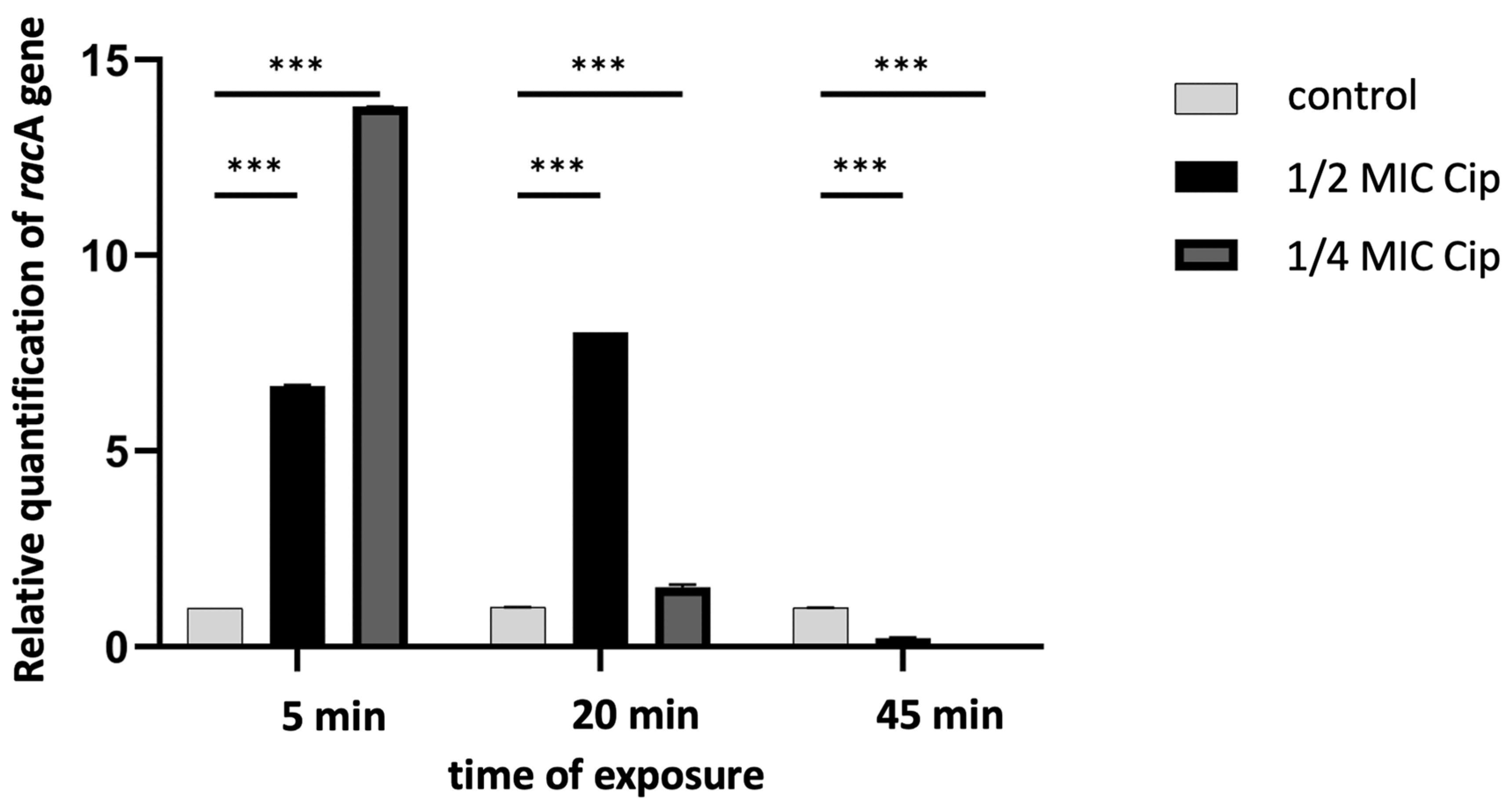
2. Results and Discussion
3. Materials and Methods
3.1. Bacterial Strains
3.2. Assessment of the Antibiotics Susceptibility
3.3. Culture Conditions for Laboratory Variants Obtaining
3.4. Determination of Mutation Frequency
3.5. RNA Isolation and Quantitative Polymerase Chain (qPCR) Analysis
3.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lederberg, J.; Lederberg, E.M. Replica plating and indirect selection of bacterial mutations. J. Bacteriol. 1952, 63, 399–406. [Google Scholar] [CrossRef]
- Rodriguez-Rojas, A.; Rodriguez-Beltran, J.; Couce, A.; Blazquez, J. Antibiotics and antibiotic resistance: A bitter fight against evolution. Int. J. Med. Microbiol. 2013, 303, 293–297. [Google Scholar] [CrossRef]
- Pereira, C.; Larsson, J.; Hjort, K.; Elf, J.; Andersson, D.I. The highly dynamic nature of bacterial heteroresistance impairs its clinical detection. Commun. Biol. 2021, 4, 521. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J.; Lederberg, J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Moll. Cell 2012, 46, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Couce, A.; Blazquez, J. Side effects of antibiotics on genetic variability. FEMS Microbiol. Rev. 2009, 33, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Jolivet-Gougeon, A.; Kovacs, B.; Le Gall-David, S.; Le Bars, H.; Bousarghin, L.; Bonnaure-Mallet, M.; Lobel, B.; Guille, F.; Soussy, C.J.; Tenke, P. Bacterial hypermutation: Clinical implications. J. Med. Microbiol. 2011, 60, 563–573. [Google Scholar] [CrossRef]
- Joseph, A.M.; Badrinarayanan, A. Visualizing mutagenic repair: Novel insights into bacterial translesion synthesis. FEMS Microbiol. Rev. 2020, 44, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Goh, E.B.; Yim, G.; Tsui, W.; McClure, J.; Surette, M.G.; Davies, J. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci. USA 2002, 99, 17025–17030. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Small molecules: The lexicon of biodiversity. J. Biotechnol. 2007, 129, 3–5. [Google Scholar] [CrossRef]
- Baharoglu, Z.; Mazel, D. Vibrio cholerae triggers SOS and mutagenesis in response to a wide range of antibiotics: A route towards multiresistance. Antimicrob. Agents Chemother. 2011, 55, 2438–2441. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Ryan, K.S. Introducing the parvome: Bioactive compounds in the microbial word. ACS Chem. Biol. 2012, 7, 252–259. [Google Scholar] [CrossRef]
- Laureti, L.; Matic, I.; Gutierrez, A. Bacterial responses and genome instability induced by subinhibitory concentrations of antibiotics. Antibiotics 2013, 2, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barceló, C.; Kojadinovic, M.; Moxon, R.; MacLean, R.C. The SOS response increases bacterial fitness, but not evolvability, under a sublethal dose of antibiotic. Proc. Biol. Sci. 2015, 282, 20150885. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.; Basu, S.; Dickens, A.; O’Sullivan, D.; McHugh, T. Effect of subinhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates. J. Antimicrob. Chemother. 2005, 56, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Spiegelman, G.B.; Yim, G. The world of subinhibitory concentrations. Curr. Opin. Microbiol. 2006, 9, 445–453. [Google Scholar] [CrossRef]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sub-lethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef]
- Kumari, H.; Balasubramanian, D.; Zincke, D.; Mathee, K. Role of Pseudomonas aeruginosa AmpR on β-lactam and non-β-lactam transient cross-resistance upon pre-exposure to subinhibitory concentrations of antibiotics. J. Med. Microbiol. 2014, 63, 544–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef]
- Drzewiecka, D.; Palusiak, A.; Siwińska, M.; Zabłotni, A. The prevailing O serogroups among the serologically differentiated clinical Proteus spp. strains in central Poland. Sci. Rep. 2021, 11, 18982. [Google Scholar] [CrossRef] [PubMed]
- Coker, C.; Poore, C.A.; Li, X.; Mobley, H.L.T. Pathogenesis of Proteus mirabilis urinary tract infections. Microb. Infect. 2000, 2, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Różalski, A.; Kwil, I.; Torzewska, A.; Baranowska, M.; Stączek, P. Bakterie z rodzaju Proteus—Cechy i czynniki chorobotwórczości. Post. Hig. Med. Dosw. 2007, 61, 204–219. [Google Scholar]
- Jacobsen, S.M.; Shirtliff, M.E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2011, 2, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Stickler, D.J. Clinical complications of urinary catheters caused by crystalline biofilms: Something needs to be done. J. Intern. Med. 2014, 276, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Torzewska, A.; Budzyńska, A.; Białczak-Kokot, M.; Różalski, A. In vitro studies of epithelium-associated crystallization caused by uropathogens during urinary calculi development. Microb. Pathog. 2014, 71–72, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Szczerbiec, D.; Bednarska-Szczepaniak, K.; Torzewska, A. Antibacterial properties and urease suppression ability of Lactobacillus inhibit the development of infectious urinary stones caused by Proteus mirabilis. Sci. Rep. 2024, 14, 943. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef]
- Wistrand-Yuen, E.; Knopp, M.; Hjort, K.; Koskiniemi, S.; Berg, O.G.; Andersson, D.I. Evolution of high-level resistance during low-level antibiotic exposure. Nat. Commun. 2018, 9, 1599. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Martínez, J.L. Antibiotics as signals that trigger specific bacterial responses. Curr. Opin. Microbiol. 2008, 11, 161–167. [Google Scholar] [CrossRef]
- Ching, C.; Orubu, E.S.F.; Sutradhar, I.; Wirtz, V.J.; Boucher, H.W.; Zaman, M.H. Bacterial antibiotic resistance development and mutagenesis following exposure to subinhibitory concentrations of fluoroquinolones in vitro: A systematic review of the literature. JAC Antimicrob. Resist. 2020, 2, dlaa068. [Google Scholar] [CrossRef]
- Lewin, C.S.; Howard, B.M.; Ratcliffe, N.T.; Smith, J.T. 4-quinolones and the SOS response. J. Med. Microbiol. 1989, 29, 139–144. [Google Scholar] [CrossRef]
- Cirz, R.T.; Jones, M.B.; Gingles, N.A.; Minogue, T.D.; Jarrahi, B.; Peterson, S.N.; Romesberg, F.E. Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J. Bacteriol. 2007, 189, 531–539. [Google Scholar] [CrossRef]
- Thi, T.D.; Lopez, E.; Rodriguez-Rojas, A.; Rodriguez-Beltran, J.; Couce, A.; Guelfo, J.R.; Castaneda-Garcia, A.; Blazquez, J. Effect of recA inactivation on mutagenesis of Escherichia coli exposed to sublethal concentrations of antimicrobials. J. Antimicrob. Chemother. 2011, 66, 531–538. [Google Scholar] [CrossRef]
- Blazquez, J.; Rodriguez-Beltran, J.; Matic, I. Antibiotic-induced Genetic Variation: How it arises and how it can be prevented. Annu. Rev. Microbiol. 2018, 72, 209–230. [Google Scholar] [CrossRef]
- Nagel, M.; Reuter, T.; Jansen, A.; Szekat, C.; Bierbaum, G. Influence of ciprofloxacin and vancomycin on mutation rate and transposition of IS256 in Staphylococcus aureus. Int. J. Med. Microbiol. 2011, 301, 229–236. [Google Scholar] [CrossRef]
- Song, L.Y.; Goff, M.; Davidian, C.; Mao, Z.; London, M.; Lam, K.; Yung, M.; Miller, J.H. Mutational Consequences of Ciprofloxacin in Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 6165–6172. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Evolution of bacterial opportunistic pathogenes. In Evolutionary Biology of Bacterial and Fungal Pathogens, 1st ed.; Baquero, F., Nombela, C., Cassell, G.H., Gutiérrez-Fuentes, J.A., Eds.; ASM Press: Washington, DC, USA, 2008; Volume 9. [Google Scholar]
- Dave, M.; Purohit, T.; Razonable, R.; Loftus, E.V. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm. Bovel Dis. 2014, 20, 196–212. [Google Scholar] [CrossRef]
- Roy, S.; Mukherjee, P.; Kundu, S.; Majumder, D.; Raychaudhuri, V.; Choudhury, L. Microbial infections in burn patients. Acute Crit. Care. 2024, 39, 214–225. [Google Scholar] [CrossRef]
- Dong, G.; Li, J.; Chen, L.; Bi, W.; Zhang, X.; Liu, H.; Zhi, X.; Zhou, T.; Cao, J. Effects of sub-minimum inhibitory concentrations of ciprofloxacin on biofilm formation and virulence factors of Escherichia coli. Braz. J. Infect. Dis. 2019, 23, 15–21. [Google Scholar] [CrossRef]
- Elhosseini, M.A.; El-Banna, T.E.; Sonbol, F.I.; El-Bouseary, M.M. Potential antivirulence activity of sub-inhibitory concentrations of ciprofloxacin against Proteus mirabilis isolates: An in-vitro and in-vivo study. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.J.; Chopra, I. Insertional inactivation of mutS in Staphylococcus aureus reveals potential for elevated mutation frequencies, although the prevalence of mutators in clinical isolates is low. J. Antimicrob. Chemother. 2002, 50, 161–169. [Google Scholar] [CrossRef]
- Henderson-Begg, S.K.; Livermore, D.M.; Hall, L.M. Effect of subinhibitory concentrations of antibiotics on mutation frequency in Streptococcus pneumoniae. J. Antimicrob. Chemother. 2006, 57, 849–854. [Google Scholar] [CrossRef]
- Didier, J.P.; Villet, R.; Huggler, E.; Lew, D.P.; Hooper, D.C.; Kelley, W.L.; Vaudaux, P. Impact of Ciprofloxacin Exposure on Staphylococcus aureus genomic alterations linked with emergence of rifampin resistance. Antimicrob. Agents Chemother. 2011, 55, 1946–1952. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, K.P.; Grinholc, M. Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sublethal treatment. Sci. Rep. 2019, 9, 9423. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, K.; Tomita, H.; Fujimoto, S.; Okuzumi, K.; Ike, Y. Fluoroquinolone enhances the mutation frequency for meropenem-selected carbapenem resistance in Pseudomonas aeruginosa, but use of the high-potency drug doripenem inhibits mutant formation. Antimicrob. Agents Chemother. 2008, 52, 3795–3800. [Google Scholar] [CrossRef]
- Kreuzer, K.N. DNA damage responses in prokaryotes: Regulating gene expression, modulating growth patterns, and manipulating replication forks. Cold Spring Harb. Perspect. Biol. 2013, 5, a012674. [Google Scholar] [CrossRef]
- Cirz, R.T.; Chin, J.K.; Andes, D.R.; de Crécy-Lagard, V.; Craig, W.A.; Romesberg, F.E. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 2005, 3, 1024–1033. [Google Scholar] [CrossRef]
- O’Sullivan, D.M.; Hinds, J.; Butcher, P.D.; Gillespie, S.H.; McHugh, T.D. Mycobacterium tuberculosis DNA repair in response to subinhibitory concentrations of ciprofloxacin. J. Antimicrob. Chemother. 2008, 62, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Mesak, L.R.; Miao, V.; Davies, J. Effect of subinhibitory concentration of antibiotics on SOS and DNA repair gene expression in Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 3394–3397. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.J. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 2007, 13, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Revitt-Mills, S.A.; Robinson, A. Antibiotic-Induced Mutagenesis: Under the Microscope. Front. Microbiol. 2020, 11, 585175. [Google Scholar] [CrossRef] [PubMed]
- Hasan, C.H.; Dutta, D.; Nguyen, A.N.T. Revisiting antibiotic resistance: Mechanistic foundations to evolutionary outlook. Antibiotics 2021, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of a resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.M.; Wassermann, T.; Jensen, P.Ø.; Hengzuang, W.; Molin, S.; Høiby, N.; Ciofu, O. Sublethal ciprofloxacin treatment leads to rapid development of high-level ciprofloxacin resistance during long-term experimental evolution of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Bonnin, R.A.; Dortet, L.; Naas, T. Genetics of acquired antibiotic resistance genes in Proteus spp. Front. Microbiol. 2020, 11, 256. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8, 10.1128. [Google Scholar] [CrossRef]
- Filipiak, A.; Chrapek, M.; Literacka, E.; Wawszczak, M.; Głuszek, S.; Majchrzak, M.; Wróbel, G.; Łysek-Gładysińska, M.; Gniadkowski, M.; Adamus-Białek, W. Pathogenic Factors Correlate With Antimicrobial Resistance Among Clinical Proteus mirabilis Strains. Front. Microbiol. 2020, 11, 579389. [Google Scholar] [CrossRef]
- Mirzaei, A.; Habibi, M.; Bouzari, S.; Asadi Karam, M.R. Characterization of antibiotic-susceptibility patterns, virulence factor profiles and clonal relatedness in Proteus mirabilis isolates from patients with urinary tract infection in Iran. Infect. Drug. Resist. 2019, 12, 3967–3979. [Google Scholar] [CrossRef]
- Literacka, E.; Izdebski, R.; Baraniak, A.; Żabicka, D.; Schneider, A.; Urbanowicz, P.; Herda, M.; Hryniewicz, W.; Gniadkowski, M. Proteus mirabilis Producing the OXA-58 Carbapenemase in Poland. Antimicrob. Agents Chemother. 2019, 63, e00106–e00119. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Gioffrè, M.E.; Chiera, D.; Ferlazzo, M.; Virgà, A.; Laganà, P. Evaluation of antibiotic resistance in Proteus spp.: A growing trend that worries Public Health. Results of 10 Years of Analysis. New Microbiol. 2022, 45, 269–277. [Google Scholar] [PubMed]
- Avrain, L.; Garvey, M.; Mesaros, N.; Glupczynski, Y.; Mingeot-Leclercq, M.P.; Piddock, L.J.V.; Tulkens, P.M.; Vanhoof, R.; Van Bambeke, F. Selection of quinolone resistance in Streptococcus pneumoniae exposed in vitro to subinhibitory drug concentrations. J. Anitimicrob. Chemother. 2007, 60, 965–972. [Google Scholar] [CrossRef][Green Version]
- Aiassa, V.; Barnes, A.I.; Smania, A.M.; Albesa, I. Sublethal ciprofloxacin treatment leads to resistance via antioxidant systems in Proteus mirabilis. FEMS Microbiol. Lett. 2012, 237, 25–31. [Google Scholar] [CrossRef]
- Fernandez, L.; Breidenstein, E.B.M.; Hancock, R.E.W. Creeping baselines and adaptive resistance to antibiotics. Drug Resist. Updat. 2011, 14, 1–21. [Google Scholar] [CrossRef]
- Tattevin, P.; Basuino, L.; Chambers, H.F. Subinhibitory fluoroquinolone exposure selects for reduced beta-lactam susceptibility in methicillin-resistant Staphylococcus aureus and alterations in the SOS-mediated response. Res. Microbiol. 2009, 160, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, L.; Rodríguez, J.C.; Escribano, I.; Royo, G. Effect of exposure to fluoroquinolones and beta-lactams on the in vitro activity of other groups of antibiotics in Salmonella spp. APMIS 2006, 114, 523–528. [Google Scholar] [CrossRef]
- Ching, C.; Zaman, M.H. Development and selection of low-level multi-drug resistance over an extended range of sub-inhibitory ciprofloxacin concentrations in Escherichia coli. Sci. Rep. 2020, 10, 8754. [Google Scholar] [CrossRef]
- Hasan, M.; Wang, J.; Ahn, J. Ciprofloxacin and Tetracycline Resistance Cause Collateral Sensitivity to Aminoglycosides in Salmonella Typhimurium. Antibiotics 2023, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Moryl, M.; Torzewska, A.; Jałmuzna, P.; Rózalski, A. Analysis of Proteus mirabilis distribution in multi-species biofilms on urinary catheters and determination of bacteria resistance to antimicrobial agents. Pol. J. Microbiol. 2013, 62, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Chen, P.C.; Chang, S.C.; Shiau, Y.R.; Wang, H.Y.; Lai, J.F.; Huang, I.W.; Tan, M.C.; Lauderdale, T.L.; TSAR Hospitals. Antimicrobial susceptibilities of Proteus mirabilis: A longitudinal nationwide study from the Taiwan surveillance of antimicrobial resistance (TSAR) program. BMC Infect. Dis. 2014, 14, 486. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Qiu, Y.; Fang, C.; Zhou, Y.; She, J.; Chen, H.; Dai, X.; Zhang, L. Genomic characteristics of clinical multidrug-resistant Proteus isolates from a tertiary care hospital in Southwest China. Front. Microbiol. 2022, 13, 977356. [Google Scholar] [CrossRef] [PubMed]
- Przekwas, J.; Gębalski, J.; Kwiecińska-Piróg, J.; Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E.; Rutkowska, D.; Skowron, K. The effect of fluoroquinolones and antioxidans on biofilm formation by Proteus mirabilis strains. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 22. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed.; CLSI Document M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. (Palo Alto, CA, USA). Statistica (Data Analysis Software System), version 13. 2017. Available online: www.statsoft.pl (accessed on 13 January 2021).
| No. | Strain | MIC of Ciprofloxacin (µg mL−1) | ||
|---|---|---|---|---|
| Clinical Strain | Laboratory Variant | |||
| “¼ MIC” | “½ MIC” | |||
| 1 | P.m. 9Bm | 0.0078 | 0.0312–0.125 | 0.0312–0.0625 |
| 2 | P.v. Bm78 | 0.0312 | 0.0039–0.0078 | 0.0625–0.125 |
| 3 | P.v. Sm95 | 0.0156 | 8–16 | 8–32 |
| 4 | P.m. Br19 | 0.0078 | 0.0312–0.0625 | 0.0312–0.0625 |
| 5 | P.m. Dr64 | 0.0625 | 0.0039–0.0078 | 0.0625 |
| 6 | P.v. Kr83 | 0.0312 | 0.125–0.025 | 0.125–0.5 |
| 7 | P.m. Dk32 | 0.0078 | 0.0156–0.0312 | 0.0156–0.0312 |
| 8 | P.m. Dk45 | 0.0156 | 0.0156 | 0.0625–0.125 |
| 9 | P.m. Bk100 * | 2 | 2 | 2 |
| (a) | |||||||||
| Strain and Their ½ and ¼ MIC Laboratory Variants | ANTIBIOTIC | ||||||||
| Penicillin | Cephalosporin | Aminoglycoside | Quinolone | ||||||
| AMP | AMX | CFL | CFX | CFZ | CFD | GEN | NOR | ||
| P.m. 9Bm | Clinical | 1 | 1 | 4 | 1 | 4 | 1 | 2 | 1 |
| ½ MIC | 256–512 | 128–512 | 256–512 | 256–512 | 256 | 1 | 0.5–0.25 | 8–16 | |
| ¼ MIC | 256–512 | 8–16 | 256–512 | 128–512 | 128 | 1 | 0.5–0.25 | 8–32 | |
| P.v. Sm95 | Clinical | 128 | 16 | 64 | 32 | 512 | 0.015 | 1 | 0.031 |
| ½ MIC | >512 | 16 | 512 | 256–512 | 512 | 1–2 | 256–512 | 32–64 | |
| ¼ MIC | >512 | 2–4 | 512 | 512 | 512 | 0.125–0.25 | 128–512 | 16–32 | |
| P.v. Bm78 | Clinical | 32 | 4 | 32 | 128 | 512 | 0.031 | 0.25 | 0.015 |
| ½ MIC | 256–512 | 4 | 128–256 | 16–32 | 512 | 0.031 | 0.25 | 0.062 | |
| ¼ MIC | 128–256 | 4 | 64–128 | 16–32 | 64 | 0.125–0.25 | 0.25 | 0.062 | |
| (b) | |||||||||
| Strain and Their ½ and ¼ MIC Laboratory Variants | ANTIBIOTIC | ||||||||
| Penicillin | Cephalosporin | Aminoglycoside | Quinolone | ||||||
| AMP | AMX | CFL | CFX | CFZ | CFD | GEN | NOR | ||
| P.m. Br19 | Clinical | 16 | 4 | 8 | 0.031 | 8 | 0.062 | 0.01 | 0.062 |
| ½ MIC | 16 | 4 | 8 | 0.5–2 | 8 | 0.25–1 | 0.5–1 | 0.062 | |
| ¼ MIC | 0.5–2 | 0.5–1 | 0.5–2 | 0.125–1 | 8 | 0.25–0.5 | 0.5–1 | 0.25–0.5 | |
| P.m. Dr64 | Clinical | 1 | 1 | 0.5 | 0.031 | 4 | 0.031 | 1 | 1 |
| ½ MIC | 1 | 0.125–0.5 | 0.5 | 0.5–1 | 0.5–1 | 0.031 | 2–4 | 0.015 | |
| ¼ MIC | 1 | 2–4 | 2–4 | 1–2 | 4 | 0.062–0.25 | 4–8 | 0.015–0.031 | |
| P.v. Kr83 | Clinical | 64 | 2 | 16 | 8 | 16 | 2 | 2 | 0.062 |
| ½ MIC | 64 | 32–128 | 256–512 | 64–128 | 256–512 | 0.031–0.062 | 2 | 8–16 | |
| ¼ MIC | 128–512 | 16–128 | 256–512 | 256–512 | 512 | 0.015–0.062 | 2 | 0.5–1 | |
| (c) | |||||||||
| Strain and Their ½ and ¼ MIC Laboratory Variants | ANTIBIOTIC | ||||||||
| Penicillin | Cephalosporin | Aminoglycoside | Quinolone | ||||||
| AMP | AMX | CFL | CFX | CFZ | CFD | GEN | NOR | ||
| P.m. Dk32 | Clinical | 512 | 128 | 512 | 128 | 256 | 0.062 | 2 | 0.125 |
| ½ MIC | 512 | 128 | 512 | 128 | 256 | 0.015–0.032 | 512 | 0.125 | |
| ¼ MIC | 512 | 4–8 | 512 | 128 | 256 | 0.062 | >512 | 0.125 | |
| P.m. Dk45 | Clinical | 0.5 | 0.5 | 0.062 | 0.015 | 0.125 | 0.015 | 0.5 | 0.062 |
| ½ MIC | 0.062–0.125 | 0.125–0.25 | 0.5–1 | 0.062–0.125 | 0.25–0.5 | 0.015 | 4–8 | 0.062 | |
| ¼ MIC | 0.062–0.125 | 0.062–0.125 | 0.5–1 | 0.062–0.125 | 0.5–1 | 0.031–0.062 | 2–8 | 1–2 | |
| P.m. Bk100 | Clinical | 32 | 4 | 64 | 16 | 16 | 0.031 | 512 | 8 |
| ½ MIC | 64–128 | 16–32 | 64 | 16 | 32–64 | 4–8 | 512 | 8 | |
| ¼ MIC | 128–256 | 4 | 64 | 16 | 16 | 0.031 | 512 | 8 | |
| No. | Strain Species | Strain Name | Source of Isolation |
|---|---|---|---|
| 1 | P. mirabilis | 9Bm | Urine |
| 2 | P. vulgaris | Bm78 | Urine |
| 3 | P. vulgaris | Sm95 | Urine |
| 4 | P. mirabilis | Br19 | Wound |
| 5 | P. mirabilis | Dr64 | Wound |
| 6 | P. vulgaris | Kr83 | Wound |
| 7 | P. mirabilis | Dk32 | Feces |
| 8 | P. mirabilis | Dk45 | Feces |
| 9 | P. mirabilis | Bk100 | Feces |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabłotni, A.; Schmidt, M.; Siwińska, M. The SOS Response Activation and the Risk of Antibiotic Resistance Enhancement in Proteus spp. Strains Exposed to Subinhibitory Concentrations of Ciprofloxacin. Int. J. Mol. Sci. 2025, 26, 119. https://doi.org/10.3390/ijms26010119
Zabłotni A, Schmidt M, Siwińska M. The SOS Response Activation and the Risk of Antibiotic Resistance Enhancement in Proteus spp. Strains Exposed to Subinhibitory Concentrations of Ciprofloxacin. International Journal of Molecular Sciences. 2025; 26(1):119. https://doi.org/10.3390/ijms26010119
Chicago/Turabian StyleZabłotni, Agnieszka, Marek Schmidt, and Małgorzata Siwińska. 2025. "The SOS Response Activation and the Risk of Antibiotic Resistance Enhancement in Proteus spp. Strains Exposed to Subinhibitory Concentrations of Ciprofloxacin" International Journal of Molecular Sciences 26, no. 1: 119. https://doi.org/10.3390/ijms26010119
APA StyleZabłotni, A., Schmidt, M., & Siwińska, M. (2025). The SOS Response Activation and the Risk of Antibiotic Resistance Enhancement in Proteus spp. Strains Exposed to Subinhibitory Concentrations of Ciprofloxacin. International Journal of Molecular Sciences, 26(1), 119. https://doi.org/10.3390/ijms26010119





