Genome-Wide Identification and Expression Analysis of YTH Gene Family for Abiotic Stress Regulation in Camellia chekiangoleosa
Abstract
1. Introduction
2. Results
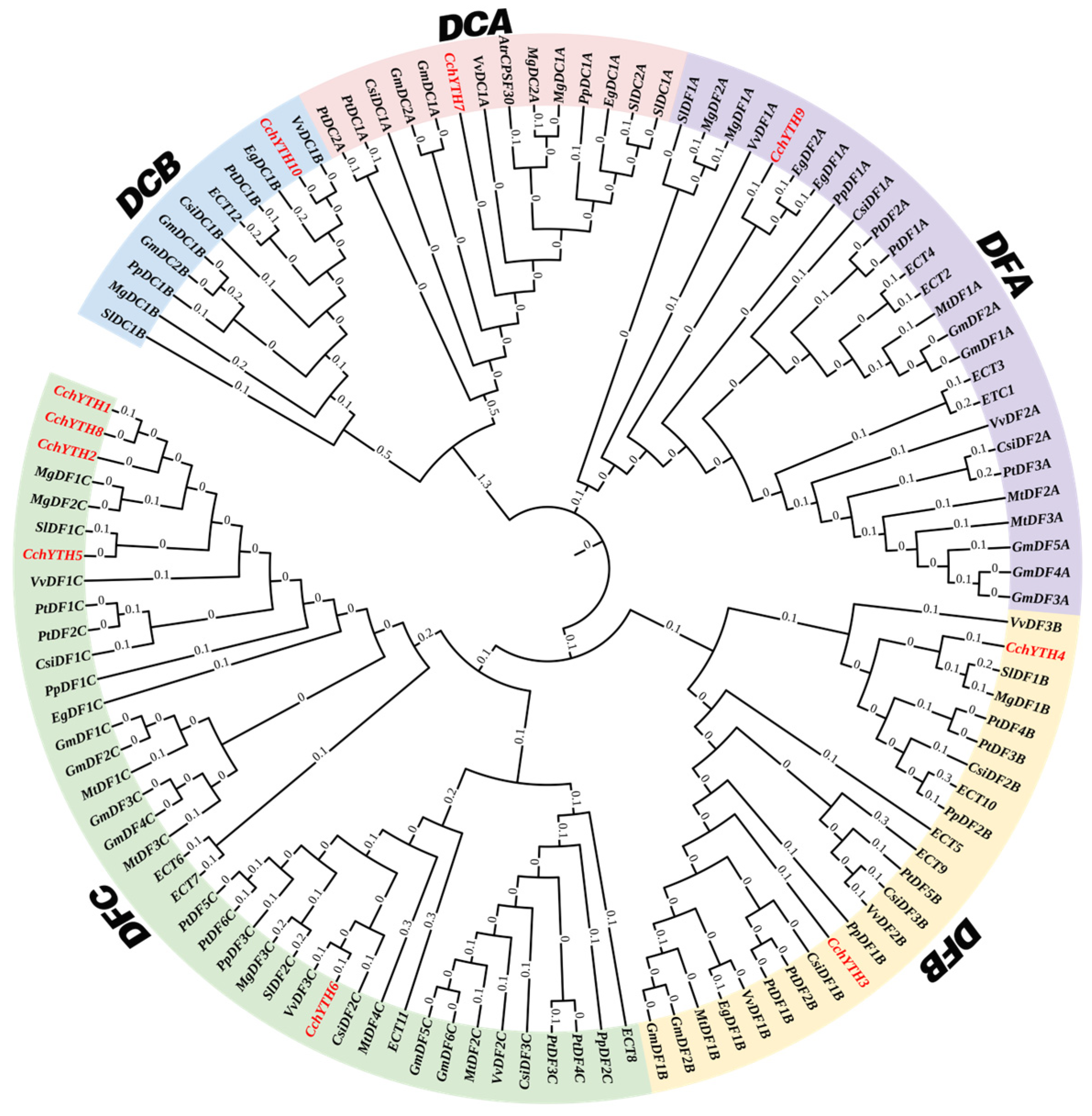
2.1. Genome-Wide Identification, Basic Physicochemical Properties, and Phylogenetic Analysis of CchYTH Genes
2.2. Chromosomal Localization Prediction of CchYTH Genes
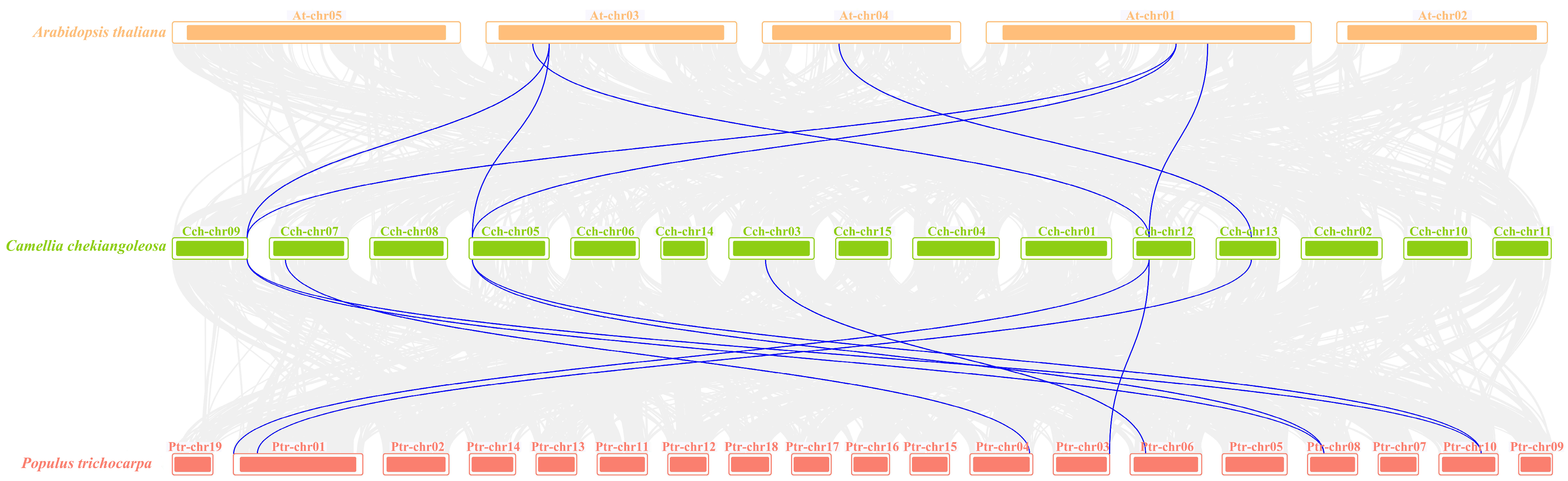
2.3. Collinearity Analysis of the YTH Family in Camellia chekiangoleosa
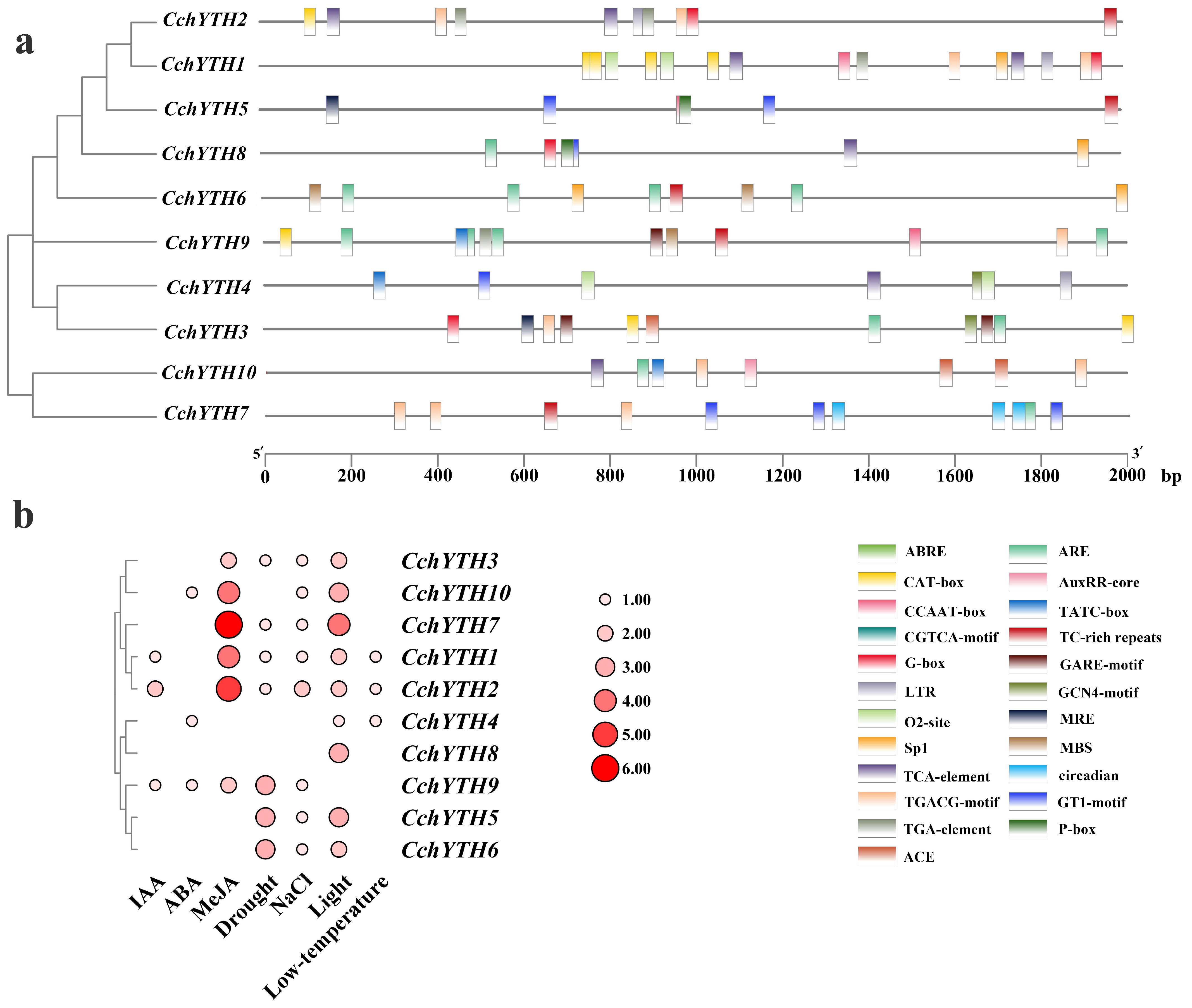
2.4. Gene Structure, Conserved Domain, and Motif Analysis of CchYTH Genes
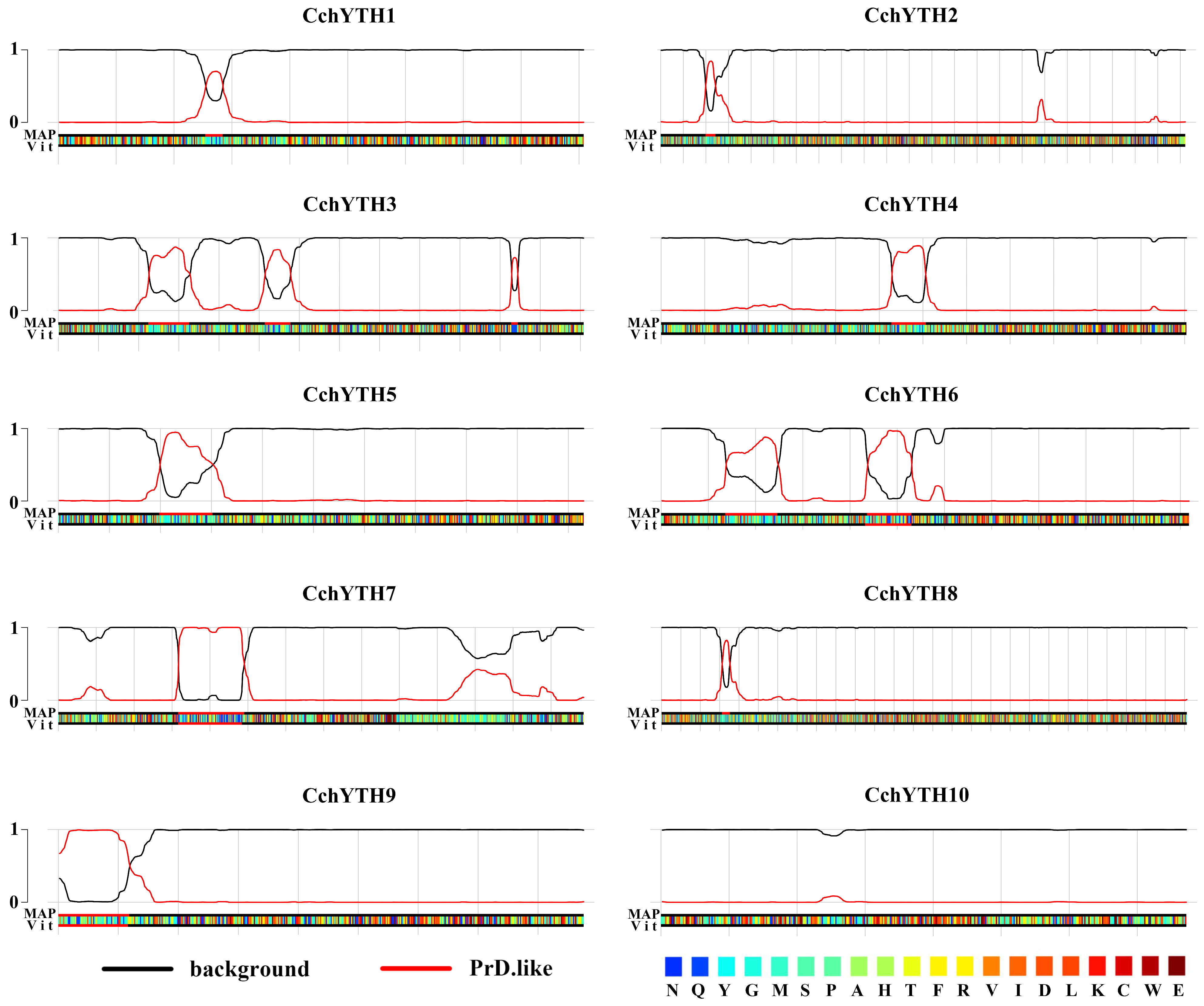
2.5. Most of the CchYTH Proteins May Participate in the Process of Liquid–Liquid Phase Separation
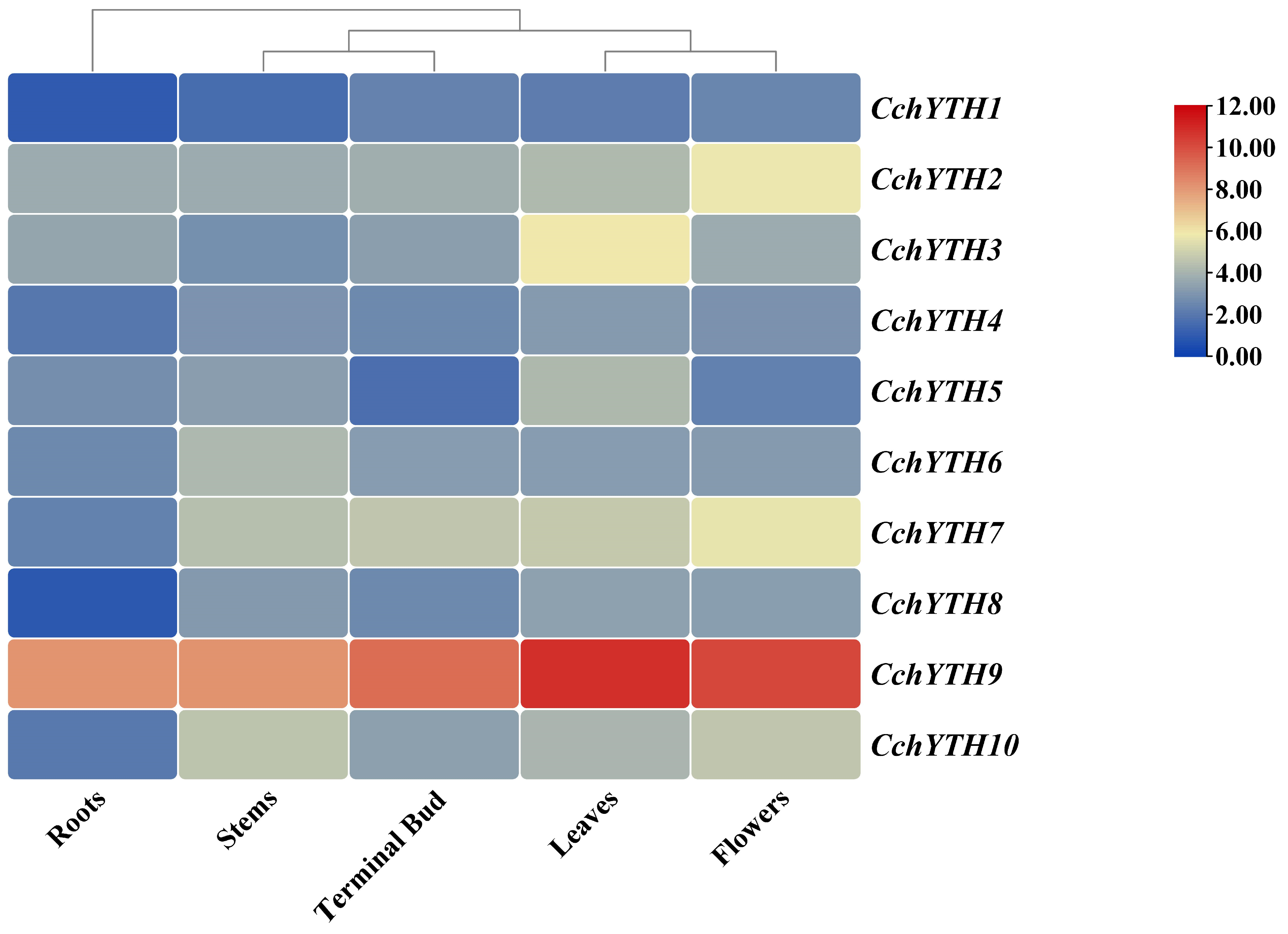
2.6. Tissue-Specific Expression Profiling of CchYTHs
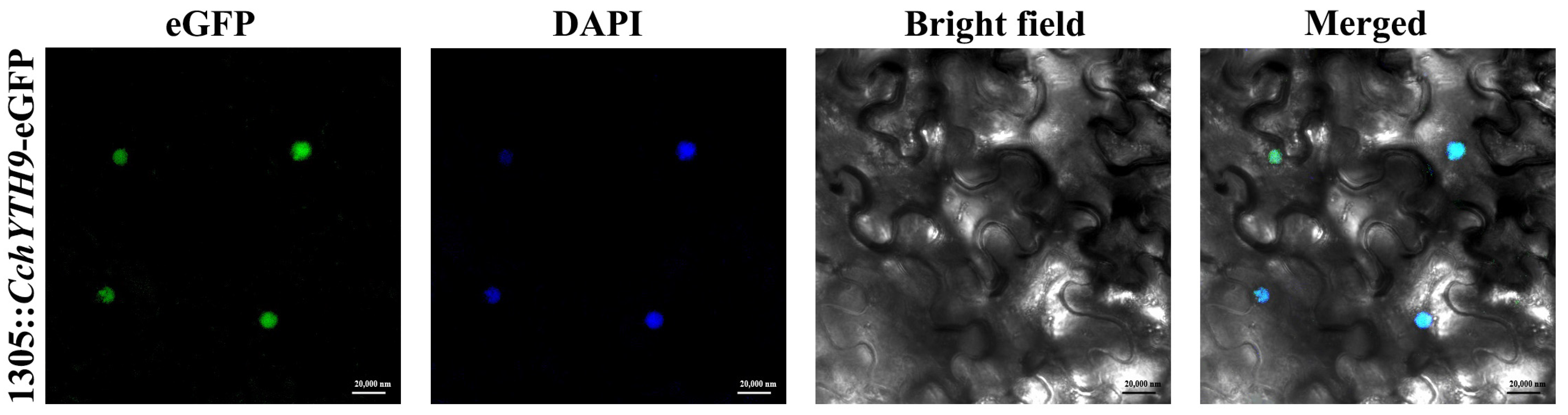
2.7. Subcellular Localization of CchYTH9
2.8. Cis-Acting Elements Analysis of the 10 CchYTH Genes Promoter
2.9. Changes in Relative Expression of the CchYTH Genes under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of Camellia chekiangoleosa YTH Genes
4.2. Basic Physicochemical Properties, Amino Acid Sequence Alignment, and Phylogenetic Analysis of Camellia chekiangoleosa YTH Genes
4.3. Chromosomal Localization Prediction and Naming of Members of YTH Family in Camellia chekiangoleosa
4.4. Duplication Events of CchYTH Genes
4.5. Structure, Conserved Domain, Motif, and Promoter Area Cis-acting Element Analysis of CchYTH
4.6. Prediction of Liquid–Liquid Phase Separation
4.7. Plant Materials and Treatments
4.8. RNA Extraction and RT-qPCR
4.9. Subcellular Localization of CchYTH9 Protein
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Klar, A.J. Asymmetric cell division via DNA strand-specific epigenetic imprinting and segregation explains eukaryotic development. Epigenet. Chromatin 2013, 6, 115. [Google Scholar] [CrossRef]
- Liu, Z.X.; Li, L.M.; Sun, H.L.; Liu, S.-M. Link between m6A modification and cancers. Front. Bioeng. Biotechnol. 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.F.; Fu, Y.E.; Zhao, X.U.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.-G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Ilona, R.; Stefan, S. YTH: A new domain in nuclear proteins. Trends Biochem. Sci. 2002, 27, 99–107. [Google Scholar]
- Zhang, Z.Y.; Theler, D.; Kaminska, K.H.; Hiller, M.; de la Grange, P.; Pudimat, R.; Rafalska, I.; Heinrich, B.; Bujnicki, J.M.; Allain, F.H.-T.; et al. The YTH domain is a novel RNA binding domain. J. Biol. Chem. 2010, 285, 14701–14710. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Nayler, O.; Schwaiger, F.W.; Obermeier, A.; Stamm, S. The interaction and colocalization of Sam68 with thesplicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59 (fyn). Mol. Biol. Cell 1999, 10, 3909–3926. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Q.; Zheng, G.; He, C.; Parisien, M.; Pan, T. N6-methyladenosine-dependent RNA structural switches regulate RNA–protein interactions. Nature 2015, 518, 560–564. [Google Scholar] [CrossRef]
- Sun, J.; Bie, X.M.; Wang, N.; Zhang, X.S.; Gao, X.-Q. Genome-wide identification and expression analysis of YTH domain-containing RNA-binding protein family in common wheat. BMC Plant Biol. 2020, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, L.F.; Jiang, L.W.; Liu, S. Genome-wide identification and expression analysis of the YTH domain-containing RNA-binding protein family in Citrus sinensis. Genes Genom. 2019, 40, 579–589. [Google Scholar] [CrossRef]
- Han, D.; Liu, J.; Chen, C.; Dong, L.; Liu, Y.; Chang, R.; Huang, X.; Liu, Y.; Wang, J.; Dougherty, U.; et al. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature 2019, 566, 270–274. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.; Luo, G.; Zhang, Z.; Wang, X.; Zhou, T.; Cui, Y.; Sha, J.; Huang, X.; Guerrero, L.; Xie, P.; et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife 2017, 6, e31311. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.G.; Xu, X.; Chen, J.M.; Yin, Y.; Zhao, Y. Genome-wide identification, characterization, and expression analysis of m6A readers-YTH domain-containing genes in alfalfa. BMC Genom. 2024, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Wang, Q.T.; Wei, L.; Liang, W.; Wang, L.; Lv, N.; Du, X.; Zhang, J.; Shen, C.; Xin, Y.; et al. Genome-wide adenine N6-methylation map reveals epigenomic regulation of lipid accumulation in Nannochloropsis. Plant Commun. 2024, 5, 100773. [Google Scholar] [CrossRef] [PubMed]
- Scutenaire, J.; Deragon, J.; Jean, V.; Benhamed, M.; Raynaud, C.; Favory, J.-J.; Merret, R.; Bousquet-Antonelli, C. The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell 2018, 30, 986–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Guo, T.; Sun, X.; Jia, X.; Wang, P.; Shao, Y.; Liang, B.; Gong, X.; Ma, F. Functions of two Malus hupehensis (Pamp.) Rehd. YTPs (MhYTP1 and MhYTP2) in biotic-and abiotic-stress responses. Plant Sci. 2017, 261, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Cui, S.; Lu, Z.; Yan, X.; Cai, L.; Lu, Y.; Cai, K.; Zhou, H.; Ma, R.; Zhou, S.; et al. YTH domain proteins play an essential role in rice growth and stress response. Plants 2022, 11, 2206. [Google Scholar] [CrossRef]
- Zhou, W.; Xiao, X.; Shen, J.; Wen, Q.; Li, T.; Zhu, H.; Yang, J.; Xun, C. Review and breeding strategy of Camellia chekiangoleosa. Germplasm Resour. 2019, 47, 20–24. [Google Scholar]
- Liao, S.X. High-yield cultivation techniques of Camellia chekiangoleosa. Mod. Agric. Sci. Technol. 2008, 9, 37–38. [Google Scholar]
- Tan, X. Advances in molecular biology of Camellia chekiangoleosa. J. Cent. South Univ. 2023, 43, 24. [Google Scholar]
- Liu, S.J.; Chen, T.T.; Ye, D.; Chen, Q.; Ni, J.; Rao, M. Prediction of distributional patterns of four major Camellia oilseed species in China under climate and land use changes. Ecol. Indic. 2023, 155, 110996. [Google Scholar] [CrossRef]
- Zhou, P.Y.; Qu, Y.S.; Wang, Z.W.; Huang, B.; Wen, Q.; Xin, Y.; Ni, Z.; Xu, L.A. Gene structural specificity and expression of MADS-Box gene family in Camellia chekiangoleosa. Int. J. Mol. Sci. 2023, 24, 3434. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, A.K.; Nutter-Upham, A.; Lindquist, S.; King, O.D. PLAAC: A web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 2014, 30, 2501–2502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.-G. Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Hernández, L.; Bressendorff, S.; Hansen, M.H.; Poulsen, C.; Erdmann, S.; Brodersen, P. An m6A-YTH module controls developmental timing and morphogenesis in Arabidopsis. Plant Cell 2018, 30, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.Z.; Zhang, K.Q.; Zhang, Q.L.; Li, H.; Zhang, W.T.; Zhang, H.Y.; Hu, S.P.; Wei, H.B. Genome-wide study of ECT gene family in rice. Chin. J. Biochem. Mol. Biol. 2022, 38, 919–925. [Google Scholar]
- Wang, N.; Yue, Z.; Liang, D.; Ma, F. Genome-wide identification of members in the YTH domain-containing RNA-binding protein family in apple and expression analysis of their responsiveness to senescence and abiotic stresses. Gene 2014, 538, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef]
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Banjade, S.; Cheng, H.C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, X.; Liu, K.; Roundtree, I.A.; Tempel, W.; Li, Y.; Lu, Z.; He, C.; Min, J. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol. 2014, 10, 927–929. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Li, L.; Sun, Z.; Li, J.; Chen, X.; Hong, G. SPX4 interacts with both PHR1 and PAP1 to regulate critical steps in phosphorus status-dependent anthocyanin biosynthesis. New Phytol. 2021, 230, 205–217. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, J.; Cheng, X.; Wang, D.; Yu, W.; Ji, K.; Yu, Q. Genome-Wide Identification and Characterization of the YTH Domain-Containing RNA-Binding Protein Family in Liriodendron chinense. Int. J. Mol. Sci. 2023, 24, 15189. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Sun, H.; Xu, C. YTH domain: A family of N6-methyladenosine (m6A) readers. Genom. Proteom. Bioinform. 2018, 16, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bodi, Z.; Zhong, S.; Mehra, S.; Song, J.; Graham, N.; Li, H.; May, S.; Fray, R.G. Adenosine methylation in Arabidopsis mRNA is associated with the 3′end and reduced levels cause developmental defects. Front. Plant Sci. 2012, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, Y.S.; Ping, X.L.; Yang, X.; Xiao, W.; Yang, Y.; Sun, H.-Y.; Zhu, Q.; Baidya, P.; Wang, X.; et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017, 27, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Li, H.; Bodi, Z.; Button, J.; Vespa, L.; Herzog, M.; Fray, R.G. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 2008, 20, 1278–1288. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Chen, C.; Chen, R.; Bai, X.; Qiang, Z.; Fu, J.; Qin, T. The genetic variation in drought resistance in eighteen perennial ryegrass varieties and the underlying adaptation mechanisms. BMC Plant Biol. 2023, 23, 451. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhang, C.; Feng, J.; Feng, A.; You, C.; Ren, Y.; Wang, D.; Sun, T.; Su, Y.; Xu, L.; et al. Genome-wide identification, characterization and expression analysis of the carotenoid cleavage oxygenase (CCO) gene family in Saccharum. Plant Physiol. Biochem. 2021, 162, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hou, C.; Wang, L.; Yu, C.; Chen, T.; Shen, B.; Hou, Y.; Li, P.; Li, T. Screening membraneless organelle participants with machine-learning models that integrate multimodal features. Proc. Natl. Acad. Sci. USA 2022, 119, e2115369119. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.; Li, M.Q.; Li, C.J.; Zheng, W.H.; Liu, R. Different Physiological Responses to Continuous Drought between Seedlings and Younger Individuals ofHaloxylon ammodendron. Plants. 2023, 21, 3683. [Google Scholar] [CrossRef] [PubMed]
- Hesselson, D.; Anderson, R.M.; Beinat, M.; Stainier, D.Y. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc. Natl. Acad. Sci. USA 2009, 106, 14896–14901. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Agassin, R.H.; Huang, Z.C.; Wang, D.; Yao, S.; Ji, K. Transcriptome-Wide Identification of TCP Transcription Factor Family Members in Pinus massoniana and Their Expression in Regulation of Development and in Response to Stress. Int. J. Mol. Sci. 2023, 21, 15938. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Number of Amino Acids | Molecular Weight (kDa) | Theoretical pI | The Instability Index | Prediction of Subcellular Localization |
|---|---|---|---|---|---|---|
| CchYTH1 | Cch01T002587.1 | 454 | 50.951 | 5.35 | 38.65 | cytoplasm |
| CchYTH2 | Cch02T002736.1 | 1161 | 130.998 | 5.83 | 39.82 | cytoplasm |
| CchYTH3 | Cch02T003694.1 | 654 | 71.922 | 5.61 | 42.17 | nucleus |
| CchYTH4 | Cch03T002158.1 | 602 | 65.914 | 5.40 | 52.69 | nucleus |
| CchYTH5 | Cch05T000069.1 | 515 | 56.843 | 5.54 | 39.66 | nucleus |
| CchYTH6 | Cch07T000910.1 | 559 | 61.303 | 7.62 | 44.71 | nucleus |
| CchYTH7 | Cch08T001554.1 | 693 | 75.739 | 5.95 | 55.16 | nucleus |
| CchYTH8 | Cch09T004551.1 | 1356 | 150.028 | 5.41 | 43.14 | cytoplasm |
| CchYTH9 | Cch12T001174.1 | 438 | 48.770 | 9.22 | 29.17 | nucleus |
| CchYTH10 | Cch13T001809.1 | 386 | 43.564 | 6.17 | 50.25 | nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Yao, S.; Zhang, J.; Wang, D.; Xu, S.; Yu, Q.; Ji, K. Genome-Wide Identification and Expression Analysis of YTH Gene Family for Abiotic Stress Regulation in Camellia chekiangoleosa. Int. J. Mol. Sci. 2024, 25, 3996. https://doi.org/10.3390/ijms25073996
Cheng X, Yao S, Zhang J, Wang D, Xu S, Yu Q, Ji K. Genome-Wide Identification and Expression Analysis of YTH Gene Family for Abiotic Stress Regulation in Camellia chekiangoleosa. International Journal of Molecular Sciences. 2024; 25(7):3996. https://doi.org/10.3390/ijms25073996
Chicago/Turabian StyleCheng, Xiang, Sheng Yao, Jingjing Zhang, Dengbao Wang, Shaojun Xu, Qiong Yu, and Kongshu Ji. 2024. "Genome-Wide Identification and Expression Analysis of YTH Gene Family for Abiotic Stress Regulation in Camellia chekiangoleosa" International Journal of Molecular Sciences 25, no. 7: 3996. https://doi.org/10.3390/ijms25073996
APA StyleCheng, X., Yao, S., Zhang, J., Wang, D., Xu, S., Yu, Q., & Ji, K. (2024). Genome-Wide Identification and Expression Analysis of YTH Gene Family for Abiotic Stress Regulation in Camellia chekiangoleosa. International Journal of Molecular Sciences, 25(7), 3996. https://doi.org/10.3390/ijms25073996






