A Rare Case of Methemoglobinemia after Ifosfamide Infusion in a 3-Year-Old Patient Treated for T-ALL
Abstract
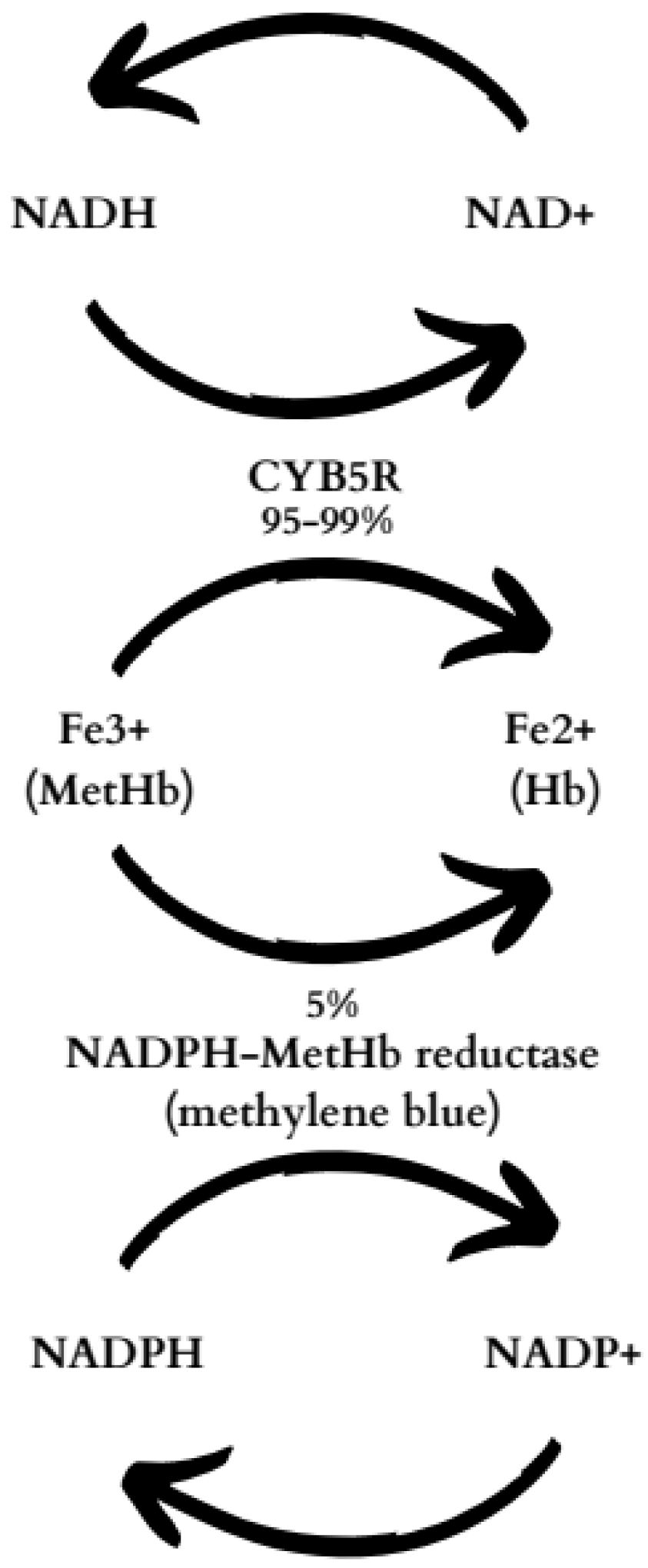
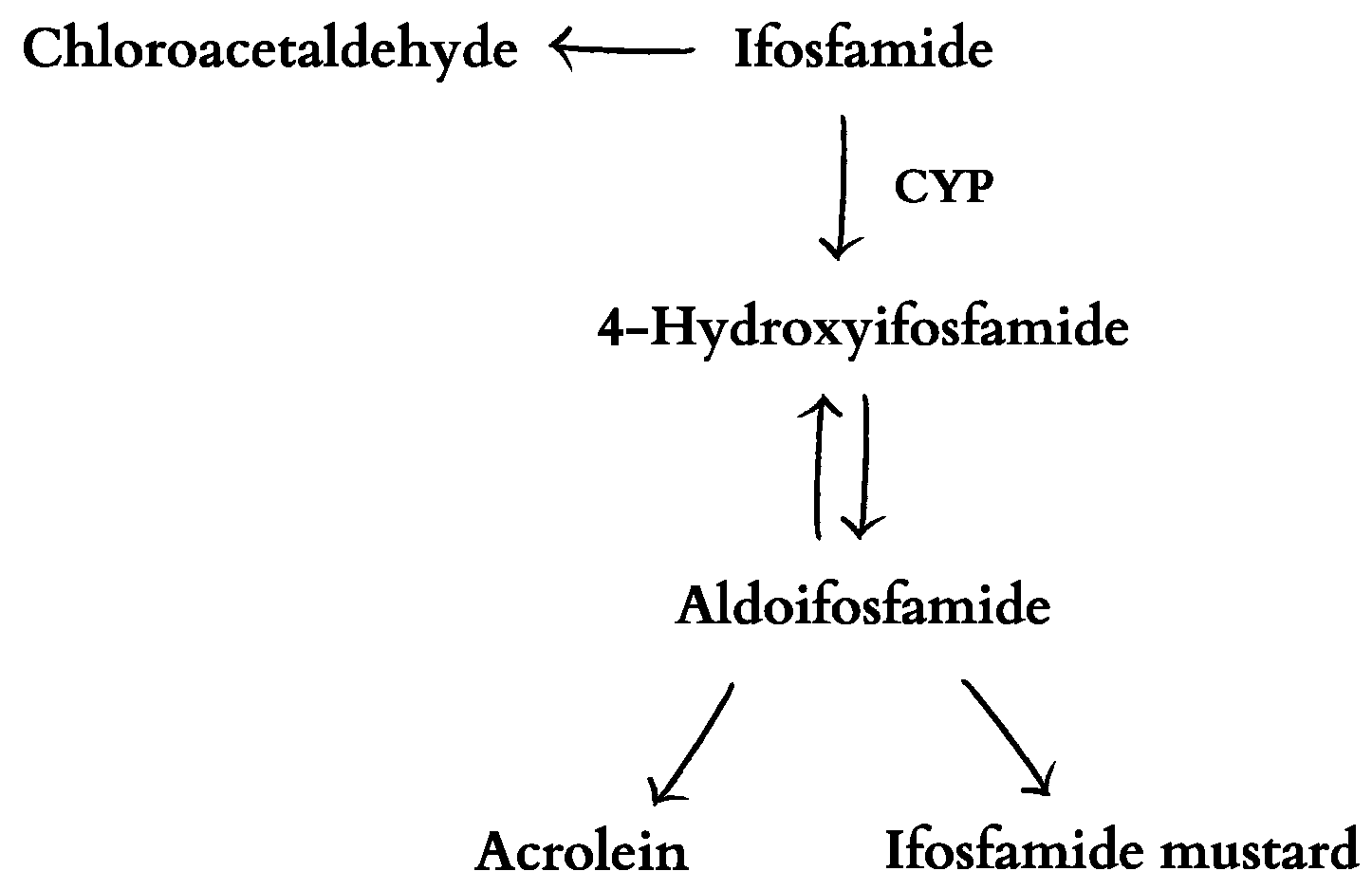
1. Introduction
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curry, S. Methemoglobinemia. Ann. Emerg. Med. 1982, 11, 214–221. [Google Scholar] [CrossRef]
- Ludlow, J.T.; Wilkerson, R.G.; Nappe, T.M. Methemoglobinemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wright, R.O.; Lewander, W.J.; Woolf, A.D. Methemoglobinemia: Etiology, Pharmacology, and Clinical Management. Ann. Emerg. Med. 1999, 34, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Pushparajah Mak, R.S.; Liebelt, E.L. Methylene Blue: An Antidote for Methemoglobinemia and Beyond. Pediatr. Emerg. Care 2021, 37, 474–477. [Google Scholar] [CrossRef]
- Iolascon, A.; Bianchi, P.; Andolfo, I.; Russo, R.; Barcellini, W.; Fermo, E.; Toldi, G.; Ghirardello, S.; Rees, D.; Van Wijk, R.; et al. Recommendations for Diagnosis and Treatment of Methemoglobinemia. Am. J. Hematol. 2021, 96, 1666. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Lurie, A.A. Concise Review: Methemoglobinemia. Am. J. Hematol. 1993, 42, 7–12. [Google Scholar] [CrossRef]
- Rangan, A.; Savedra, M.E.; Dergam-Larson, C.; Swanson, K.C.; Szuberski, J.; Go, R.S.; Porter, T.R.; Brunker, S.E.; Shi, M.; Nguyen, P.L.; et al. Interpreting Sulfhemoglobin and Methemoglobin in Patients with Cyanosis: An Overview of Patients with M-Hemoglobin Variants. Int. J. Lab. Hematol. 2021, 43, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Gordeuk, V.R.; Shah, B.N.; Zhang, X.; Thuma, P.E.; Zulu, S.; Moono, R.; Reading, N.S.; Song, J.; Zhang, Y.; Nouraie, M.; et al. The CYB5R3c.350C>G and G6PD A Alleles Modify Severity of Anemia in Malaria and Sickle Cell Disease. Am. J. Hematol. 2020, 95, 1269–1279. [Google Scholar] [CrossRef]
- Bradberry, S.M. Occupational Methaemoglobinaemia. Mechanisms of Production, Features, Diagnosis and Manage-309 ment Including the Use of Methylene Blue. Toxicol. Rev. 2003, 22, 13–27. [Google Scholar] [CrossRef]
- Mary, A.M.; Bhupalam, L. Metoclopramide-Induced Methemoglobinemia in an Adult. J. Ky. Med. Assoc. 2000, 98, 245–247. [Google Scholar]
- Jackson, S.H.; Barker, S.J. Methemoglobinemia in a Patient Receiving Flutamide. Anesthesiology 1995, 82, 1065–1067. [Google Scholar] [CrossRef]
- Chou, T.D.; Gibran, N.S.; Urdahl, K.; Lin, E.Y.; Heimbach, D.M.; Engrav, L.H. Methemoglobinemia Secondary to Topical Silver Nitrate Therapy—A Case Report. Burns 1999, 25, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, X.J. Methemoglobinemia Induced by Trimethoprim-Sulfamethoxazole in a Boy With Acute Lymphoblastic Leukemia. Clin. Pediatr. 2023, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, N.; Dansey, R.; Seen, S.; Abella, E. Cyclophosphamide-Induced Methemoglobinemia. Bone Marrow Transplant. 2003, 32, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Sanchez, T.; Norgbe, S.; Picking, C.R.; Millner, P.G. Rasburicase-Induced Methemoglobinemia. Cureus 2021, 13, e14406. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.S.; Edwards, E.M.; Egelund, T.A. Methemoglobinemia Induced by Rasburicase in a Pediatric Patient: A Case Report and Literature Review. J. Oncol. Pharm. Pract. 2012, 18, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, C.; Brown, B.; Benner, K.; Prabhakaran, P.; Hayes, L. A Novel Use of Methylene Blue in the Pediatric ICU. Pediatrics 2015, 136, e1030–e1034. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.N. Hemoglobin Metabolism. In Rodak’s Hematology, 6th ed.; Clinical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–103. [Google Scholar] [CrossRef]
- Crooks, G.M.; Sato, J.K. Ifosfamide and Etoposide in Recurrent Childhood Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 1995, 17, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Raetz, E.A.; Teachey, D.T. T-Cell Acute Lymphoblastic Leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Zalupski, M.; Baker, L.H. Ifosfamide. J. Natl. Cancer Inst. 1988, 80, 556–566. [Google Scholar] [CrossRef]
- Abahssain, H.; Moukafih, B.; Essangri, H.; Mrabti, H.; Meddah, B.; Guessous, F.; Fadhil, F.Z.; Souadka, A.; Errihani, H. Methylene Blue and Ifosfamide-Induced Encephalopathy: Myth or Reality? J. Oncol. Pharm. Pract. 2021, 27, 143–149. [Google Scholar] [CrossRef]
- Klastersky, J. Side Effects of Ifosfamide. Oncology 2003, 65 (Suppl. S2), 7–10. [Google Scholar] [CrossRef]
- Hadjiliadis, D.; Govert, J.A. Methemoglobinemia after Infusion of Ifosfamide Chemotherapy: First Report of a Potentially Serious Adverse Reaction Related to Ifosfamide. Chest 2000, 118, 1208–1210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kerbusch, T.; De Kraker, J.; Keizer, H.J.; Van Putten, J.W.G.; Groen, H.J.M.; Jansen, R.L.H.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics and Pharmacodynamics of Ifosfamide and Its Metabolites. Clin. Pharmacokinet. 2001, 40, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.D. Ifosfamide Pharmacokinetics. Investig. New Drugs 1991, 9, 305–311. [Google Scholar] [CrossRef]
- Momerency, G.; Van Cauwenberghe, K.; Highley, M.S.; Harper, P.G.; Van Oosterom, A.T.; De Bruijn, E.A. Partitioning of Ifosfamide and Its Metabolites between Red Blood Cells and Plasma. J. Pharm. Sci. 1996, 85, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Highley, M.S.; De Bruijn, E.A. Erythrocytes and the Transport of Drugs and Endogenous Compounds. Pharm. Res. 1996, 13, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Valiev, T.T.; Korkina, Y.S. Ifosfamide/Cyclofosfamide-Induced Methemoglobinemia in Pediatric Patients with Hemoblastoses. J. Mod. Oncol. 2020, 22, 139–142. [Google Scholar] [CrossRef]
- Balsamo, P.; Hardy, W.R.; Scott, E.M. Hereditary Methemoglobinemia Due to Diaphorase Deficiency in Navajo Indians. J. Pediatr. 1964, 65, 928–931. [Google Scholar] [CrossRef]
- Cafaro, R.P. Hypoxia: Its Causes and Symptoms. J. Am. Dent. Soc. Anesthesiol. 1960, 7, 4–8. [Google Scholar]
- Jones, R.H.; Sabiston, D.C. Pulmonary Embolism. Surg. Clin. N. Am. 1976, 56, 891–907. [Google Scholar] [CrossRef]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and Organ Distribution of Intravenous and Oral Methylene Blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rakesh, K.; Agarwal, R.; Tripathi, P.P.; Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Hans, R.; Sharma, R.; Sharma, N.; et al. Therapeutic Whole Blood Exchange in the Management of Methaemoglobinemia: Case Series and Systematic Review of Literature. Transfus. Med. 2020, 30, 231–239. [Google Scholar] [CrossRef] [PubMed]
| Date: | 13 May 2023 | 14 May 2023 | |||
|---|---|---|---|---|---|
| Hour: | 0 | 6 | 9 | 23:04 | 9:12 |
| Methylene blue | ↓ | ↓ | |||
| Exchange transfusion | ↓ | ||||
| MetHb [%] | 57.10 | 28 | 10.3 | - | 0.7 |
| O2Hb [%] | 42.7 | 72 | 87.4 | - | 98 |
| HHb [%] | 0.00 | 0.00 | 1.8 | - | 0.7 |
| COHb [%] | 0.2 | 0.1 | 0.4 | - | 0.6 |
| THb [g/dL] | 10.38 | 9.34 | 9.68 | - | 11.79 |
| RBC [106/μL] | 3.67 | - | 3.21 | 3.89 | |
| HCT [%] | 31.5 | - | 26.4 | 32 | |
| MCV [fl] | 85.8 | - | 82.2 | 82.3 | |
| PLT [103/μL] | 274 | - | 98 | 102 | |
| WBC [103/μL] | 6.38 | - | 1.17 | 8.21 | |
| Serum glucose [mg/dL] | 103 | - | - | - | 53 |
| VBG: | not available | ||||
| blood pH | - | - | 7.639 | - | 7.413 |
| anion gap [mmol/L] | - | - | −2.4 | - | −5.3 |
| pCO2 [mmHg] | - | - | 16.1 | - | 29.1 |
| pO2 [mmHg] | - | - | 79 | - | 14.9 |
| bicarbonates [mmol/L] | - | - | 16.9 | - | 18.1 |
| CRP [mg/L] | <0.6 | - | - | - | <0.6 |
| ALAT [U/L] | 103 | - | - | - | 87 |
| ASP [U/L] | - | - | - | - | 74 |
| bilirubin [mg/dL] | 0.48 | - | - | - | 0.87 |
| creatinine [mg/dL] | 0.43 | - | - | - | 0.39 |
| urea [mg/dL] | 2 | - | - | - | - |
| uric acid [mg/dL] | 2 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suprunowicz, M.; Marcinkiewicz, K.; Leszczyńska, E.; Krętowska-Grunwald, A.; Płonowski, M.; Tałałaj, M.; Dakowicz, Ł.; Krawczuk-Rybak, M.; Sawicka-Żukowska, M. A Rare Case of Methemoglobinemia after Ifosfamide Infusion in a 3-Year-Old Patient Treated for T-ALL. Int. J. Mol. Sci. 2024, 25, 3789. https://doi.org/10.3390/ijms25073789
Suprunowicz M, Marcinkiewicz K, Leszczyńska E, Krętowska-Grunwald A, Płonowski M, Tałałaj M, Dakowicz Ł, Krawczuk-Rybak M, Sawicka-Żukowska M. A Rare Case of Methemoglobinemia after Ifosfamide Infusion in a 3-Year-Old Patient Treated for T-ALL. International Journal of Molecular Sciences. 2024; 25(7):3789. https://doi.org/10.3390/ijms25073789
Chicago/Turabian StyleSuprunowicz, Maria, Katarzyna Marcinkiewicz, Elżbieta Leszczyńska, Anna Krętowska-Grunwald, Marcin Płonowski, Mariola Tałałaj, Łucja Dakowicz, Maryna Krawczuk-Rybak, and Małgorzata Sawicka-Żukowska. 2024. "A Rare Case of Methemoglobinemia after Ifosfamide Infusion in a 3-Year-Old Patient Treated for T-ALL" International Journal of Molecular Sciences 25, no. 7: 3789. https://doi.org/10.3390/ijms25073789
APA StyleSuprunowicz, M., Marcinkiewicz, K., Leszczyńska, E., Krętowska-Grunwald, A., Płonowski, M., Tałałaj, M., Dakowicz, Ł., Krawczuk-Rybak, M., & Sawicka-Żukowska, M. (2024). A Rare Case of Methemoglobinemia after Ifosfamide Infusion in a 3-Year-Old Patient Treated for T-ALL. International Journal of Molecular Sciences, 25(7), 3789. https://doi.org/10.3390/ijms25073789