Bioengineering Skin Substitutes for Wound Management—Perspectives and Challenges
Abstract
1. Introduction
2. Wound Healing Complications—Clinical Need for Advanced Products
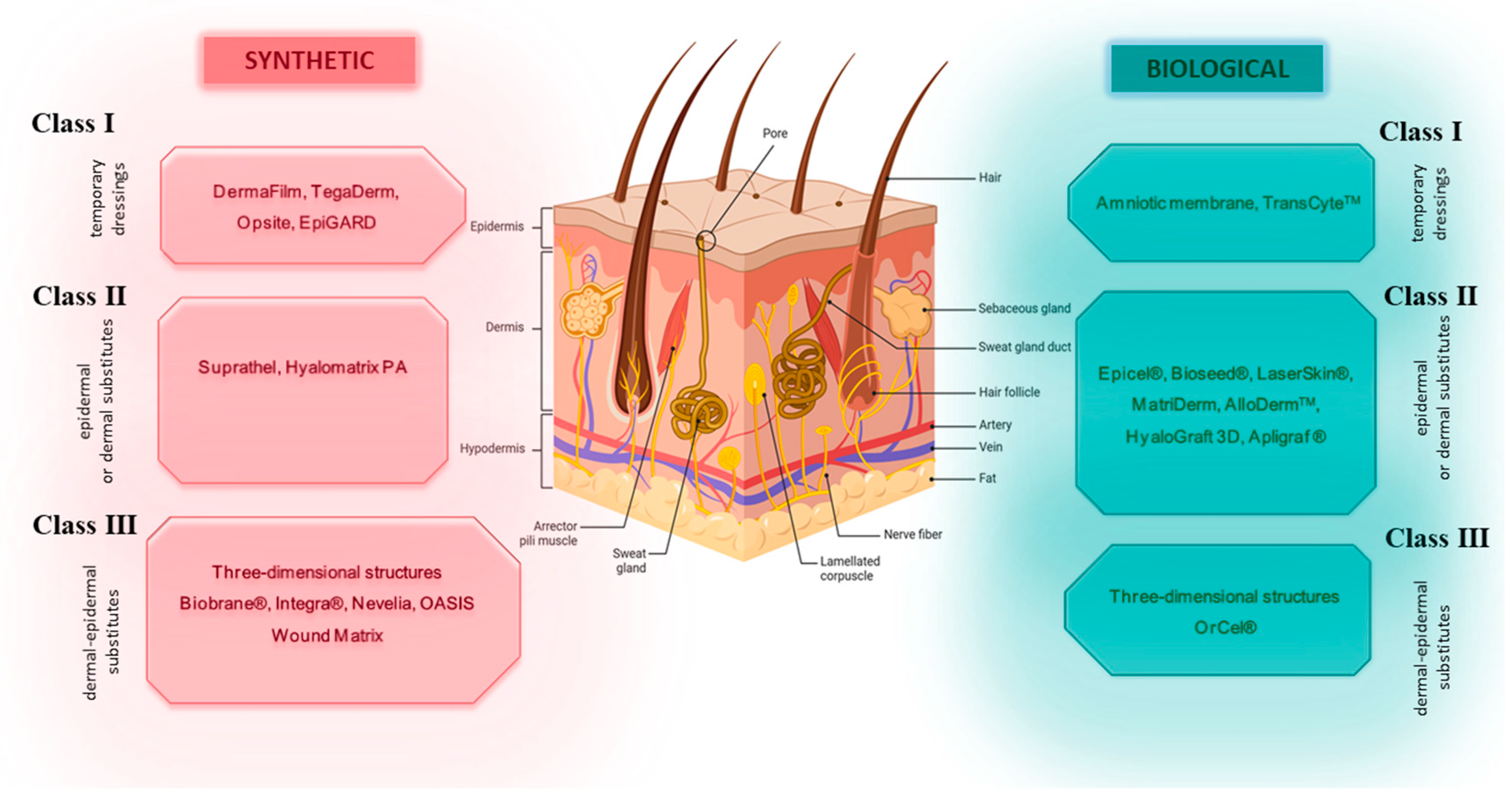
3. Skin Substitutes—Characteristics and Clinical Application
- Class I—one- or two-layer temporary dressing materials;
- Class II—single-layer skin substitutes (epidermal or dermal);
- Class III—complex skin substitutes (dermal–epidermal).
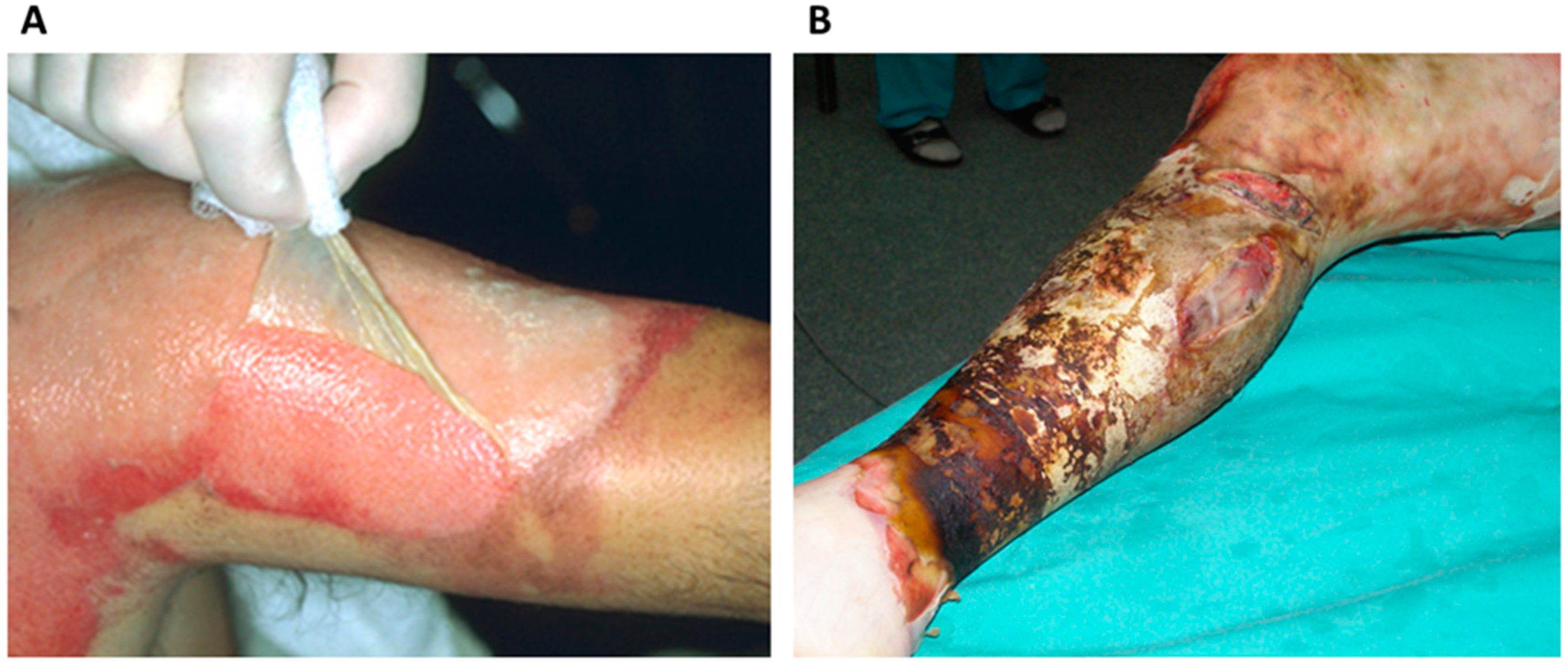
3.1. Class I Skin Substitutes
3.2. Class II Skin Substitutes
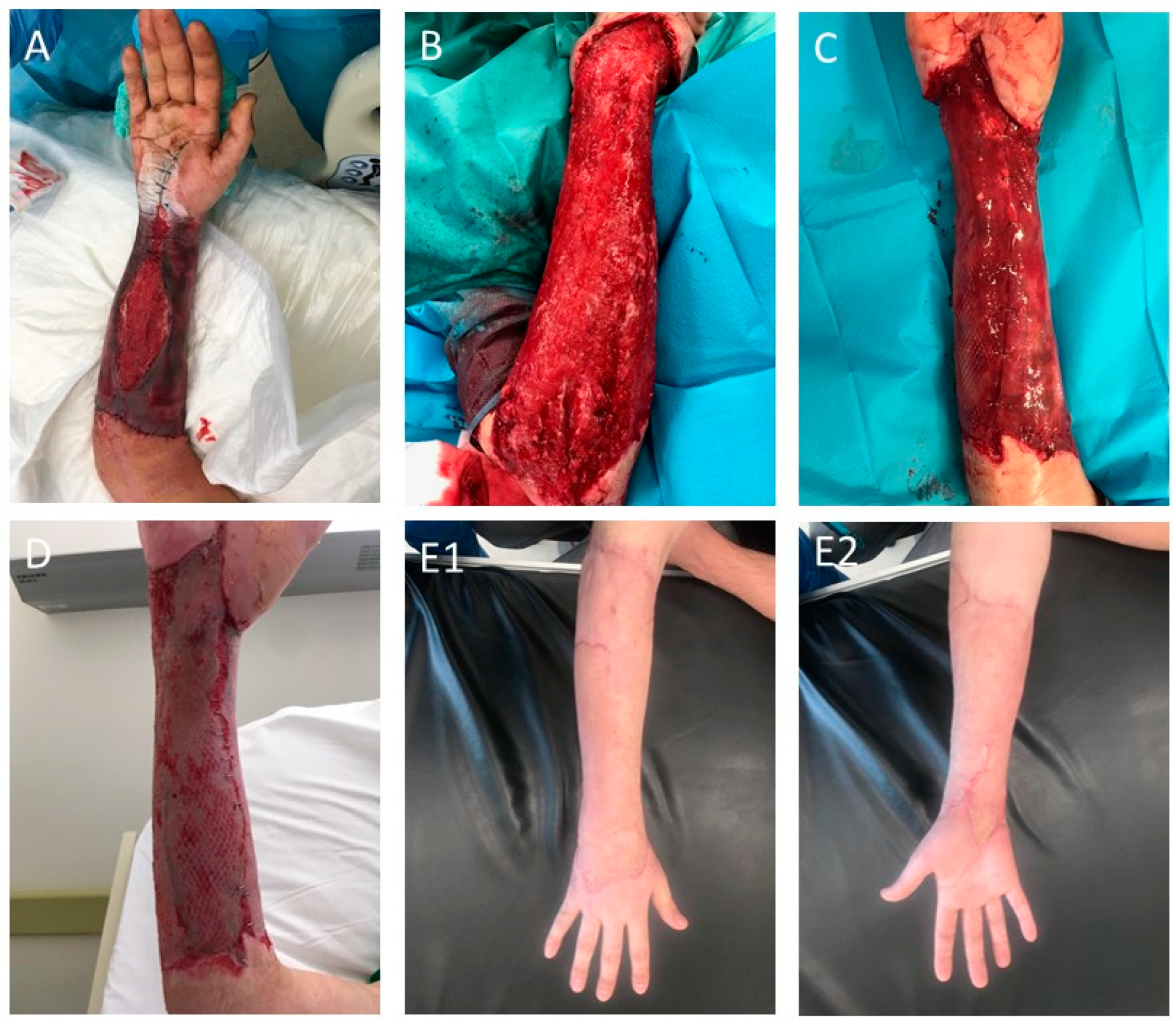
3.3. Class III—Complex Skin Substitutes (Dermal–Epidermal)
4. 3D Bioprinting Skin Substitutes
5. Immune Reaction to Skin Substitutes
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470443/ (accessed on 21 February 2024).
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Deptuła, M.; Zieliński, J.; Wardowska, A.; Pikuła, M. Wound healing complications in oncological patients: Perspectives for cellular therapy. Adv. Dermatol. Allergol. 2018, 36, 139–146. [Google Scholar] [CrossRef]
- Aisa, J.; Parlier, M. Local wound management: A review of modern techniques and products. Vet. Dermatol. 2022, 33, 463–478. [Google Scholar] [CrossRef]
- Goldberg, S.R.; Diegelmann, R.F. What Makes Wounds Chronic. Surg. Clin. N. Am. 2020, 100, 681–693. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Rahmani Del Bakhshayesh, A.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Derm. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, A.; Wise, L.; Hibma, M. The role of Langerhans cells in pathologies of the skin. Immunol. Cell Biol. 2019, 97, 700–713. [Google Scholar] [CrossRef] [PubMed]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Tardáguila-García, A.; García-Morales, E.; García-Alamino, J.M.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen. 2019, 27, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Stojadinovic, O.; Brem, H.; Lee, B.; Vouthounis, C.; Entero, H.; Tomic-Canic, M. 138 Molecular Pathogenesis of Chronic Wounds: The Role of β-Catenin and C-MYC. Wound Repair Regen. 2004, 12, A36. [Google Scholar] [CrossRef]
- Bonnici, L.; Suleiman, S.; Schembri-Wismayer, P.; Cassar, A. Targeting Signalling Pathways in Chronic Wound Healing. Int. J. Mol. Sci. 2024, 25, 50. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, C.; Graves, D.T. Abnormal Cell Responses and Role of TNF-α in Impaired Diabetic Wound Healing. Biomed. Res. Int. 2013, 2013, 754802. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1216321 (accessed on 12 February 2024). [CrossRef] [PubMed]
- Canecki-Varžić, S.; Prpić-Križevac, I.; Mihaljević, S.; Bilić-Ćurčić, I.; Alkhamis, T.; Wagner, J.; Škrlec, I.; Barbić, J. Association Between Interleukin-10 Gene (-1082g/A) Polymorphism and Type 2 Diabetes, Diabetes-Related Traits, and Microvascular Complications in the Croatian Population. Acta Clin. Croat. 2018, 57, 71–81. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Lingen, M.W.; Chang, B.-D.; Shen, M.; Swift, M.; Curry, J.; Bacon, P.; Bodner, B.; Roninson, I.B. Tumor Suppressor Maspin Is Up-Regulated during Keratinocyte Senescence, Exerting a Paracrine Antiangiogenic Activity. Cancer Res. 2004, 64, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Advanced Biomedical Applications of Multifunctional Natural and Synthetic Biomaterials. Processes 2023, 11, 2696. [Google Scholar] [CrossRef]
- Zhou, J.; He, W.; Luo, G.; Wu, J. Fundamental Immunology of Skin Transplantation and Key Strategies for Tolerance Induction. Arch. Immunol. Ther. Exp. 2013, 61, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Shakespeare, P.G. The role of skin substitutes in the treatment of burn injuries. Clin. Dermatol. 2005, 23, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Wound Care Market Size, Share and Trends Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/wound-care-market (accessed on 22 March 2024).
- Li, D.; Cao, W.; Zhou, Q.; Wu, X.; Song, X.; Qin, H. COVID-19 and primary wound healing: A new insights and advance. Int. Wound J. 2023, 20, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- DiFazio, L.T.; Curran, T.; Bilaniuk, J.W.; Adams, J.M.; Durling-Grover, R.; Kong, K.; Nemeth, Z.H. The Impact of the COVID-19 Pandemic on Hospital Admissions for Trauma and Acute Care Surgery. Am. Surg. 2020, 86, 901–903. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, J.; Zhong, Y.; Zhang, T.; Liu, X.; Xing, D. Advanced function, design and application of skin substitutes for skin regeneration. Mater. Today Bio. 2024, 24, 100918. [Google Scholar] [CrossRef] [PubMed]
- Biological Skin Substitutes Market Size & Share Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/biological-skin-substitutes-market-report (accessed on 22 March 2024).
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Advances in Skin Tissue Bioengineering and the Challenges of Clinical Translation. Front. Surg. 2021, 8, 640879. Available online: https://www.frontiersin.org/articles/10.3389/fsurg.2021.640879 (accessed on 16 February 2024). [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.S.; Iqbal, S.A.; Hodgkinson, T.; Morris, J.; Benatar, B.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen. 2015, 23, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Shores, J.T.; Gabriel, A.; Gupta, S. Skin Substitutes and Alternatives: A Review. Adv. Ski. Wound Care 2007, 20, 493. [Google Scholar] [CrossRef]
- Santema, T.B.K.; Poyck, P.P.C.; Ubbink, D.T. Systematic review and meta-analysis of skin substitutes in the treatment of diabetic foot ulcers: Highlights of a Cochrane systematic review. Wound Repair Regen. 2016, 24, 737–744. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, A. Updated Classification of Skin Substitutes. Indian J. Plast. Surg. 2023, 56, 388–389. [Google Scholar] [CrossRef]
- van der Veen, V.C.; Boekema, B.K.H.L.; Ulrich, M.M.W.; Middelkoop, E. New dermal substitutes. Wound Repair Regen. 2011, 19 (Suppl. S1), s59–s65. [Google Scholar] [CrossRef]
- Halim, A.S.; Khoo, T.L.; Mohd Yussof, S.J. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. 2010, 43, S23–S28. [Google Scholar] [CrossRef]
- Violas, P.; Abid, A.; Darodes, P.; Galinier, P.; de Gauzy, J.S.; Cahuzac, J.-P. Integra artificial skin in the management of severe tissue defects, including bone exposure, in injured children. J. Pediatr. Orthop. B 2005, 14, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Heitland, A.; Piatkowski, A.; Noah, E.M.; Pallua, N. Update on the use of collagen/glycosaminoglycate skin substitute-six years of experiences with artificial skin in 15 German burn centers. Burns 2004, 30, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Brinci, L.; Spallone, D.; Tati, E.; Palla, L.; Lucarini, L.; De Angelis, B. The use of MatriDerm® and skin grafting in post-traumatic wounds. Int. Wound J. 2011, 8, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Vecin, N.M.; Kirsner, R.S. Skin substitutes as treatment for chronic wounds: Current and future directions. Front. Med. 2023, 10, 1154567. [Google Scholar] [CrossRef] [PubMed]
- Hansbrough, J. Dermagraft-TC for partial-thickness burns: A clinical evaluation. J. Burn. Care Rehabil. 1997, 18, S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Dougherty, W.R.; Blome-Eberwein, S. Use of Transcyte® and dermabrasion to treat burns reduces length of stay in burns of all size and etiology. Burns 2006, 32, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.J.; Kimble, R.M.; Boots, R.; Pegg, S.P. Treatment of partial-thickness burns: A prospective, randomized trial using TranscyteTM. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- See, A.; Wright, S.; Denham, J.W. A pilot study of dermofilm in acute radiation-induced desquamative skin reactions. Clin. Oncol. 1998, 10, 182–185. [Google Scholar] [CrossRef]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef]
- Koob, T.J.; Lim, J.J.; Zabek, N.; Massee, M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Abul, A.; Karam, M.; Rahman, S. Human Amniotic Membrane: A New Option for Graft Donor Sites—Systematic Review and Meta-analysis. Int. Wound J. 2020, 17, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Aghayan, H.R.; Hosseini, M.S.; Gholami, M.; Mohamadi-Jahani, F.; Tayanloo-Beik, A.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Abdollahi, M.; Larijani, B.; et al. Mesenchymal stem cells’ seeded amniotic membrane as a tissue-engineered dressing for wound healing. Drug Deliv. Transl. Res. 2022, 12, 538–549. [Google Scholar] [CrossRef]
- Schmiedova, I.; Dembickaja, A.; Kiselakova, L.; Nowakova, B.; Slama, P. Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds. Membranes 2021, 11, 941. [Google Scholar] [CrossRef]
- Haugh, A.M.; Witt, J.G.; Hauch, A.; Darden, M.; Parker, G.; Ellsworth, W.A.; Buell, J.F. Amnion Membrane in Diabetic Foot Wounds: A Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1302. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Fulmer, J.; Shebetka, K.A.; Regulski, M.; Vayser, D.; Fried, D.; Kashefsky, H.; Owings, T.M.; Nadarajah, J.; Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: Results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 2014, 11, 554–560. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, L.; Yan, J. Amniotic membrane for treating skin graft donor sites: A systematic review and meta-analysis. Burns 2020, 46, 621–629. [Google Scholar] [CrossRef]
- Yang, C.; Xiong, A.B.; He, X.C.; Bin Ding, X.; Tian, X.L.; Li, Y.; Yan, H. Efficacy and feasibility of amniotic membrane for the treatment of burn wounds: A meta-analysis. J. Trauma Acute Care Surg. 2021, 90, 744–755. [Google Scholar] [CrossRef]
- James, J.H.; Watson, A.C. The use of Opsite, a vapour permeable dressing, on skin graft donor sites. Br. J. Plast. Surg. 1975, 28, 107–110. [Google Scholar] [CrossRef]
- Fong, P.H.; Wong, K.L. Opsite, a synthetic burns dressing. Ann. Acad. Med. Singap. 1985, 14, 387–390. [Google Scholar]
- Wilde, J.M.; Loudon, M.A. Modified Opsite sandwich for temporary abdominal closure: A non-traumatic experience. Ann. R. Coll. Surg. Engl. 2007, 89, 57–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López-Parra, M.; Gil-Rey, D.; López-González, E.; González-Rodríguez, E.-M.; Simó-Sánchez, I.; Zamora-Carmona, F.; Roqueta-Andreu, L.; Arizu-Puigvert, M.; Abril-Sabater, D.; Moreno-Álvarez, À.; et al. Open-label randomized controlled trial to compare wound dressings for patients undergoing hip and knee arthroplasty: Study protocol for a randomized controlled trial. Trials 2018, 19, 357. [Google Scholar] [CrossRef]
- Hawthorne, B.; Simmons, J.K.; Stuart, B.; Tung, R.; Zamierowski, D.S.; Mellott, A.J. Enhancing wound healing dressing development through interdisciplinary collaboration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1967–1985. [Google Scholar] [CrossRef] [PubMed]
- Mac Kinnon, J.L.; Cleek, P.J. The Penetration of Ultraviolet Light Through Transparent Dressings: A Case Report. Phys. Ther. 1984, 64, 204. [Google Scholar] [CrossRef] [PubMed]
- Bhoyar, S.D.; Malhotra, K.; Madke, B. Dressing Materials: A Comprehensive Review. J. Cutan. Aesthet Surg. 2023, 16, 81–89. [Google Scholar] [CrossRef]
- Navarro-Triviño, F.J.; Sierra Cerdán, M.J.; Ruiz-Villaverde, R. TegadermTM I.V. Advanced can prevent eczematous reaction caused by Dexcom glucose monitoring system. Int. J. Dermatol. 2020, 59, e166–e168. [Google Scholar] [CrossRef]
- Barnett, A.; Berkowitz, R.L.; Mills, R.; Vistnes, L.M. Comparison of synthetic adhesive moisture vapor permeable and fine mesh gauze dressings for split-thickness skin graft donor sites. Am. J. Surg. 1983, 145, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.D.; Gowda, P.; DCunha, P.; Gowda, P. A Randomized Controlled Trial Comparing Two Wound Dressings Used in Obstetric and Gynecological Surgeries: Trushield NXT vs. Tegaderm. Cureus 2023, 15, e49207. Available online: https://www.cureus.com/articles/206032-a-randomized-controlled-trial-comparing-two-wound-dressings-used-in-obstetric-and-gynecological-surgeries-trushield-nxt-vs-tegaderm (accessed on 22 February 2024). [CrossRef] [PubMed]
- Anilakumari, D.; Singla, D.; Agarwal, A.; Kumari, R. Comparative efficacy of MicroporeTM surgical dressing, TegadermTM and Lockit plus® for lumbar epidural catheter fixation in children: A prospective parallel group randomized controlled trial. Rev. Española De Anestesiol. Y Reanim. (Engl. Ed.) 2023, 70, 429–437. [Google Scholar] [CrossRef]
- Lucas, D.; Di Rocco, D.; Müller, C.T.; Jurjus, A.R.; Raffoul, W.; di Summa, P.G.; Watfa, W. Application of Dermal Skin Substitutes for Hand and Finger Palmar Soft Tissue Loss. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2551. [Google Scholar] [CrossRef]
- Grassner, L.; Marhold, F.; Yousif, M.; Grillhösl, A.; Ungersboeck, K.; Schulz, J.; Strowitzki, M. Experiences with a temporary synthetic skin substitute after decompressive craniectomy: A retrospective two-center analysis. Acta Neurochir. 2019, 161, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, J.A.; Eseme, E.A.; Gohritz, A.; Tremp, M.; Merat, R.; Kalbermatten, D.F.; Oranges, C.M. Multistage Reconstruction of Large Arm Defect Using Keystone Type I Flap and Temporary Synthetic Skin Substitute. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4745. [Google Scholar] [CrossRef] [PubMed]
- Takami, Y.; Yamaguchi, R.; Ono, S.; Hyakusoku, H. Clinical application and histological properties of autologous tissue-engineered skin equivalents using an acellular dermal matrix. J. Nippon Med. Sch. 2014, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Carsin, H.; Ainaud, P.; Le Bever, H.; Rives, J.M.; Lakhel, A.; Stephanazzi, J.; Lambert, F.; Perrot, J. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: A five year single-center experience with 30 patients. Burns 2000, 26, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kimia, R.; Azoury, S.C.; Allukian, M.; Nguyen, P.D. Cultured Epidermal Autograft for Total Scalp Reconstruction in a Neonate Following Necrotizing Fasciitis. Ann. Plast. Surg. 2020, 85, 276–280. [Google Scholar] [CrossRef]
- Vanscheidt, W.; Ukat, A.; Horak, V.; Brüning, H.; Hunyadi, J.; Pavlicek, R.; Emter, M.; Hartmann, A.; Bende, J.; Zwingers, T.; et al. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant: A multinational randomized controlled clinical trial. Wound Repair Regen. 2007, 15, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.; Ermuth, T.; Tanczos, E.; Bannasch, H.; Horch, R.; Zschocke, I.; Peschen, M.; Schöpf, E.; Vanscheidt, W.; Augustin, M. Treatment of therapy-refractive ulcera cruris of various origins with autologous keratinocytes in fibrin sealant. Vasa 2005, 34, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.K.; Chan, E.S.; To, E.W.; Lau, C.H.; Yen, S.C.; King, W.W. Development and evaluation of a new composite Laserskin graft. J. Trauma 1999, 47, 918–922. [Google Scholar] [CrossRef]
- Pajardi, G.; Rapisarda, V.; Somalvico, F.; Scotti, A.; Russo, G.L.; Ciancio, F.; Sgrò, A.; Nebuloni, M.; Allevi, R.; Torre, M.L.; et al. Skin substitutes based on allogenic fibroblasts or keratinocytes for chronic wounds not responding to conventional therapy: A retrospective observational study. Int. Wound J. 2016, 13, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hundeshagen, G.; Collins, V.N.; Wurzer, P.; Sherman, W.; Voigt, C.D.; Cambiaso-Daniel, J.; Nunez Lopez, O.; Sheaffer, J.; Herndon, D.N.; Finnerty, C.C.; et al. A Prospective, Randomized, Controlled Trial Comparing the Outpatient Treatment of Pediatric and Adult Partial-Thickness Burns with Suprathel or Mepilex Ag. J. Burn Care Res. 2018, 39, 261–267. [Google Scholar] [CrossRef]
- Keck, M.; Selig, H.F.; Lumenta, D.B.; Kamolz, L.P.; Mittlböck, M.; Frey, M. The use of Suprathel® in deep dermal burns: First results of a prospective study. Burns 2012, 38, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Cheema, L.; Manzoor, S.; Khalid, U.; Shamim, R.; Hashaam; Tayyab, Z.; Bashir, M. Suprathel Dressing at Split Thickness Skin Graft Donor Site for Pain Control and Wound Healing. Pak. J. Med. Health Sci. 2022, 16, 116. [Google Scholar]
- Krasner, D. Painful venous ulcers: Themes and stories about living with the pain and suffering. J. Wound Ostomy Cont. Nurs. 1998, 25, 158–168. [Google Scholar] [CrossRef]
- Zaulyanov, L.; Kirsner, R.S. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2007, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Foley, E.; Robinson, A.; Maloney, M. Skin Substitutes and Dermatology: A Review. Curr. Derm. Rep. 2013, 2, 101–112. [Google Scholar] [CrossRef]
- Jackson, S.R.; Roman, S. Matriderm and Split Skin Grafting for Full-Thickness Pediatric Facial Burns. J. Burn. Care Res. 2019, 40, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Hones, K.M.; Hones, J.; Satteson, E.S.; Chim, H. Treatment of complex extremity wounds with MatriDerm: First clinical experience in the US. J. Wound Care 2023, 32, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Orabona, G.D.; Maffia, F.; Audino, G.; Abbate, V.; Germano, C.; Bonavolontà, P.; Romano, A.; Villari, R.; Mormile, M.; Califano, L. The Use of Matriderm® for Scalp Full-Thickness Defects Reconstruction: A Case Series. J. Clin. Med. 2022, 11, 6041. [Google Scholar] [CrossRef]
- Wester, J.L.; Pittman, A.L.; Lindau, R.H.; Wax, M.K. AlloDerm with split-thickness skin graft for coverage of the forearm free flap donor site. Otolaryngol. Head Neck Surg. 2014, 150, 47–52. [Google Scholar] [CrossRef]
- Widmyer, A.S.; Mirhaidari, S.J.; Wagner, D.S. Implant-based Breast Reconstruction Outcomes Comparing Freeze-dried Aseptic Alloderm and Sterile Ready-to-use Alloderm. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2530. [Google Scholar] [CrossRef] [PubMed]
- Beers, P.J.; Adgerson, C.N.; Millan, S.B. Porcine tri-layer wound matrix for the treatment of stage IV pressure ulcers. JAAD Case Rep. 2016, 2, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019, 2019, e1286054. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-D.; Kang, T.J.; Lee, C.H.; Lee, A.-Y.; Noh, M. HaCaT Keratinocytes and Primary Epidermal Keratinocytes Have Different Transcriptional Profiles of Cornified Envelope-Associated Genes to T Helper Cell Cytokines. Biomol. Ther. 2012, 20, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Maarof, M.; Mh Busra, M.F.; Lokanathan, Y.; Bt Hj Idrus, R.; Rajab, N.F.; Chowdhury, S.R. Safety and efficacy of dermal fibroblast conditioned medium (DFCM) fortified collagen hydrogel as acellular 3D skin patch. Drug Deliv. Transl. Res. 2019, 9, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Gravante, G.; Sorge, R.; Merone, A.; Tamisani, A.M.; Di Lonardo, A.; Scalise, A.; Doneddu, G.; Melandri, D.; Stracuzzi, G.; Onesti, M.G.; et al. Hyalomatrix PA in Burn Care Practice: Results from a National Retrospective Survey, 2005 to 2006. Ann. Plast. Surg. 2010, 64, 69. [Google Scholar] [CrossRef]
- Caravaggi, C.; De Giglio, R.; Pritelli, C.; Sommaria, M.; Dalla Noce, S.; Faglia, E.; Mantero, M.; Clerici, G.; Fratino, P.; Dalla Paola, L.; et al. HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: A prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 2003, 26, 2853–2859. [Google Scholar] [CrossRef]
- You, H.J.; Han, S.K.; Rhie, J.W. Randomised controlled clinical trial for autologous fibroblast-hyaluronic acid complex in treating diabetic foot ulcers. J. Wound Care 2014, 23, 521–522+524+526–530. [Google Scholar] [CrossRef]
- Melnik, I.; Mnouskin, Y.; Verdiger Kurzbart, E.; Yoffe, B. Evaluation of a Porcine Dermal Collagen (Permacol) Implant for Abdominal Wall Reconstruction in a Pediatric Multitrauma Patient. Case Rep. Emerg. Med. 2014, 2014, e585723. [Google Scholar] [CrossRef]
- Mitchell, I.C.; Garcia, N.M.; Barber, R.; Ahmad, N.; Hicks, B.A.; Fischer, A.C. Permacol: A potential biologic patch alternative in congenital diaphragmatic hernia repair. J. Pediatr. Surg. 2008, 43, 2161–2164. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Ignotz, R.; Montilla, R.; Broderick, G.B.; Christakis, A.; Dunn, R.M. Long-term histologic and mechanical results of a PermacolTM abdominal wall explant. Hernia 2011, 15, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Baltazar, T.; Merola, J.; Catarino, C.M.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.Y.K.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Ju, H.W.; Lee, J.M.; Kim, D.-K.; Lee, O.J.; Moon, B.M.; Park, H.J.; Jeong, J.Y.; Yeon, Y.K.; Park, C.H. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1567–1574. [Google Scholar] [CrossRef]
- Piejko, M.; Radziun, K.; Bobis-Wozowicz, S.; Waligórska, A.; Zimoląg, E.; Nessler, M.; Chrapusta, A.; Madeja, Z.; Drukała, J. Adipose-Derived Stromal Cells Seeded on Integra® Dermal Regeneration Template Improve Post-Burn Wound Reconstruction. Bioengineering 2020, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cao, D.; Xie, J.; Li, H.; Chen, Z.; Bao, Q. Clinical experience of the use of Integra in combination with negative pressure wound therapy: An alternative method for the management of wounds with exposed bone or tendon. J. Plast. Surg. Hand Surg. 2021, 55, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatol. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Driver, V.R.; Lavery, L.A.; Reyzelman, A.M.; Dutra, T.G.; Dove, C.R.; Kotsis, S.V.; Kim, H.M.; Chung, K.C. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015, 23, 891–900. [Google Scholar] [CrossRef]
- Hicks, C.W.M.; Zhang, G.Q.; Canner, J.K.M.; Mathioudakis, N.M.; Coon, D.M.; Sherman, R.L.D.; Abularrage, C.J. Outcomes and Predictors of Wound Healing among Patients with Complex Diabetic Foot Wounds Treated with a Dermal Regeneration Template (Integra). Plast. Reconstr. Surg. 2020, 146, 893–902. [Google Scholar] [CrossRef]
- Srivastava, A.; Maniakas, A.; Myers, J.; Chambers, M.S.; Cardoso, R. Reconstruction of intraoral oncologic surgical defects with Integra® bilayer wound matrix. Clin. Case Rep. 2021, 9, 213–219. [Google Scholar] [CrossRef]
- Kok, Y.O.; Chong, S.J.; Basuki, A.; Tan, B.K. Early definitive treatment of partial-thickness alkali burns with tangential excision and biobrane. Arch. Plast. Surg. 2018, 45, 193. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-J.; See, J.L.; Choke, A.; Ooi, A.; Chong, S.J. BiobraneTM for burns of the pubic region: Minimizing dressing changes. Mil. Med. Res. 2018, 5, 29. [Google Scholar] [PubMed]
- Bairagi, A.; Griffin, B.; Tyack, Z.; Vagenas, D.; McPhail, S.M.; Kimble, R. Comparative effectiveness of Biobrane®, RECELL® Autologous skin Cell suspension and Silver dressings in partial thickness paediatric burns: BRACS randomised trial protocol. Burn. Trauma 2019, 7, s41038-019-0165-0. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.S.; Vasconez, H.C. Wound Healing: Biologics, Skin Substitutes, Biomembranes and Scaffolds. Healthcare 2014, 2, 356–400. [Google Scholar] [CrossRef]
- Still, J.; Glat, P.; Silverstein, P.; Griswold, J.; Mozingo, D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 2003, 29, 837–841. [Google Scholar] [CrossRef]
- Yeh, D.D.; Nazarian, R.M.; Demetri, L.; Mesar, T.; Dijkink, S.; Larentzakis, A.; Velmahos, G.; Sadik, K.W. Histopathological assessment of OASIS Ultra on critical-sized wound healing: A pilot study. J. Cutan. Pathol. 2017, 44, 523–529. [Google Scholar] [CrossRef]
- Brown-Etris, M.; Milne, C.T.; Hodde, J.P. An extracellular matrix graft (Oasis® wound matrix) for treating full-thickness pressure ulcers: A randomized clinical trial. J. Tissue Viability 2019, 28, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Tresoldi, M.M.; Malovini, A.; Visaggio, M.; Faga, A.; Scevola, S. Versatile use of dermal substitutes: A retrospective survey of 127 consecutive cases. Indian J. Plast. Surg. 2018, 51, 46–53. [Google Scholar] [CrossRef]
- Yiğitbaş, H.; Yavuz, E.; Beken Özdemir, E.; Önen, Ö.; Pençe, H.; Meriç, S.; Çelik, A.; Çelebi, F.; Yasti, A.C.; Sapmaz, T. Our experience with dermal substitute Nevelia® in the treatment of severely burned patients. Ulus Travma Acil Cerrahi Derg 2019, 25, 520–526. [Google Scholar] [CrossRef]
- De Angelis, B.; Orlandi, F.; D’autilio, M.F.L.M.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int. Wound J. 2018, 15, 695–706. [Google Scholar] [CrossRef]
- Manita, P.G.; Garcia-Orue, I.; Santos-Vizcaino, E.; Hernandez, R.M.; Igartua, M. 3D Bioprinting of Functional Skin Substitutes: From Current Achievements to Future Goals. Pharmaceuticals 2021, 14, 362. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Dańczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Jiao, T.; Zhao, T.; Wang, H.; Yang, S.; Li, D. 3D Bioprinted Skin Substitutes for Accelerated Wound Healing and Reduced Scar. J. Bionic Eng. 2021, 18, 900–914. [Google Scholar] [CrossRef]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef]
- Levin, A.A.; Karalkin, P.A.; Koudan, E.V.; Senatov, F.S.; Parfenov, V.A.; Lvov, V.A.; Petrov, S.V.; Pereira, F.D.A.S.; Kovalev, A.V.; Osidak, E.O.; et al. Commercial articulated collaborative in situ 3D bioprinter for skin wound healing. Int. J. Bioprint. 2023, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Abaci, H.E.; Coffman, A.; Doucet, Y.; Chen, J.; Jacków, J.; Wang, E.; Guo, Z.; Shin, J.U.; Jahoda, C.A.; Christiano, A.M. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun. 2018, 9, 5301. [Google Scholar] [CrossRef]
- Chen, L.; Yan, D.; Wu, N.; Zhang, W.; Yan, C.; Yao, Q.; Zouboulis, C.C.; Sun, H.; Fu, Y. 3D-Printed Poly-Caprolactone Scaffolds Modified with Biomimetic Extracellular Matrices for Tarsal Plate Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 219. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2020.00219 (accessed on 12 February 2024). [CrossRef]
- Yao, B.; Wang, R.; Wang, Y.; Zhang, Y.; Hu, T.; Song, W.; Li, Z.; Huang, S.; Fu, X. Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration. Sci. Adv. 2020, 6, eaaz1094. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kisiel, M.A.; Biedermann, T.; Klar, A.S. Immunomodulation of Skin Repair: Cell-Based Therapeutic Strategies for Skin Replacement (A Comprehensive Review). Biomedicines 2022, 10, 118. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, T.; Tang, Y.; Luo, G.; Liang, G.; He, W. Epigenetic regulation of macrophage polarization in wound healing. Burn. Trauma 2023, 11, tkac057. [Google Scholar] [CrossRef] [PubMed]

| Factor Type | Cells/Molecules | Role in Wound Pathology | References |
|---|---|---|---|
| Cells | Macrophages | Macrophages show impaired bacterial phagocytosis, removal of apoptotic cells and reduced polarization capacity. | [11] |
| Cells | Neutrophils | Exhibit cytotoxic activity. They are less susceptible to removal by macrophages and apoptosis. | [12] |
| Cells | Langerhans cells | Cells with impaired migration in chronic wounds, resulting in reduced epithelialization. | [13] |
| Metalloproteinases | Serine proteases | Degrade extracellular matrix components and growth factors. | [14] |
| Metalloproteinases | Collagenases (MMP1, MMP8, MMP13) | Abnormal regulation of inflammation. Overexpression in keratinocytes delays re-epithelialization. | [15] |
| Metalloproteinases | Gelatinases (MMP2, MMP9) | Secreted by fibroblasts and keratinocytes. Exert an antifibrotic effect. | [16] |
| Metalloproteinases | Stromelysins (MMP3) | Secreted by keratinocytes. Interferes with wound contraction. | [17] |
| Growth factors and other | B-cathenin | Inhibition of keratinocyte migration. | [18] |
| Growth factors and other | c-myc | Inhibition of keratinocyte migration. | [19] |
| Growth factors and other | TNF-α | Prolong the inflammatory phase, increase metalloproteinase activity. | [20] |
| Growth factors and other | IL-1 | Prolong the inflammatory phase, increase metalloproteinase activity. | [21] |
| Growth factors and other | IL-6 | Interleukin associated with cellular aging and increased inflammatory response. | [22] |
| Growth factors and other | IL-10 | Interleukin associated with phagocytosis. | [23] |
| Growth factors and other | Maspin | Anti-angiogenic factor secreted by keratinocytes. | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondej, K.; Zawrzykraj, M.; Czerwiec, K.; Deptuła, M.; Tymińska, A.; Pikuła, M. Bioengineering Skin Substitutes for Wound Management—Perspectives and Challenges. Int. J. Mol. Sci. 2024, 25, 3702. https://doi.org/10.3390/ijms25073702
Kondej K, Zawrzykraj M, Czerwiec K, Deptuła M, Tymińska A, Pikuła M. Bioengineering Skin Substitutes for Wound Management—Perspectives and Challenges. International Journal of Molecular Sciences. 2024; 25(7):3702. https://doi.org/10.3390/ijms25073702
Chicago/Turabian StyleKondej, Karolina, Małgorzata Zawrzykraj, Katarzyna Czerwiec, Milena Deptuła, Agata Tymińska, and Michał Pikuła. 2024. "Bioengineering Skin Substitutes for Wound Management—Perspectives and Challenges" International Journal of Molecular Sciences 25, no. 7: 3702. https://doi.org/10.3390/ijms25073702
APA StyleKondej, K., Zawrzykraj, M., Czerwiec, K., Deptuła, M., Tymińska, A., & Pikuła, M. (2024). Bioengineering Skin Substitutes for Wound Management—Perspectives and Challenges. International Journal of Molecular Sciences, 25(7), 3702. https://doi.org/10.3390/ijms25073702








