Semaglutide Improves Liver Steatosis and De Novo Lipogenesis Markers in Obese and Type-2-Diabetic Mice with Metabolic-Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Results
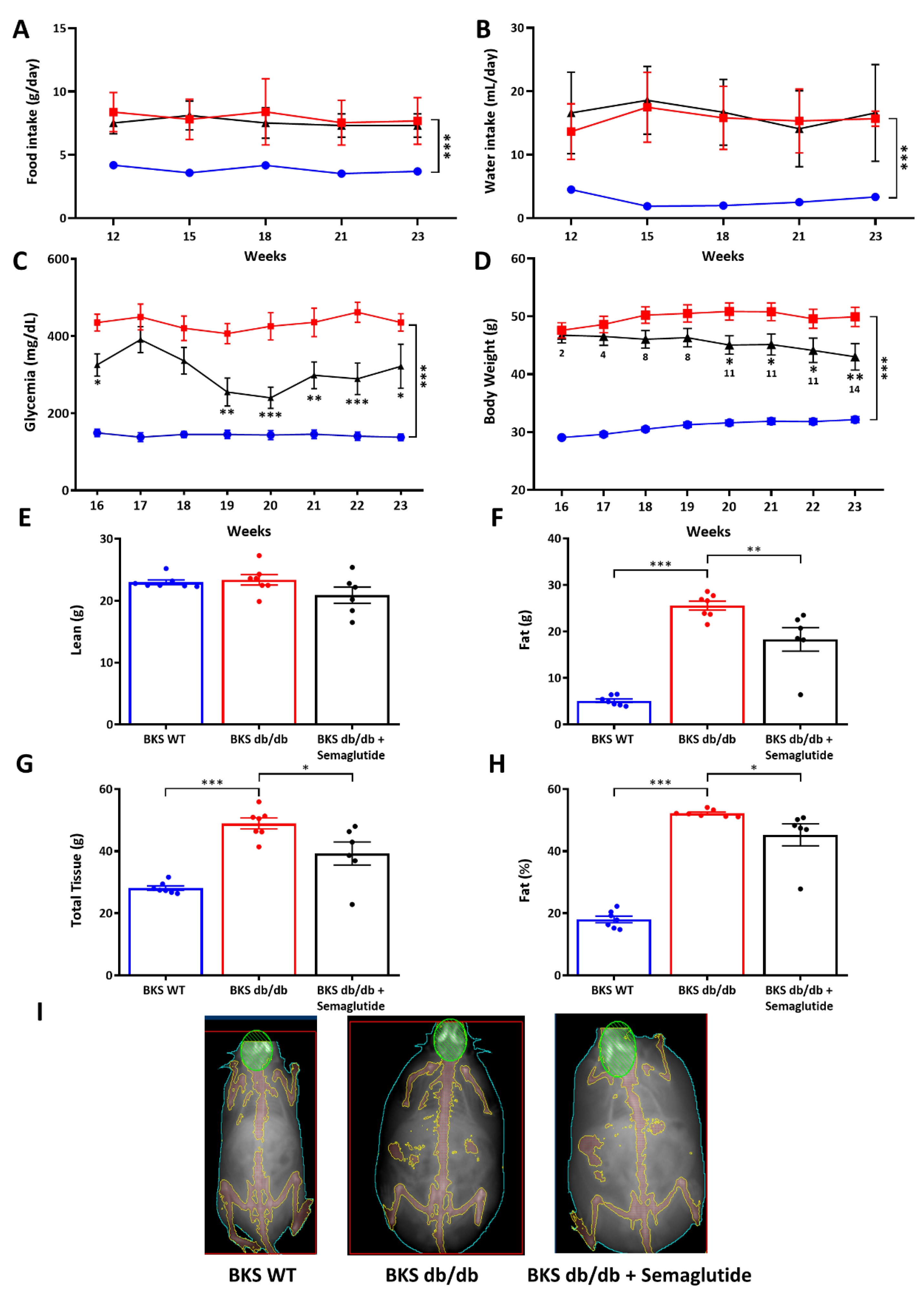
2.1. Semaglutide Improves Systemic Metabolic Parameters, Hepatic Function and Body Composition in BKS db/db Mice
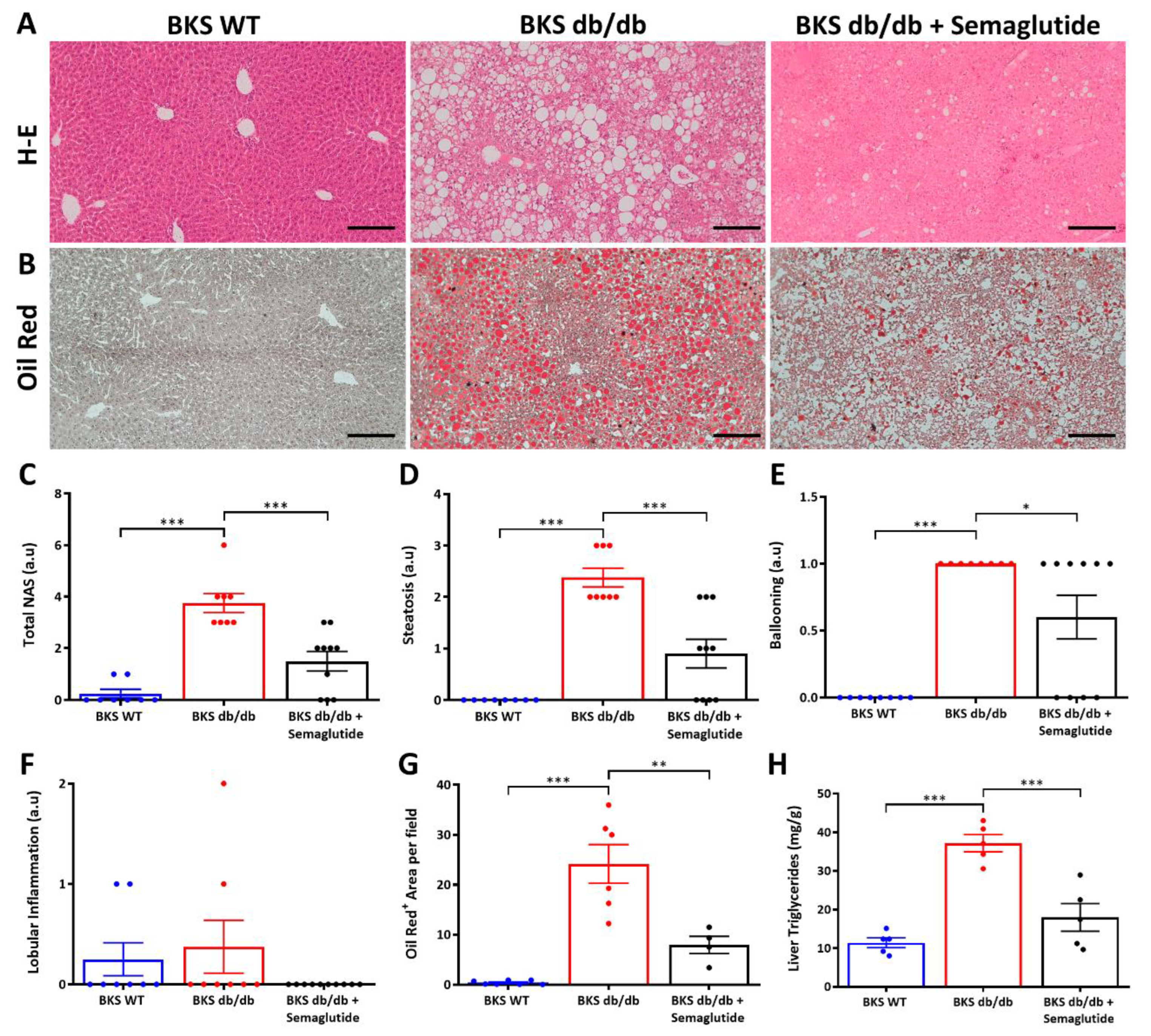
2.2. Treatment with Semaglutide Ameliorates NAFLD Activity Score and Reduces Intrahepatic Lipids
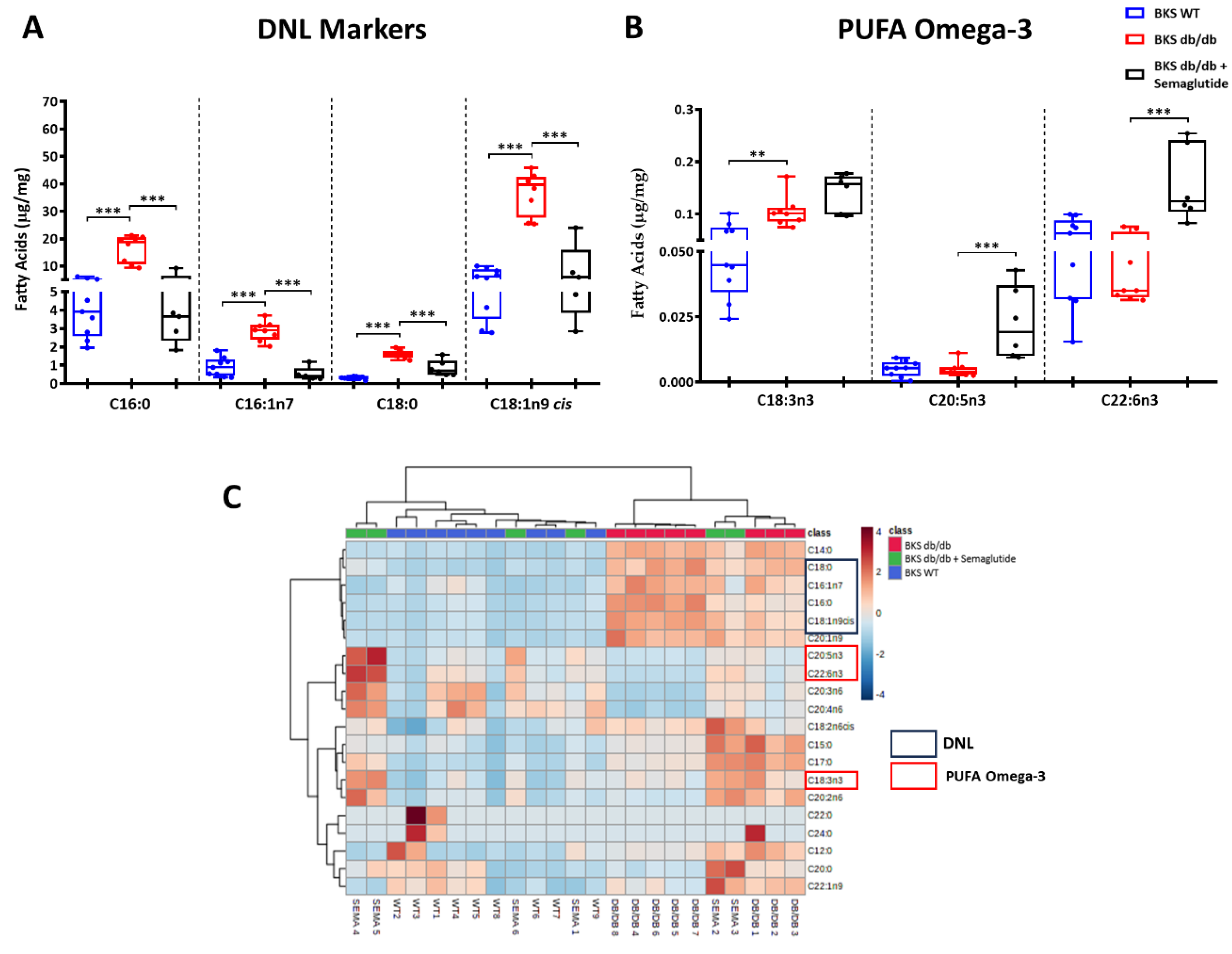
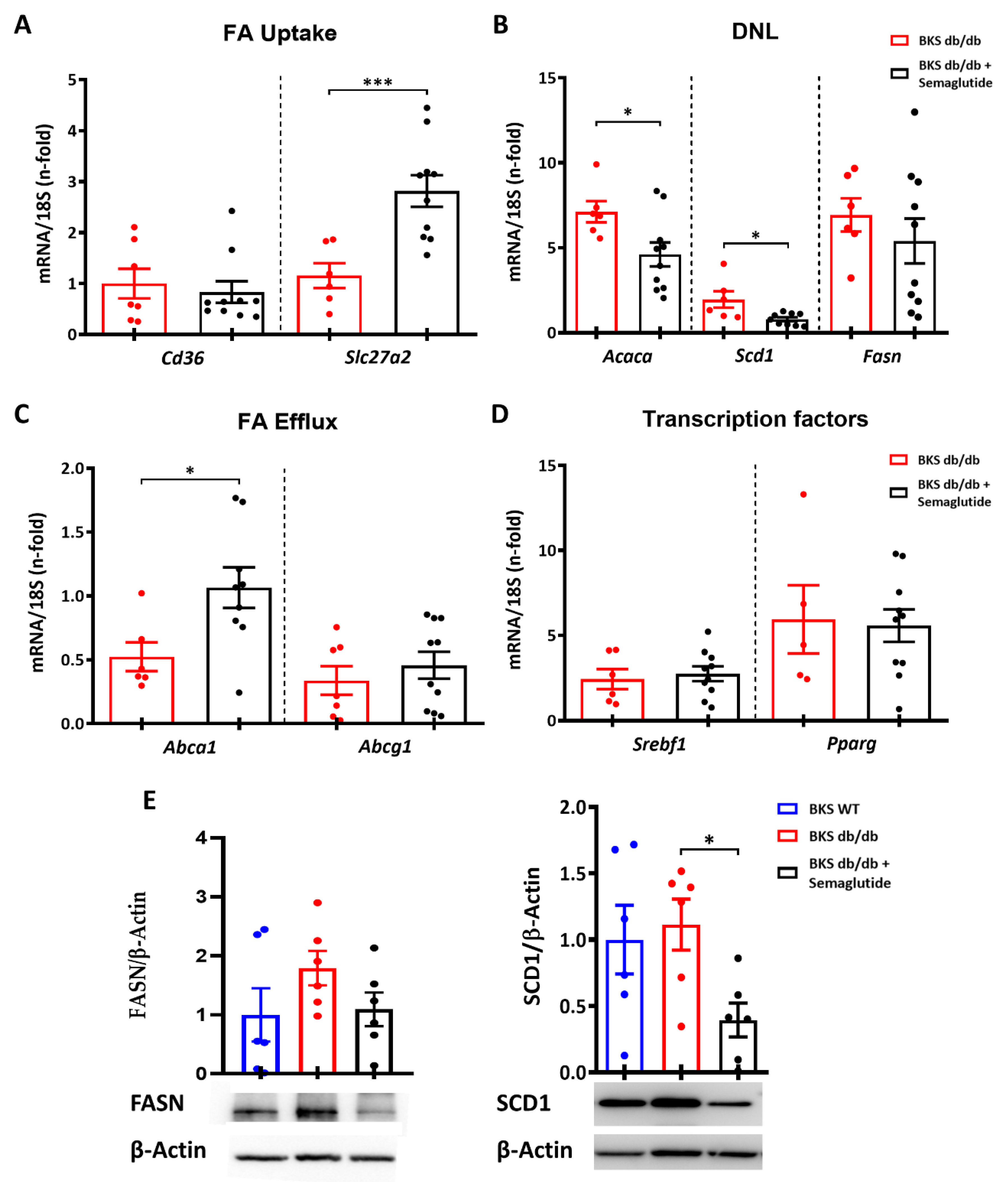
2.3. Semaglutide Modifies Lipidomic Profile and De Novo Lipogenesis Markers in Liver of BKS db/db Mice
3. Discussion
4. Material and Methods
4.1. Experimental Model
4.2. Biochemical Parameters
4.3. Dual-Energy X-ray Absorptiometry (DXA)
4.4. Liver Morphological Analysis
4.5. Lipid Profile Determination
4.6. Gene Expression Studies
4.7. Protein Studies
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abca1 | ATP-binding cassette, sub-family A member 1 |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| AP | alkaline phosphatase |
| c-HDL | high-density lipoprotein cholesterol |
| Diabesity | diabetes and obesity |
| DXA | dual-energy X-ray absorptiometry |
| FA | fatty acid |
| FATP | fatty acid transport protein |
| GLP1-RA | glucagon-like peptide 1 receptor agonist |
| MASLD | metabolic-dysfunction-associated steatotic liver disease |
| MASH | metabolic-dysfunction-associated steatohepatitis |
| NAFLD | non-alcoholic fatty liver disease |
| NAS | NAFLD activity score |
| PUFA | polyunsaturated fatty acid |
| SCD1 | Stearoyl-CoA desaturase-1 |
| T2DM | type 2 diabetes mellitus |
| TGs | triglycerides |
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 29, 101133. [Google Scholar]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Sung, K.C.; Jeong, W.S.; Wild, S.H.; Byrne, C.D. Combined Influence of Insulin Resistance, Overweight/Obesity, and Fatty Liver as Risk Factors for Type 2 Diabetes. Diabetes Care 2012, 35, 717–722. [Google Scholar] [CrossRef]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef]
- Somm, E.; Montandon, S.A.; Loizides-Mangold, U.; Gaïa, N.; Lazarevic, V.; De Vito, C.; Perroud, E.; Bochaton-Piallat, M.L.; Dibner, C.; Schrenzel, J.; et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl. Res. J. Lab. Clin. Med. 2021, 227, 75–88. [Google Scholar] [CrossRef]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes. Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Rajeev, S.P.; Wilding, J. GLP-1 as a target for therapeutic intervention. Curr. Opin. Pharmacol. 2016, 31, 44–49. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, H.Y. Therapeutic Mechanisms and Clinical Effects of Glucagon-like Peptide 1 Receptor Agonists in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 9324. [Google Scholar] [CrossRef]
- Mells, J.E.; Fu, P.P.; Sharma, S.; Olson, D.; Cheng, L.; Handy, J.A.; Saxena, N.K.; Sorescu, D.; Anania, F.A. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G225–G235. [Google Scholar] [CrossRef]
- Aldawsari, M.; Almadani, F.A.; Almuhammadi, N.; Algabsani, S.; Alamro, Y.; Aldhwayan, M. The Efficacy of GLP-1 Analogues on Appetite Parameters, Gastric Emptying, Food Preference and Taste Among Adults with Obesity: Systematic Review of Randomized Controlled Trials. Diabetes Metab. Syndr. Obesity Targets Ther. 2023, 16, 575–595. [Google Scholar] [CrossRef]
- Cornell, S. A review of GLP-1 receptor agonists in type 2 diabetes: A focus on the mechanism of action of once-weekly agents. J. Clin. Pharm. Ther. 2020, 45 (Suppl. S1), 17–27. [Google Scholar] [CrossRef]
- Panjwani, N.; Mulvihill, E.E.; Longuet, C.; Yusta, B.; Campbell, J.E.; Brown, T.J.; Streutker, C.; Holland, D.; Cao, X.; Baggio, L.L.; et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology 2013, 154, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.K.; Karuppasamy, M.; Sahoo, B.M. Therapeutic Potential of Semaglutide, a Newer GLP-1 Receptor Agonist, in Abating Obesity, Non-Alcoholic Steatohepatitis and Neurodegenerative diseases: A Narrative Review. Pharm. Res. 2022, 39, 1233. [Google Scholar] [CrossRef]
- Patel Chavez, C.; Cusi, K.; Kadiyala, S. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD. J. Clin. Endocrinol. Metab. 2022, 107, 29–38. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. Innov. Hepatol. 2019, 1, 312–328. [Google Scholar] [CrossRef]
- Sharma, S.; Mells, J.E.; Fu, P.P.; Saxena, N.K.; Anania, F.A. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS ONE 2011, 6, e25269. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; for the STEP 8 Investigators; et al. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Loomba, R.; Abdelmalek, M.F.; Armstrong, M.J.; Jara, M.; Kjær, M.S.; Krarup, N.; Lawitz, E.; Ratziu, V.; Sanyal, A.J.; Schattenberg, J.M.; et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 511–522. [Google Scholar] [CrossRef]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Terrault, N.; Chalasani, N.P.; Abdelmalek, M.F.; McCullough, A.J.; Shringarpure, R.; Ferguson, B.; Lee, L.; et al. Factors Associated with Histologic Response in Adult Patients with Nonalcoholic Steatohepatitis. Gastroenterology 2019, 156, 88–95.e5. [Google Scholar] [CrossRef]
- Singh, S.; Wright, E.E.; Kwan, A.Y.M.; Thompson, J.C.; Syed, I.A.; Korol, E.E.; Waser, N.A.; Yu, M.B.; Juneja, R. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes. Metab. 2017, 19, 228–238. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Drewe, J.; Göke, B.; Schmidt, H.; Rohrer, B.; Lareida, J.; Beglinger, C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am. J. Physiol. 1999, 276, R1541–R1544. [Google Scholar] [CrossRef]
- Inia, J.A.; Stokman, G.; Morrison, M.C.; Worms, N.; Verschuren, L.; Caspers, M.P.M.; Menke, A.L.; Petitjean, L.; Chen, L.; Petitjean, M.; et al. Semaglutide Has Beneficial Effects on Non-Alcoholic Steatohepatitis in Ldlr−/−.Leiden Mice. Int. J. Mol. Sci. 2023, 24, 8494. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Ding, X.; Saxena, N.K.; Lin, S.; Gupta, N.; Anania, F.A. Exendin-4, a Glucagon-Like Protein-1 (GLP-1) Receptor Agonist, Reverses Hepatic Steatosis in ob/ob Mice. Hepatology 2006, 43, 173. [Google Scholar] [CrossRef]
- Niu, S.; Chen, S.; Chen, X.; Ren, Q.; Yue, L.; Pan, X.; Zhao, H.; Li, Z.; Chen, X. Semaglutide ameliorates metabolism and hepatic outcomes in an NAFLD mouse model. Front. Endocrinol. 2022, 13, 1046130. [Google Scholar] [CrossRef]
- Pontes-da-Silva, R.M.; de Souza Marinho, T.; de Macedo Cardoso, L.E.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Obese mice weight loss role on nonalcoholic fatty liver disease and endoplasmic reticulum stress treated by a GLP-1 receptor agonist. Int. J. Obes. 2021, 46, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Michurina, S.V.; Ishenko, I.J.; Klimontov, V.V.; Archipov, S.A.; Myakina, N.E.; Cherepanova, M.A.; Zavjalov, E.L.; Koncevaya, G.V.; Konenkov, V.I. Linagliptin alleviates fatty liver disease in diabetic db/db mice. World J. Diabetes 2016, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.; Dearlove, D.J.; Hodson, L. Nutritional regulation of hepatic de novo lipogenesis in humans. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 65–71. [Google Scholar] [CrossRef]
- Jeyakumar, S.M.; Vajreswari, A. Stearoyl-CoA desaturase 1: A potential target for non-alcoholic fatty liver disease?-perspective on emerging experimental evidence. World J. Hepatol. 2022, 14, 168. [Google Scholar] [CrossRef]
- Wang, W.; Kong, Y.; Wang, X.; Wang, Z.; Tang, C.; Li, J.; Yang, Q.; Chen, Y.Q.; Zhu, S. Identification of novel SCD1 inhibitor alleviates nonalcoholic fatty liver disease: Critical role of liver-adipose axis. Cell Commun. Signaling CCS 2023, 21, 268. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl–CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482. [Google Scholar] [CrossRef]
- Miyazaki, M.; Flowers, M.T.; Sampath, H.; Chu, K.; Otzelberger, C.; Liu, X.; Ntambi, J.M. Hepatic Stearoyl-CoA Desaturase-1 Deficiency Protects Mice from Carbohydrate-Induced Adiposity and Hepatic Steatosis. Cell Metab. 2007, 6, 484–496. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016, 64, 399. [Google Scholar] [CrossRef]
- Ben-Shlomo, S.; Zvibel, I.; Shnell, M.; Shlomai, A.; Chepurko, E.; Halpern, Z.; Barzilai, N.; Oren, R.; Fishman, S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J. Hepatol. 2011, 54, 1214–1223. [Google Scholar] [CrossRef]
- Xu, X.; Poulsen, K.L.; Wu, L.; Liu, S.; Miyata, T.; Song, Q.; Wei, Q.; Zhao, C.; Lin, C.; Yang, J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target. Ther. 2022, 7, 287. [Google Scholar] [CrossRef]
- Santos-Laso, A.; Gutiérrez-Larrañaga, M.; Alonso-Peña, M.; Medina, J.M.; Iruzubieta, P.; Arias-Loste, M.T.; López-Hoyos, M.; Crespo, J. Pathophysiological Mechanisms in Non-Alcoholic Fatty Liver Disease: From Drivers to Targets. Biomedicines 2022, 10, 46. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313. [Google Scholar] [CrossRef]
- Cavelier, C.; Lorenzi, I.; Rohrer, L.; von Eckardstein, A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim. Biophys. Acta 2006, 1761, 655–666. [Google Scholar] [CrossRef]
- Melton, E.M.; Cerny, R.L.; Watkins, P.A.; DiRusso, C.C.; Black, P.N. Human Fatty Acid Transport Protein 2a/Very Long Chain Acyl-CoA Synthetase 1 (FATP2a/Acsvl1) Has a Preference in Mediating the Channeling of Exogenous n-3 Fatty Acids into Phosphatidylinositol. J. Biol. Chem. 2011, 286, 30670. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; de Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011, 31, 1285–1297. [Google Scholar] [CrossRef]
- Bhaswant, M.; Poudyal, H.; Brown, L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J. Nutr. Biochem. 2015, 26, 571–584. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Itou, M.; Taniguchi, E.; Sata, M. Exendin-4, aglucagon-like peptide-1 receptor agonist, modulateshepatic fatty acid composition and -Δ5-desaturase index in a murine model of non-alcoholic steatohepatitis. Int. J. Mol. Med. 2014, 34, 782–787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Opazo-Ríos, L.; Soto-Catalán, M.; Lázaro, I.; Sala-Vila, A.; Jiménez-Castilla, L.; Orejudo, M.; Moreno, J.A.; Egido, J.; Mas-Fontao, S. Meta-Inflammation and De Novo Lipogenesis Markers Are Involved in Metabolic Associated Fatty Liver Disease Progression in BTBR ob/ob Mice. Int. J. Mol. Sci. 2022, 23, 3965. [Google Scholar] [CrossRef]
| Variables | BKS WT | BKS db/db | BKS db/db + Semaglutide |
|---|---|---|---|
| Glycemia (mg/dL) | 164.0 (155.8–176.8) | 431.0 (374.8–502.3) ### | 308.0 (158.3–490.0) * |
| Body Weight (g) | 31.7 (30.9–33.5) | 50.3 (46.0–53.9) ### | 42.2 (38.6–48.6) ** |
| Liver Weight (g) | 1.4 (1.1–1.8) | 5.2 (4.6–5.3) ### | 3.8 (2.9–4.1) *** |
| AST (IU/L) | 95.0 (65.0–129.0) | 140.0 (117.0–241.0) | 94.0 (82.5–119.8) * |
| ALT (IU/L) | 42.0 (32.0–48.0) | 195.0 (179.0–318.0) ### | 74.0 (60.3–113.8) *** |
| AP (IU/L) | 83.5 (70.8–94.8) | 211.0 (180.0–257.0) ### | 113.0 (106.0–134.0) *** |
| TGs (mg/dL) | 73.5 (54.3–84.3) | 94.0 (78.0–198.0) # | 65.0 (47.0–143.0) |
| Total Cholesterol (mg/dL) | 88.0 (82.2–91.2) | 165.0 (145.3–192.2) ### | 140.0 (129.0–171.0) |
| c-HDL (mg/dL) | 85.0 (78.0–92.0) | 135.0 (121.0–154.3) ### | 113.0 (106.5–138.5) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Catalán, M.; Opazo-Ríos, L.; Quiceno, H.; Lázaro, I.; Moreno, J.A.; Gómez-Guerrero, C.; Egido, J.; Mas-Fontao, S. Semaglutide Improves Liver Steatosis and De Novo Lipogenesis Markers in Obese and Type-2-Diabetic Mice with Metabolic-Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2024, 25, 2961. https://doi.org/10.3390/ijms25052961
Soto-Catalán M, Opazo-Ríos L, Quiceno H, Lázaro I, Moreno JA, Gómez-Guerrero C, Egido J, Mas-Fontao S. Semaglutide Improves Liver Steatosis and De Novo Lipogenesis Markers in Obese and Type-2-Diabetic Mice with Metabolic-Dysfunction-Associated Steatotic Liver Disease. International Journal of Molecular Sciences. 2024; 25(5):2961. https://doi.org/10.3390/ijms25052961
Chicago/Turabian StyleSoto-Catalán, Manuel, Lucas Opazo-Ríos, Hernán Quiceno, Iolanda Lázaro, Juan Antonio Moreno, Carmen Gómez-Guerrero, Jesús Egido, and Sebastian Mas-Fontao. 2024. "Semaglutide Improves Liver Steatosis and De Novo Lipogenesis Markers in Obese and Type-2-Diabetic Mice with Metabolic-Dysfunction-Associated Steatotic Liver Disease" International Journal of Molecular Sciences 25, no. 5: 2961. https://doi.org/10.3390/ijms25052961
APA StyleSoto-Catalán, M., Opazo-Ríos, L., Quiceno, H., Lázaro, I., Moreno, J. A., Gómez-Guerrero, C., Egido, J., & Mas-Fontao, S. (2024). Semaglutide Improves Liver Steatosis and De Novo Lipogenesis Markers in Obese and Type-2-Diabetic Mice with Metabolic-Dysfunction-Associated Steatotic Liver Disease. International Journal of Molecular Sciences, 25(5), 2961. https://doi.org/10.3390/ijms25052961






