O26 Polysaccharides as Key Players in Enteropathogenic E. coli Immune Evasion and Vaccine Development
Abstract
1. Introduction
2. Results
2.1. Visualization of the Polysaccharide Capsule
2.2. Gas Chromatography Analysis
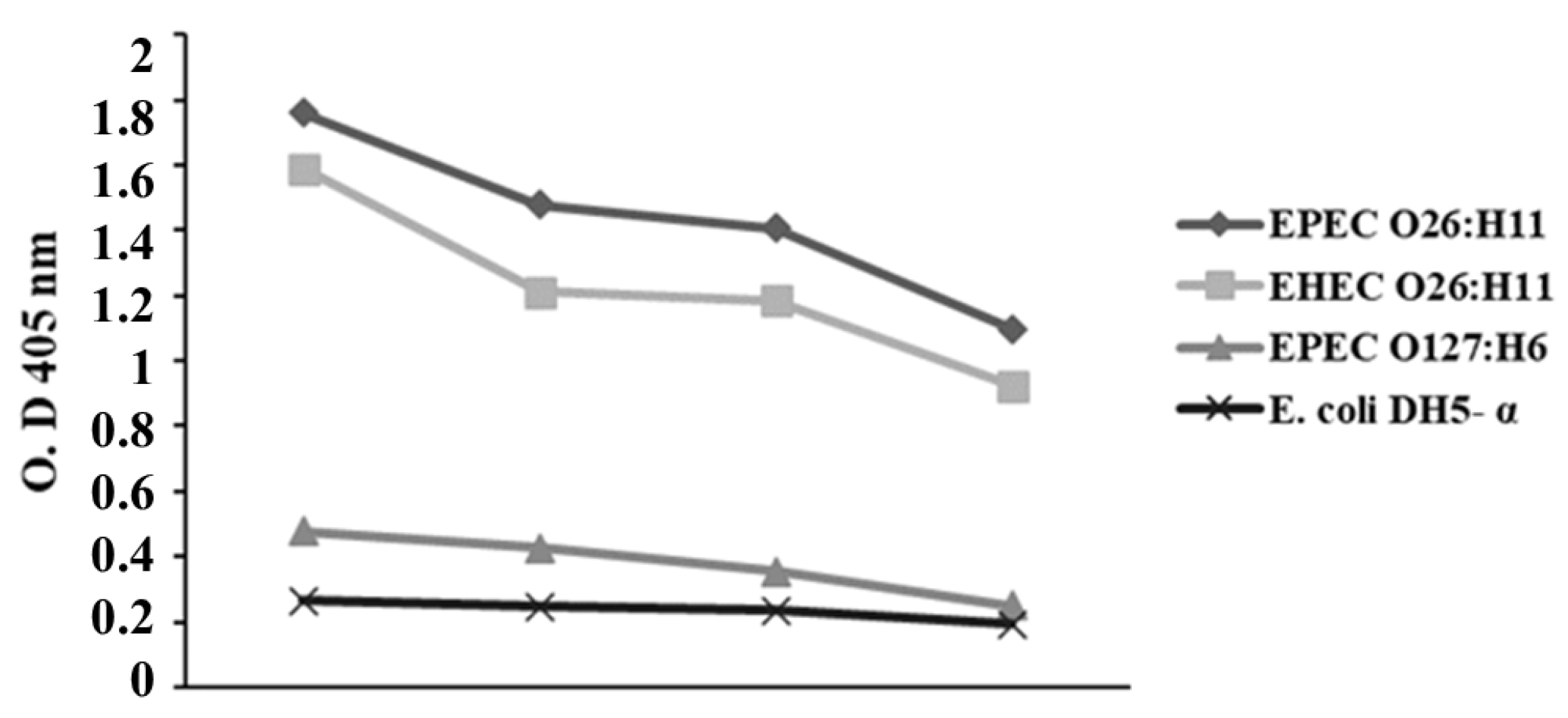
2.3. Influence of Anti-O26 Polysaccharide Antibodies on E. coli Recognition
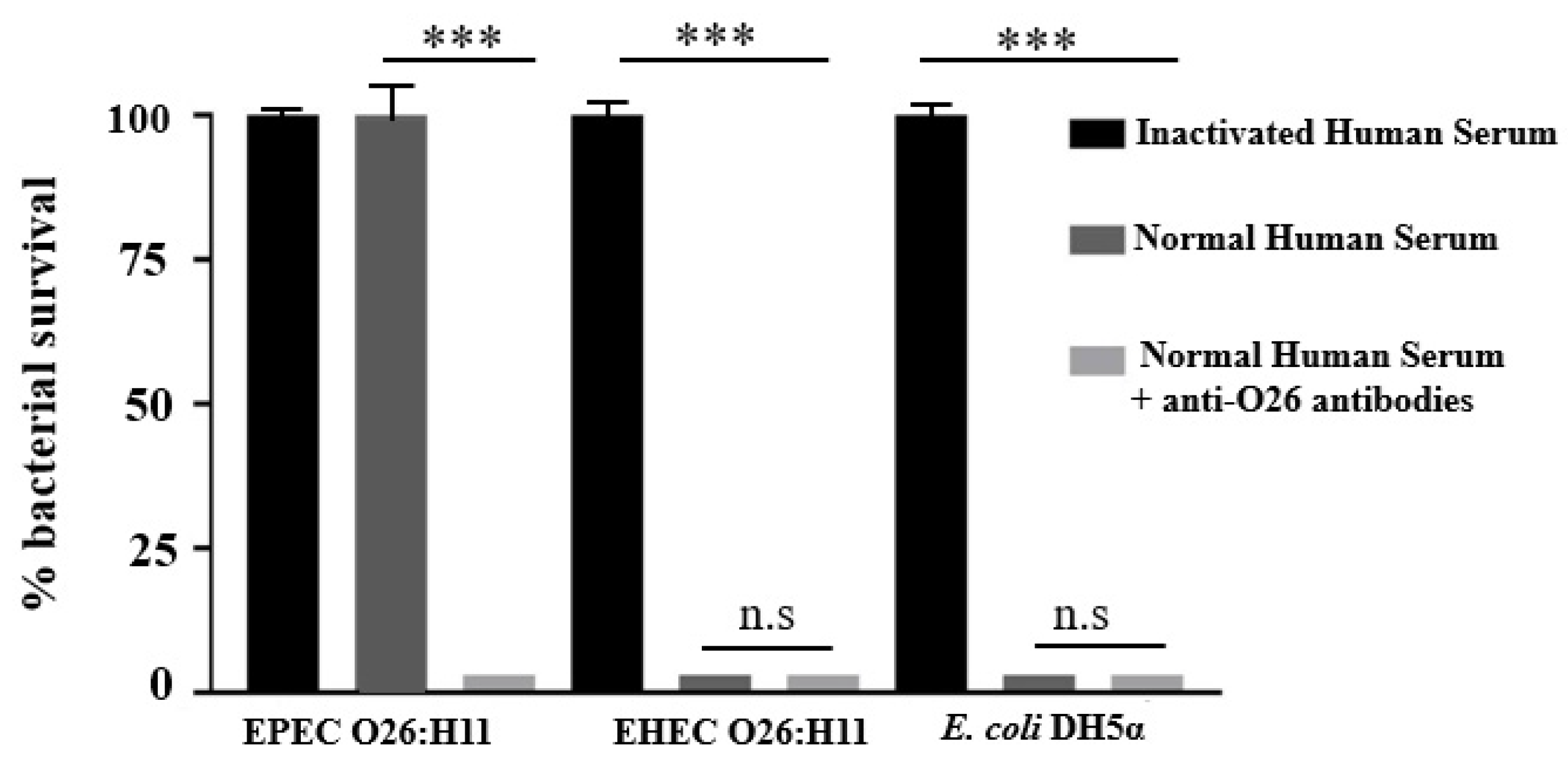
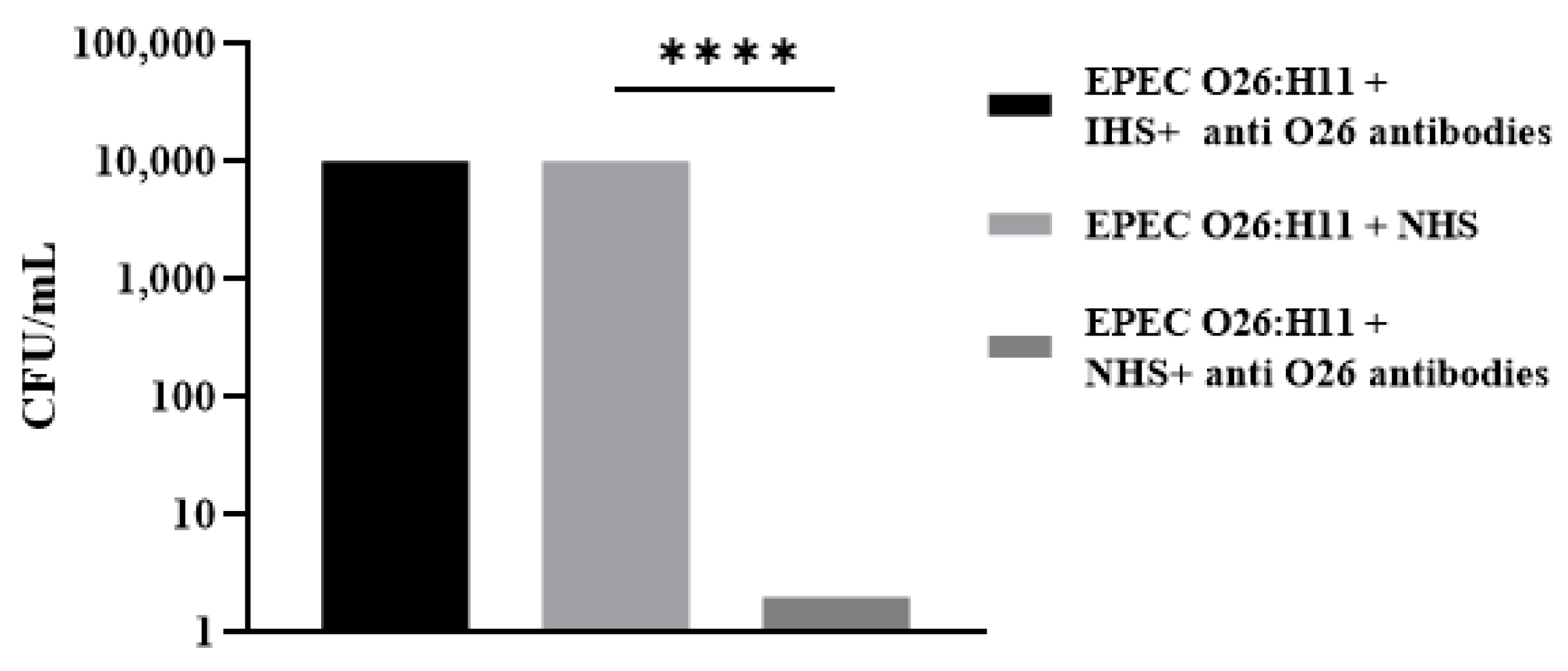
2.4. Influence of Anti-O26 Antibodies on Complement Response
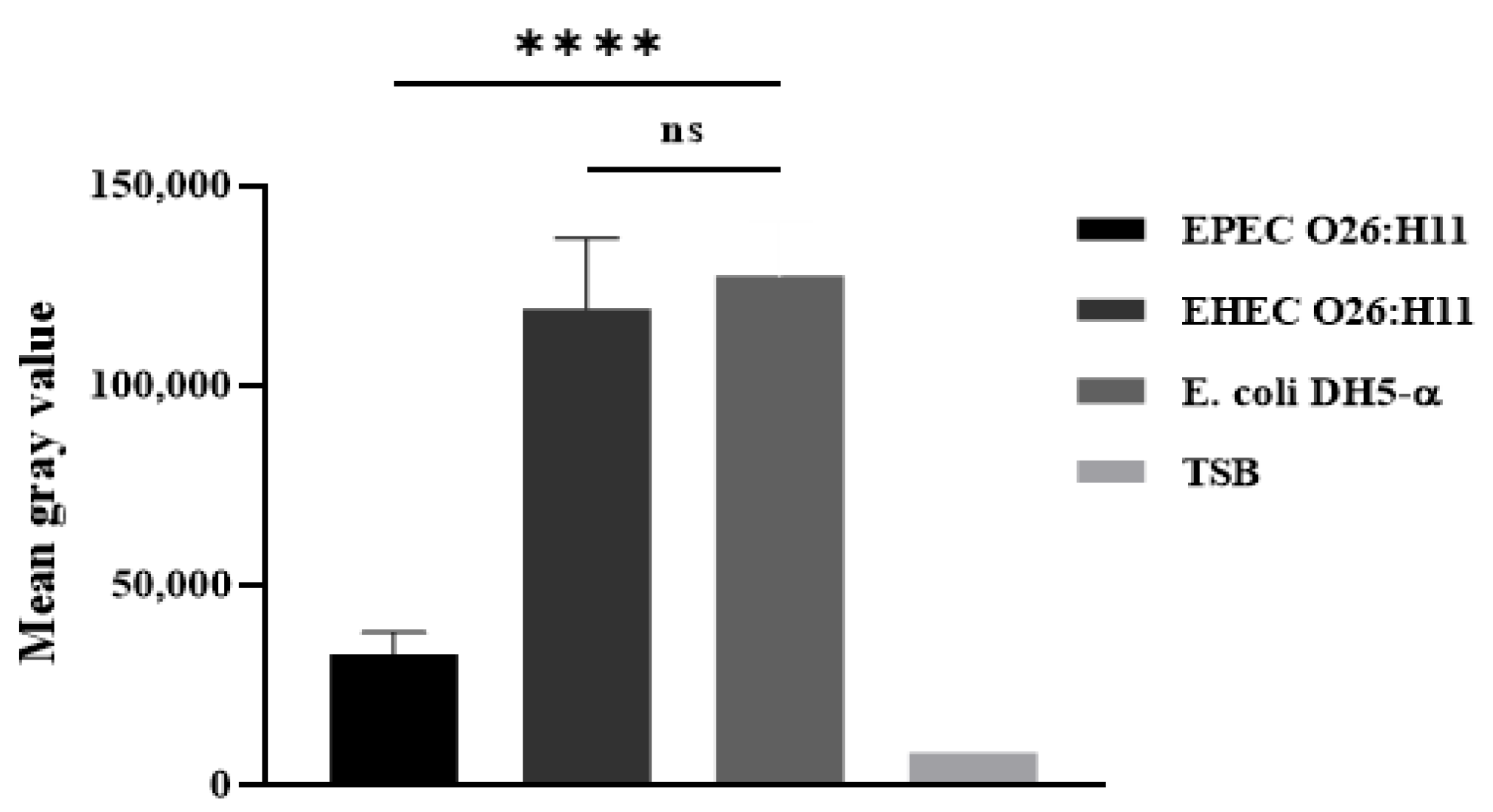
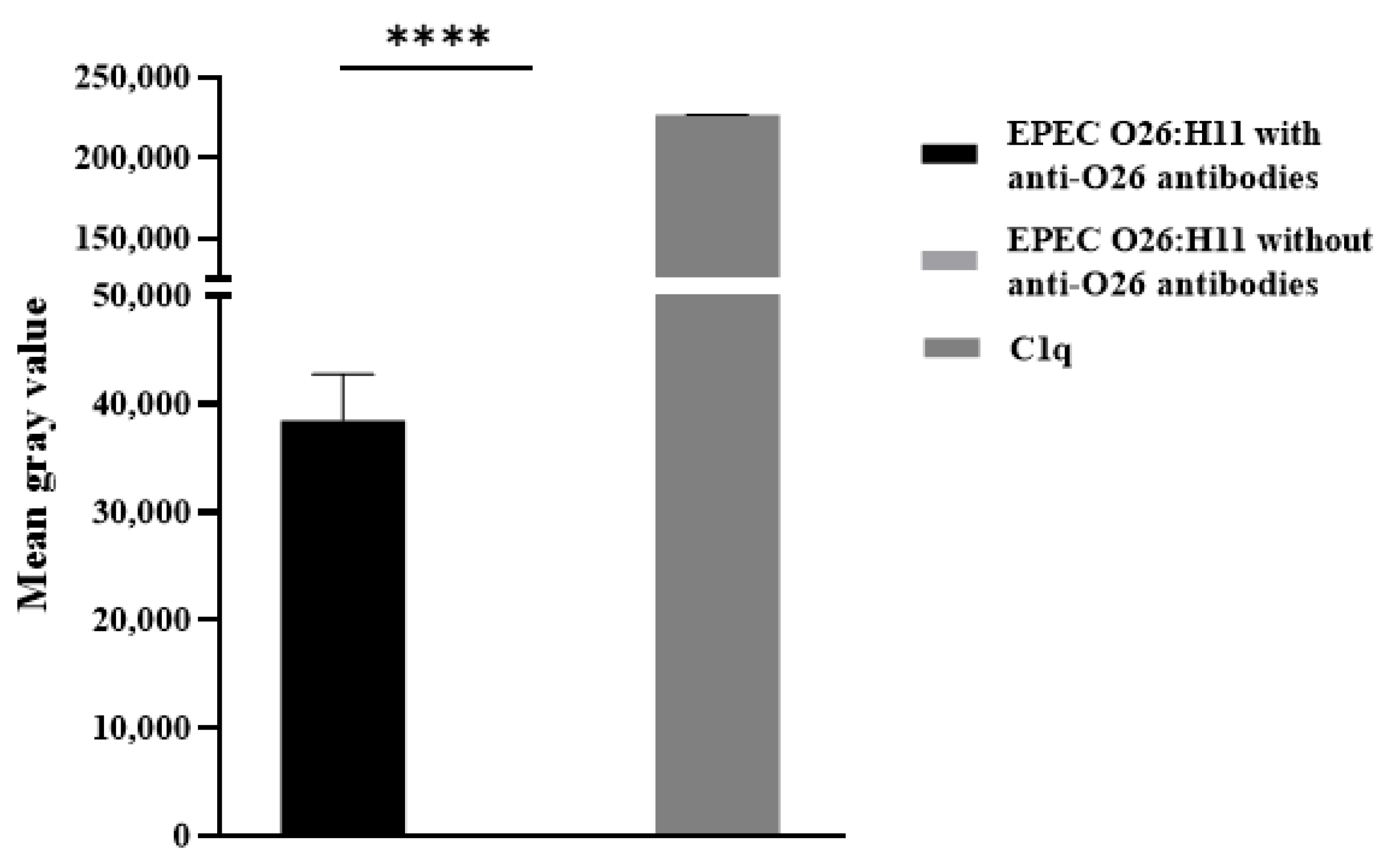
2.5. Deposition of C3b and C1q on the Bacterial Surface
2.6. Influence of Anti-O26 Antibodies on Phagocytosis
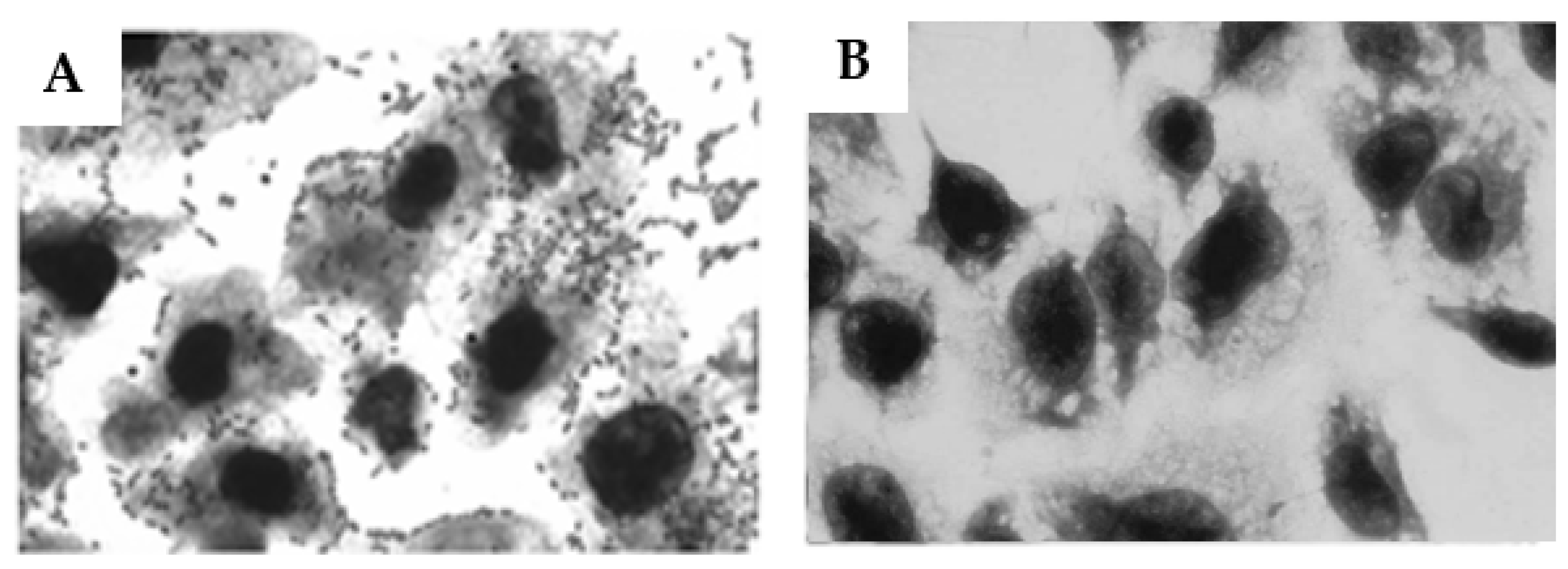
2.7. Influence of Anti-O26 Antibodies on the Adherence of Capsulated aEPEC O26:H11 to Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Cell Lines
4.3. Antibodies against O26 Polysaccharides
4.4. Pseudo-Capsule Visualization
4.5. Extraction and Purification of LPS
4.6. Sugar Analysis
4.7. Serum Resistance Assay
4.8. C3b Deposition on the Bacterial Surface
4.9. C1q Deposition on the Bacterial Surface
4.10. Phagocytosis
4.11. Inhibition of Bacterial Adhesion to Epithelial Cells
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piazza, R.M.F.; Delannoy, S.; Fach, P.; Saridakis, H.O.; Pedroso, M.Z.; Rocha, L.B.; Gomes, T.A.T.; Vieira, M.A.M.; Beutin, L.; Guth, B.E.C. Diarrheagenic E. coli O26 Strains Isolated in Brazil. J. ASM 2013, 79, 6847–6854. [Google Scholar] [CrossRef]
- Multistate Outbreaks of Shiga Toxin-Producing Escherichia coli O26 Infections Linked to Chipote Mexican Grill Restaurants (Final Update). E. coli (Escherichia coli). Centers for Disease Control and Prevention. CDC. Available online: www.cdc.gov/ecoli/2015/o26-11-15 (accessed on 4 December 2023).
- Outbreak of E. coli Infections Linked to Ground Beef. Centers for Disease. Control and Prevention. CDC. Available online: https://www.cdc.gov/ecoli/2018/o26-09-18/index.html#print (accessed on 1 December 2022).
- Minary, K.; Tanne, C.; Kwon, T.; Faudeux, C.; Clave, S.; Longevin, L.; Pietrment, C.; Enoch, C.; Parmentier, C.; Mariani-Kikdjian, P.; et al. Outbreak of hemolytic uremic syndrome with unusually severe clinical presentation caused by Shiga toxin-producing Escherichia coli O26:H11 in France. Arch. De Pediatr. 2022, 29, 448–452. [Google Scholar] [CrossRef]
- Muniesa, A.; Schembri, M.A.; Hauf, N.; Chakraborty, T. Active genetic elements present in the locus of enterocyte effacement in Escherichia coli O26 and their role in mobility. Infect. Immun. 2006, 74, 4190–4199. [Google Scholar] [CrossRef][Green Version]
- Bielaszewska, A.; Sonntag, A.; Schmidt, M.A.; Karch, H. Presence of virulence and fitness gene modules of enterohemorrhagic Escherichia coli in atypical enteropathogenic Escherichia coli 026. Microbes Infect. 2007, 9, 891–897. [Google Scholar] [CrossRef]
- Whitfield, C. Biosynthesis and Assembly of Capsular Polysaccharides in Escherichia coli. Rev. Biochem. 2006, 75, 39–69. [Google Scholar] [CrossRef]
- Biran, D.; Rosenshine, I.; Ron, E.Z. Escherichia coli O-antigen capsule (group 4) is essential for serum resistance. Res. Microbiol. 2020, 171, 99–101. [Google Scholar] [CrossRef]
- Shifrin, Y.; Peleg, A.; Ilan, O.; Nadler, C.; Kobi, S.; Baruch, K.; Yerushalmi, G.; Berdichevsky, T.; Altuvia, S.; Elgrably-Weiss, M.; et al. Transient shielding of intimin and the type III secretion system of enterohemorrhagic and enteropathogenic Escherichia coli by a group4 capsule. J. Bacteriol. 2008, 190, 5063.e74. [Google Scholar] [CrossRef] [PubMed]
- Caboni, M.; Pedron, T.; Rossi, O.; Goulding, D.; Pickard, D.; Citiulo, F.; MacLennan, C.A.; Dougan, G.; Thomson, N.R.; Saul, A.; et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015, 11, e1004749. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, P.; Martorana, M.; Polissi, A. The lipopolysaccharide transport (Lpt) machinery: A nonconventional transporter for lipopolysaccharide assembly at the outer membrane of Gram-negative bacteria. J. Biol. Chem. 2017, 44, 17981–17990. [Google Scholar] [CrossRef]
- Peleg, A.; Shifrin, Y.; Ilan, O.; Nadler-Yona, C.; Nov, S.; Koby, S.; Baruch, K.; Altuvia, S.; Elgrably-Weiss, M.; Abe, C.M.; et al. Identification of an Escherichia coli Operon Required for Formation of the O-Antigen Capsule. J. Bacteriol. 2005, 187, 52595266. [Google Scholar] [CrossRef]
- Thomassin, J.L.; Lee, M.J.; Brannon, J.R.; Sheppard, D.C.; Gruenheid, S.; Moual, H.L. Both Group 4 Capsule and Lipopolysaccharide O-antigen Contribute to nteropathogenic Escherichia coli Resistance to Human α-Defensin 5. PLoS ONE 2013, 8, e82475. [Google Scholar] [CrossRef]
- Santos, M.F.; New, R.R.C.; Andrade, G.R.; Ozaki, C.Y.; Sant’Anna, O.A.; Mendonça-Previato, L.; Trabulsi, L.R.; Domingos, M.O. Lipopolysaccharide as an antigen Target for the Formulation of a Universal Vaccine against Escherichia coli O111 strains. Clin. Vaccine Immul. 2010, 11, 1172–1780. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.R.; New, R.R.C.; Sant’Anna, O.A.; Williams, N.A.; Alves, R.C.B.; Pimenta, D.C.; Viferelli, H.; Melo, B.S.; Rocha, L.B.; Piazza, R.M.F.; et al. A universal polysaccharide conjugated vaccine against O111 E. coli. Hum. Vaccines Immunother. 2014, 10, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.G.S.; Franzolin, M.R.; Anjos, G.F.; Barbosa, A.S.; Santos, L.F.; Miranda, K.F.; Marques, R.M.; Souza, M.C.; Piazza, R.M.F.; Domingos, M.O. O55 Polysaccharides Are Good Antigen Targets for the Formulation of Vaccines against O55 STEC and Capsulated aEPEC Strains. Pathogens 2022, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Kafmann, S.; Dorhoi, A. Molecular determinants in phagocyte-bacteria interactions. Immunity 2016, 44, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Sahly, H.; Podschun, R.; Oelschlaeger, T.A.; Greiwe, M.; Parolis, H.; Hasty, D.; Kekow, J.; Ullmann, U.; Ofek, I.; Sela, S. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect. Immun. 2000, 68, 6744–6749. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M. Ficolins: Complement-Activating Lectins Involved in Innate Immunity. J. Innate Immun. 2010, 2, 4–32. [Google Scholar] [CrossRef]
- Tytgat, H.L.P.; Lebeer, S. The Sweet Tooth of Bacteria: Common Themes in Bacterial Glycoconjugates. Microbiol. Mol. Biol. Rev. 2014, 78, 372–417. [Google Scholar] [CrossRef]
- Walsh, A.G.; Matewish, M.J.; Burrows, L.L.; Monteiro, M.A.; Perry, M.B.; Lam, J.S. Lipopolysaccharide core phosphates arerequired or viability and intrinsic drug resistance in Pseudomonas aeruginosa. Mol. Microbiol. 2000, 35, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, F.; Wang, L.; Li, H.; Gu, G.; Li, E. Rhamnose-containing compounds: Biosynthesis and Applications. Molecules 2022, 27, 5315. [Google Scholar] [CrossRef]
- Ridley, B.L.; Jeyaretnam, B.S.; Carlson, R.W. The type and yield of lipopolysaccharide from symbiotically deficient Rhizobium lipopolysaccharide mutants vary depending on the extraction method. Glycobiology 2000, 10, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Lopes Alves, L.; Travassos, L.R.; Previato, J.O.; Mendonça-Previato, L. Novel antigenic determinants from peptidorhamonomannans of Sporothrix schenckii. Glycobiology 1994, 4, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.; Gutierrez, A.L.; Mattos, K.A.; Jones, C.; Wait, R.; Previato, J.O.; Mendonça-Previato, L. Molecular analysis of a novel family of complex glycoinositolphosphoryl ceramides from Cryptococcus neoformans: Structural differences between encapsulated and acapsular yeast forms. Glycobiology 2002, 12, 409–420. [Google Scholar] [CrossRef]
- Baron, F.; Cochet, M.F.; Ablain, W.; Grosset, N.; Madec, M.N.; Gonnet, F.; Jan, S.; Gautier, M. Rapid and cost-effective method for micro-organism enumeration based on miniaturization of conventional plate-couting technique. Lait INRA Ed. 2006, 86, 251–257. [Google Scholar] [CrossRef]
- Beck, N.K.; Callahan, K.; Nappier, S.P.; Kim, H.; Sobsey, M.D.; Meschke, J.S. Development of a Spot-Titer Culture assay for Quantifying bacteria and viral. J. Rapid Methods Autom. Microbiol. 2009, 17, 455–464. [Google Scholar] [CrossRef]

| Monosaccharide | Molar Ratio a | |
|---|---|---|
| EPEC O26:H11 | EHEC O26:H11 | |
| Rha | 2.8 | 2.5 |
| RhaNAc | 1.0 | 1.0 |
| Glc | 10.0 | 1.69 |
| Hep | 3.6 | 0.89 |
| Kdo | 2.6 | 0.46 |
| GlcNAx | 4.5 | 2.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemos, T.J.d.S.; Silva, H.G.d.S.; Previato, J.O.; Mendonça-Previato, L.; Freitas, E.O.d.; Barbosa, A.S.; Franzolin, M.R.; Santos, L.F.d.; Melo, B.d.S.; Anjos, G.F.d.; et al. O26 Polysaccharides as Key Players in Enteropathogenic E. coli Immune Evasion and Vaccine Development. Int. J. Mol. Sci. 2024, 25, 2878. https://doi.org/10.3390/ijms25052878
Lemos TJdS, Silva HGdS, Previato JO, Mendonça-Previato L, Freitas EOd, Barbosa AS, Franzolin MR, Santos LFd, Melo BdS, Anjos GFd, et al. O26 Polysaccharides as Key Players in Enteropathogenic E. coli Immune Evasion and Vaccine Development. International Journal of Molecular Sciences. 2024; 25(5):2878. https://doi.org/10.3390/ijms25052878
Chicago/Turabian StyleLemos, Thiago Jordão da Silva, Herbert Guimarães de Sousa Silva, José Osvaldo Previato, Lucia Mendonça-Previato, Elisangela Oliveira de Freitas, Angela Silva Barbosa, Marcia Regina Franzolin, Luis Fernando dos Santos, Bruna de Sousa Melo, Geovana Ferreira dos Anjos, and et al. 2024. "O26 Polysaccharides as Key Players in Enteropathogenic E. coli Immune Evasion and Vaccine Development" International Journal of Molecular Sciences 25, no. 5: 2878. https://doi.org/10.3390/ijms25052878
APA StyleLemos, T. J. d. S., Silva, H. G. d. S., Previato, J. O., Mendonça-Previato, L., Freitas, E. O. d., Barbosa, A. S., Franzolin, M. R., Santos, L. F. d., Melo, B. d. S., Anjos, G. F. d., Gonçalves, R. H. N., & Domingos, M. d. O. (2024). O26 Polysaccharides as Key Players in Enteropathogenic E. coli Immune Evasion and Vaccine Development. International Journal of Molecular Sciences, 25(5), 2878. https://doi.org/10.3390/ijms25052878






