Exposure to Light of the Abaxial versus Adaxial Side of Detached Kalanchoë blossfeldiana Leaves Affects Anthocyanin Content and Composition Differently
Abstract
1. Introduction
2. Results and Discussion
2.1. Anthocyanin Profiles and Their Changes in K. blossfeldiana Leaves
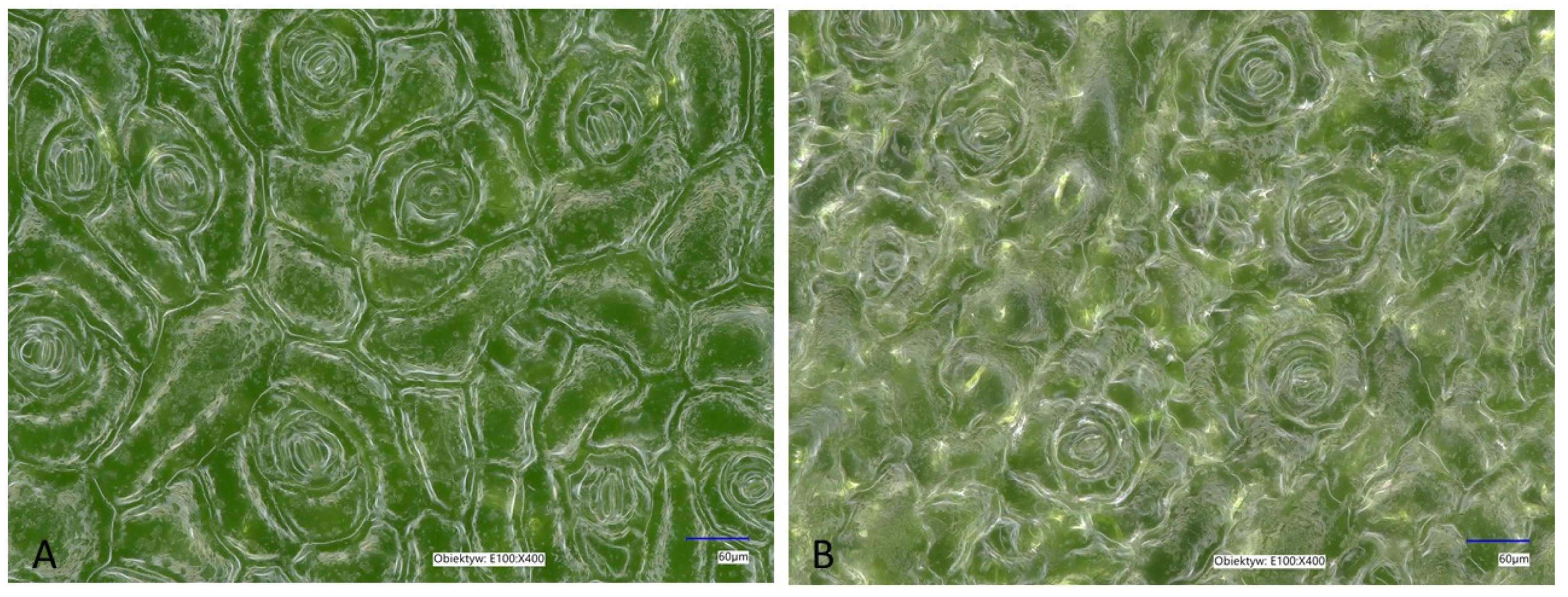
2.2. Histological Observations of K. blossfeldiana Leaves
3. Materials and Methods
3.1. Plant Materials
3.2. Anthocyanin Extraction and Analysis
3.3. Histological Analyses of K. blossfeldiana Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marko, D.; Puppel, N.; Tjaden, Z.; Jakobs, S.; Pahlke, G. The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol. Nutr. Food Res. 2004, 48, 318–325. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Riedl, K.; Otegui, M.S.; Grotewold, E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta 2014, 240, 931–940. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 2015, 10, e1027850. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Do anthocyanins function as osmoregulators in leaf tissues? Adv. Bot. Res. 2002, 37, 103–106. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L. The ecophysiology of foliar anthocyanin. Bot. Rev. 2003, 69, 149–161. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Plant stress response and adaptation via anthocyanins: A review. Plant Stress 2023, 10, 100230. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 1995, 12, 639–657. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Gould, K.S.; Markham, K.R.; Smith, R.H.; Gortis, J.J. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 2000, 521, 1107–1115. Available online: http://www.jstor.org/stable/23696619 (accessed on 10 October 2023). [CrossRef]
- Gould, K.S.; Neill, S.O.; Vogelmann, T.C. A unified explanation for anthocyanins in leaves? Adv. Bot. Res. 2002, 37, 167–192. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by over accumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Gordeeva, E.I.; Arbuzova, V.S.; Khlestkina, E.K. Anthocyanins participate in the protection of wheat seedlings from osmotic stress. Cerel Res. Commun. 2017, 45, 47–56. [Google Scholar] [CrossRef]
- Jezek, M.; Allan, A.C.; Jones, J.J.; Geilfus, C.M. Why do plants blush when they are hungry? New Phytol. 2023, 239, 494–505. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Agati, G.; Guidi, L.; Landi, M.; Tattini, M. Anthocyanins in phytoprotection: Knowing the actors in play to solve this complex ecophysiological issue. New Phytol. 2021, 232, 2228–2235. [Google Scholar] [CrossRef]
- Tattini, M.; Landi, M.; Brunetti, C.; Giordano, C.; Remorini, D.; Gould, K.S.; Guidi, L. Epidermal coumaroyl anthocyanins protect sweet basil against excess light stress: Multiple consequences of light attenuation. Physiol. Plant. 2014, 152, 585–598. [Google Scholar] [CrossRef]
- Dębski, H.; Wiczkowski, W.; Szawara-Nowak, D.; Bączek, N.; Szwed, M.; Horbowicz, M. Enhanced light intensity increases flavonol and anthocyanin concentrations but reduces flavone levels in the cotyledons of common buckwheat seedlings. Cereal Res. Commun. 2017, 45, 225–233. [Google Scholar] [CrossRef]
- Jaakola, L.; Määttä-Riihinen, K.; Kärenlampi, S.; Hohtola, A. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L) leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef]
- Zhang, K.M.; Yua, H.J.; Shia, K.; Zhou, Y.H.; Yu, J.Q.; Xia, X.J. Photo protective roles of anthocyanins in Begonia semperflorens. Plant Sci. 2010, 179, 202–208. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Takahashi, T.; Katsube, T.; Kudo, A.; Kuramitsu, O.; Ishiwata, M.; Matsumoto, S. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem. 2013, 141, 552–556. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B. Light-stress-induced pigment changes and evidence for anthocyanin photo protection in apples. J. Photochem. Photobiol. B Biol. 2000, 55, 155–163. [Google Scholar] [CrossRef]
- Dębski, H.; Szwed, M.; Wiczkowski, W.; Szawara-Nowak, D.; Bączek, N.; Horbowicz, M. UV-B radiation increases anthocyanin levels in cotyledons and inhibits the growth of common buckwheat seedlings. Acta Biol. Hung. 2016, 67, 403–411. [Google Scholar] [CrossRef]
- Li, W.; Tan, L.; Zou, Y.; Tan, X.; Huang, J.; Chen, W.; Tang, Q. The effects of ultraviolet A/B treatments on anthocyanin accumulation and gene expression in dark-purple tea cultivar ‘Ziyan’ (Camellia sinensis). Molecules 2020, 25, 354. [Google Scholar] [CrossRef]
- Balsa, C.; Albert, G.; Brulffert, J.; Queiroz, O.; Boudet, A.M. Photoperiodic control of phenolic metabolism in Kalanchoe blossfeldiana. Phytochemistry 1979, 18, 1159–1163. [Google Scholar] [CrossRef]
- Delessert, C.; Wilson, I.W.; Van Der Straeten, D.; Dennis, E.S.; Dolferus, R. Spatial and temporal analysis of the local response to wounding in Arabidopsis leaves. Plant Mol Biol. 2004, 55, 165–181. [Google Scholar] [CrossRef]
- Saniewski, M.; Ueda, J.; Miyamoto, K. Interaction of ethylene with jasmonates in the regulation of some physiological processes in plants. In Biology and Biotechnology the Plant Hormone Ethylene, 2nd ed.; Pech, J.C., Grierson, D., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 173–180. [Google Scholar]
- Xu, Y.; Chang, P.F.L.; Liu, D.; Narasimhan, M.L.; Raghothama, K.G.; Hasegawa, P.M.; Bressan, R.A. Plant Defense Genes Are Synergistically Induced by Ethylene and Methyl Jasmonate. Plant Cell 1994, 6, 1077–1085. [Google Scholar] [CrossRef]
- Morker, K.H.; Roberts, M.R. Light exerts multiple levels of influence on the Arabidopsis wound response. Plant Cell Environ. 2011, 34, 717–728. [Google Scholar] [CrossRef]
- Kimberlin, A.N.; Holtsclaw, R.E.; Zhang, T.; Mulaudzi, T.; Koo, A.J. On the initiation of jasmonate biosynthesis in wounded leaves. Plant Physiol. 2022, 189, 1925–1942. [Google Scholar] [CrossRef]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14 (Suppl. S1), S131–S151. [Google Scholar] [CrossRef]
- Onkokesung, N.; Gális, I.; von Dahl, C.C.; Matsuoka, K.; Saluz, H.P.; Baldwin, I.T. Jasmonic acid and ethylene modulate local responses to wounding and simulated herbivory in Nicotiana attenuata leaves. Plant Physiol. 2010, 153, 785–798. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, C.M.; Kim, H.S.; Kim, C.; Jeon, J.H.; Lee, H.J. Ethylene signals modulate the survival of Arabidopsis leaf explants. BMC Plant Biol. 2023, 23, 281. [Google Scholar] [CrossRef]
- Saniewski, M.; Węgrzynowicz-Lesiak, E. Methyl jasmonate-induced leaf abscission in Kalanchoe blossfeldiana. Acta Hortic. 1995, 394, 315–324. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Neyland, M.; Ng, Y.L.; Thimann, K.V. Formation of anthocyanin in leaves of Kalanchoe blossfeldiana—A photoperiodic response. Plant Physiol. 1963, 38, 447–451. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Olsen, C.E.; Moller, B.L. Flavonoids in flowers of 16 Kalanchoe blossfeldiana varieties. Phytochemistry 2005, 66, 2829–2835. [Google Scholar] [CrossRef]
- Saniewski, M.; Horbowicz, M.; Puchalski, J.; Ueda, J. Methyl jasmonate stimulates the formation and the accumulation of anthocyanin in Kalanchoe blossfeldiana. Acta Physiol. Plant. 2003, 25, 143–149. [Google Scholar] [CrossRef]
- Góraj-Koniarska, J.; Stochmal, A.; Oleszek, W.; Mołdoch, J.; Saniewski, M. Elicitation of anthocyanin production in roots of Kalanchoe blossfeldiana by methyl jasmonate. Acta Biol. Cracoviensia Ser. Bot. 2015, 57, 141–148. [Google Scholar] [CrossRef]
- Saniewski, M.; Szablińska-Piernik, J.; Marasek-Ciołakowska, A.; Mitrus, J.; Góraj-Koniarska, J.; Lahuta, L.B.; Wiczkowski, W.; Miyamoto, K.; Ueda, J.; Horbowicz, M. Accumulation of anthocyanins in detached leaves of Kalanchoë blossfeldiana: Relevance to the effect of methyl jasmonate on this process. Int. J. Mol. Sci. 2023, 24, 626. [Google Scholar] [CrossRef]
- Dey, P.K.; Goswami, A.; Mitra, A. A new targeted approach of postharvest accumulation on anthocyanin in fragrant leaves of Melissa officinalis L. Ind. Crops Prod. 2023, 196, 116479. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef]
- Sipos, M.; Bunta, D. Histo-anatomy of vegetative organs at Kalanchoe blossfeldiana Poellnitz. Analele Univ. Craiova Biol. Hortic. Tehnol. Prelucr. Prod. Agric. Ing. Mediu. 2011, 26, 380–393. [Google Scholar]
- Jeong, W.G.; Kim, J.S.; Kim, C.S.; Sung, M.W. Epidermal Structure and Stomatal Types in Various Parts of Each Organ of Kalanchoe. Korean J. Bot. 1987, 30, 79–84. [Google Scholar]
- Chernetskyy, M.; Woźniak, A.; Skalska-Kamińska, A.; Żuraw, B.; Blicharska, E.; Rejdak, R.; Donica, H.; Weryszko-Chmielewska, E. Structure of leaves and phenolic acids in Kalanchoë daigremontiana Raym.-Hamet & H. Perrier. Acta Sci. Pol. Hortorum Cultus 2018, 17, 137–155. [Google Scholar] [CrossRef]
- Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Subcellular-level resolution MALDI-MS imaging of maize leaf metabolites by MALDI-linear ion trap-Orbitrap mass spectrometer. Anal. Bioanal. Chem. 2015, 407, 2301–2309. [Google Scholar] [CrossRef]
- Suzuki, T.; Kim, S.-J.; Yamaguchi, H.; Takigawa, S.; Honda, J.; Mukasa, J. Characterization of a flavonoid 3-O-glucotransferase and its activity during cotyledon growth in buckwheat (Fagopyrum esculentum). Plant Sci. 2005, 169, 943–948. [Google Scholar] [CrossRef]
- Li, B.; Neumann, E.K.; Ge, J.; Gao, W.; Yang, H.; Li, P.; Sweedler, J.V. Interrogation of spatial metabolome of Ginkgo biloba with high-resolution matrix-assisted laser desorption/ionization and laser desorption/ionization mass spectroscopy imaging. Plant Cell Environ. 2018, 41, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Collins, T.M. Phylogenetic and ontogenetic influences on the distribution of anthocyanins and betacyanins in leaves of tropical plants. Int. J. Plant Sci. 2001, 162, 1141–1153. [Google Scholar] [CrossRef]
- Fernandez-Marin, B.; Esteban, R.; Miguez, F.; Artetxe, U.; Castaneda, V.; Pinto-Marijuan, M.; Becerril, J.M.; Garcia-Plazaola, J.I. Ecophysiological role of abaxial anthocyanins in a perennial understorey herb from temperate deciduous forests. AoB Plants 2015, 7, plv042. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.M.; Vogelmann, T.C.; Smith, W.K. Optical effects of abaxial anthocyanin on absorption of red wavelengths by understorey species: Revisiting the back-scatter hypothesis. J. Exp. Bot. 2008, 59, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Filho, H.A.; Bruno, O.M. Plants with purple abaxial leaves. A repository of metrics from stomata distribution. bioRxiv 2018, 294553, preprint. [Google Scholar] [CrossRef]
- Hughes, N.M.; Smith, W.K. Attenuation of incident light in Galax urceolata (Diapensiaceae): Concerted influence of adaxial and abaxial anthocyanic layers on photoprotection. Am. J. Bot. 2007, 94, 784–790. [Google Scholar] [CrossRef]
- Hughes, N.M.; Lev-Yadun, S. Why do some plants have leaves with red or purple undersides? Environ. Exp. Bot. 2023, 205, 105–126. [Google Scholar] [CrossRef]
- Momose, T.; Ozeki, Y. Regulatory effect of stems on sucrose-induced chlorophyll degradation and anthocyanin synthesis in Egeria densa leaves. J. Plant Res. 2013, 126, 859–867. [Google Scholar] [CrossRef]




| Anthocyanin | [M]+ (m/z) | MS/MS (m/z) |
|---|---|---|
| Delphinidin-rhamnoside | 449 | 303 |
| Delphinidin-rhamnoside-glucoside | 611 | 449/303 |
| Cyanidin-glucoside-glucoside | 611 | 449/287 |
| Cyanidin-rhamnoside | 433 | 287 |
| Cyanidin-rhamnoside-glucoside | 595 | 433/287 |
| Cyanidin-rhamnoside-rhamnoside-glucoside | 741 | 579/433/287 |
| Delphinidin-(coumaroyl)-glucoside-rhamnoside | 757 | 449/303 |
| Delphinidin derivative 1 | 473 | 303 |
| Cyanidin derivative 1 | 484 | 287 |
| Petunidin derivative 1 | 487 | 317 |
| Petunidin derivative 2 | 501 | 317 |
| Cyanidin-rhamnoside-rhamnoside | 579 | 433/287 |
| Delphinidin-(feruloyl)-rhamnoside | 625 | 449/303 |
| Delphinidin-(feruloyl)-glucoside | 641 | 465/303 |
| Cyanidin-rhamnoside-rhamnoside-xyloside | 711 | 579/433/287 |
| Antho-cyanin | Large (Older) Leaves | Small (Younger) Leaves | ||||
|---|---|---|---|---|---|---|
| On Plant | Detached and Kept in Natural Position | Detached and Kept in Inverted Position | On Plant | Detached and Kept in Natural Position | Detached and Kept in Inverted Position | |
| 1 | 0.334 ± 0.008 a | 0.279 ± 0.006 b | 0.261 ± 0.004 b | 0.343 ± 0.007 a | 0.323 ± 0.006 a | 0.278 ± 0.008 b |
| 2 | 0.151 ± 0.002 a | 0.144 ± 0.003 a | 0.142 ± 0.002 a | 0.144 ± 0.003 a | 0.145 ± 0.002 a | 0.144 ± 0.003 a |
| 3 | 0.112 ± 0.001 a | 0.105 ± 0.002 b | 0.092 ± 0.002 c | 0.115 ± 0.002 a | 0.102 ± 0.002 b | 0.104 ± 0.002 b |
| 4 | 0.034 ± 0.002 a | 0.032 ± 0.002 a | 0.028 ± 0.002 a | 0.031 ± 0.001 a | 0.033 ± 0.001 a | 0.032 ± 0.001 a |
| 5 | 0.021 ± 0.001 a | 0.016 ± 0.002 a | 0.021 ± 0.001 a | 0.024 ± 0.002 a | 0.024 ± 0.002 a | 0.026 ± 0.002 a |
| 6 | 0.052 ± 0.002 a | 0.051 ± 0.001 a | 0.048 ± 0.002 a | 0.046 ± 0.002 a | 0.049 ± 0.002 a | 0.044 ± 0.003 a |
| 7 | 0.018 ± 0.001 a | 0.019 ± 0.001 a | 0.020 ± 0.001 a | 0.022 ± 0.002 a | 0.022 ± 0.002 a | 0.023 ± 0.002 a |
| 8 | 0.114 ± 0.002 d | 0.090 ± 0.001 e | 0.094 ± 0.002 e | 0.155 ± 0.004 a | 0.134 ± 0.001 b | 0.129 ± 0.002 c |
| 9 | 0.071 ± 0.002 a | 0.063 ± 0.002 a | 0.042 ± 0.001 b | 0.036 ± 0.001 c | 0.017 ± 0.001 d | 0.018 ± 0.001 d |
| 10 | 2.896 ± 0.056 a | 2.396 ± 0.086 bc | 2.206 ± 0.038 c | 3.058 ± 0.071 a | 3.022 ± 0.060 a | 2.578 ± 0.065 b |
| 11 | 0.170 ± 0.003 b | 0.174 ± 0.002 b | 0.150 ± 0.001 c | 0.210 ± 0.003 a | 0.164 ± 0.004 b | 0.183 ± 0.004 b |
| Antho-cyanin | Leaves on Plant | Leaves Detached and Kept in Natural Position | Leaves Detached and Kept in Inverted Position | |||
|---|---|---|---|---|---|---|
| Not Treated | Treated with Lanolin | Treated with 0.5% MJ in Lanolin | Treated with 0.1% MJ in Lanolin | |||
| 1 | 0.432 ± 0.004 a | 0.375 ± 0.006 b | 0.348 ± 0.004 c | 0.392 ± 0.008 b | 0.388 ± 0.008 b | 0.383 ± 0.009 b |
| 2 | 0.213 ± 0.004 a | 0.175 ± 0.005 b | 0.158 ± 0.004 b | 0.171 ± 0.005 b | 0.167 ± 0.003 b | 0.176 ± 0.004 b |
| 3 | 0.141 ± 0.002 a | 0.133 ± 0.003 a | 0.132 ± 0.003 a | 0.150 ± 0.006 a | 0.132 ± 0.004 a | 0.124 ± 0.006 a |
| 4 | 0.034 ± 0.002 a | 0.031 ± 0.002 a | 0.035 ± 0.002 a | 0.038 ± 0.003 a | 0.035 ± 0.002 a | 0.037 ± 0.002 a |
| 5 | 0.021 ± 0.001 a | 0.025 ± 0.002 a | 0.021 ± 0.002 a | 0.024 ± 0.001 a | 0.017 ± 0.002 a | 0.019 ± 0.002 a |
| 6 | 0.066 ± 0.003 a | 0.057 ± 0.002 a | 0.054 ± 0.002 a | 0.064 ± 0.003 a | 0.054 ± 0.002 a | 0.056 ± 0.002 a |
| 7 | 0.022 ± 0.001 a | 0.017 ± 0.003 a | 0.020 ± 0.002 a | 0.024 ± 0.002 a | 0.016 ± 0.002 a | 0.017 ± 0.002 a |
| 8 | 0.094 ± 0.002 a | 0.073 ± 0.002 b | 0.076 ± 0.002 b | 0.094 ± 0.002 a | 0.078 ± 0.002 b | 0.077 ± 0.002 b |
| 9 | 0.049 ± 0.002 ab | 0.033 ± 0.002 c | 0.048 ± 0.002 ab | 0.061 ± 0.003 a | 0.047 ± 0.001 b | 0.042 ± 0.002 bc |
| 10 | 2.199 ± 0.088 ab | 1.502 ± 0.068 c | 2.104 ± 0.080 ab | 2.396 ± 0.038 a | 1.872 ± 0.060 b | 1.936 ± 0.035 b |
| 11 | 0.158 ± 0.004 bc | 0.122 ± 0.003 d | 0.155 ± 0.004 b | 0.175 ± 0.003 ab | 0.132 ± 0.002 cd | 0.143 ± 0.003 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiczkowski, W.; Saniewski, M.; Marasek-Ciołakowska, A.; Góraj-Koniarska, J.; Mitrus, J.; Horbowicz, M. Exposure to Light of the Abaxial versus Adaxial Side of Detached Kalanchoë blossfeldiana Leaves Affects Anthocyanin Content and Composition Differently. Int. J. Mol. Sci. 2024, 25, 2875. https://doi.org/10.3390/ijms25052875
Wiczkowski W, Saniewski M, Marasek-Ciołakowska A, Góraj-Koniarska J, Mitrus J, Horbowicz M. Exposure to Light of the Abaxial versus Adaxial Side of Detached Kalanchoë blossfeldiana Leaves Affects Anthocyanin Content and Composition Differently. International Journal of Molecular Sciences. 2024; 25(5):2875. https://doi.org/10.3390/ijms25052875
Chicago/Turabian StyleWiczkowski, Wiesław, Marian Saniewski, Agnieszka Marasek-Ciołakowska, Justyna Góraj-Koniarska, Joanna Mitrus, and Marcin Horbowicz. 2024. "Exposure to Light of the Abaxial versus Adaxial Side of Detached Kalanchoë blossfeldiana Leaves Affects Anthocyanin Content and Composition Differently" International Journal of Molecular Sciences 25, no. 5: 2875. https://doi.org/10.3390/ijms25052875
APA StyleWiczkowski, W., Saniewski, M., Marasek-Ciołakowska, A., Góraj-Koniarska, J., Mitrus, J., & Horbowicz, M. (2024). Exposure to Light of the Abaxial versus Adaxial Side of Detached Kalanchoë blossfeldiana Leaves Affects Anthocyanin Content and Composition Differently. International Journal of Molecular Sciences, 25(5), 2875. https://doi.org/10.3390/ijms25052875






