Lipidomics Reveals Myocardial Lipid Composition in a Murine Model of Insulin Resistance Induced by a High-Fat Diet
Abstract
1. Introduction
2. Results
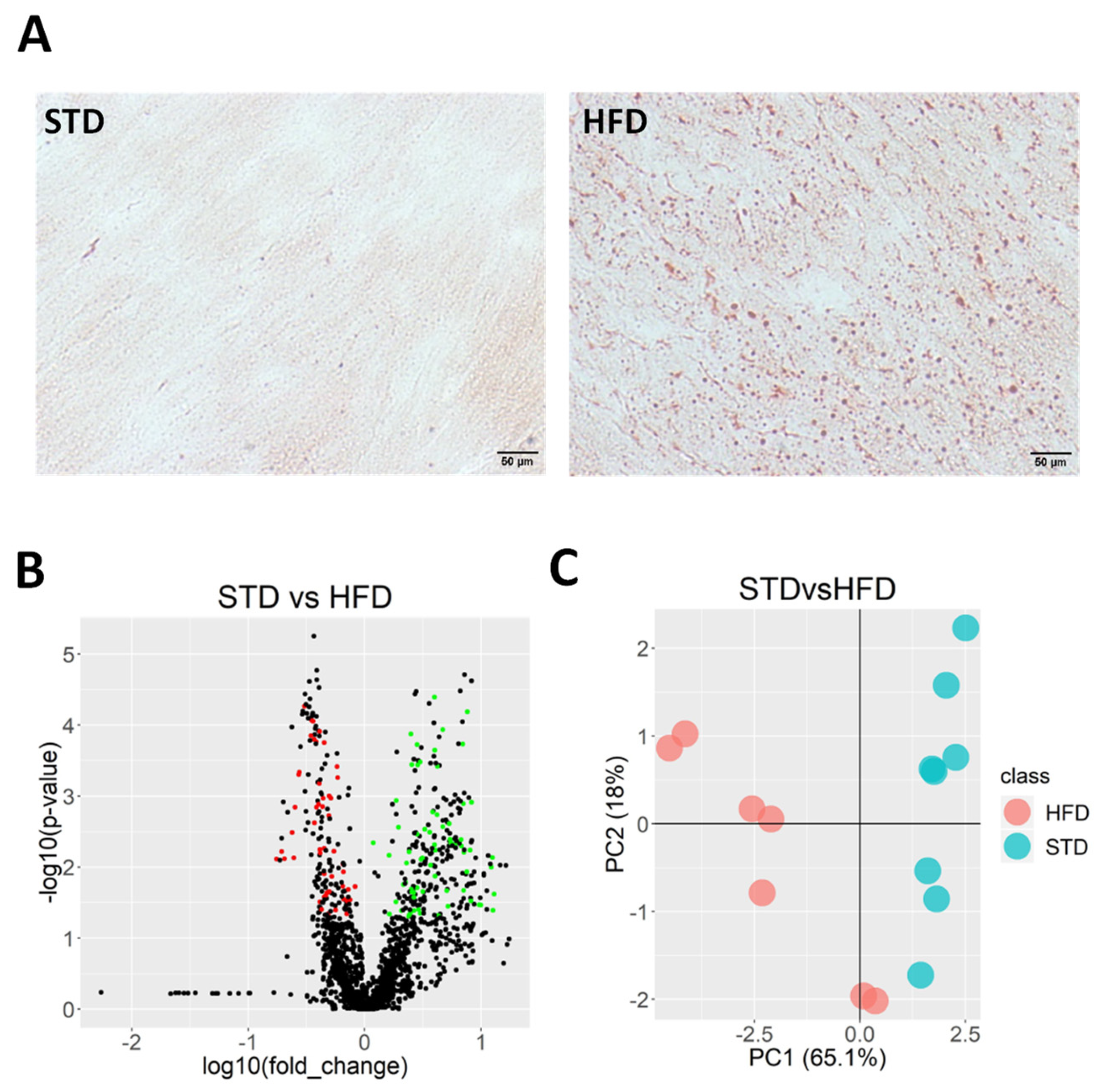
2.1. HFD Induces Ectopic Fat Accumulation in Heart
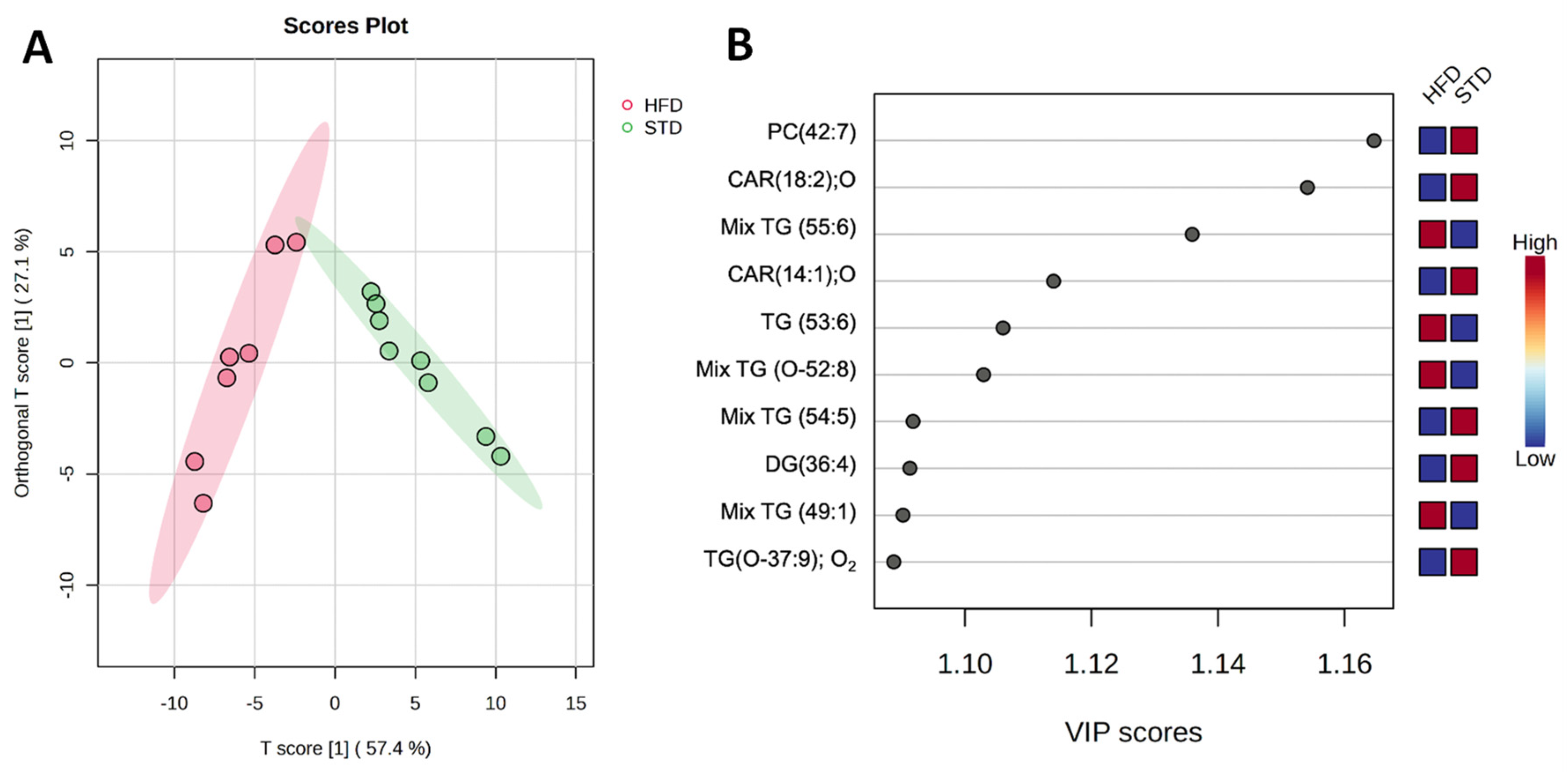
2.2. Lipidomics Reveals Myocardial Lipid Composition in HFD-Fed Mice
2.3. Identified Metabolites Classify Both the STD and the HFD Groups
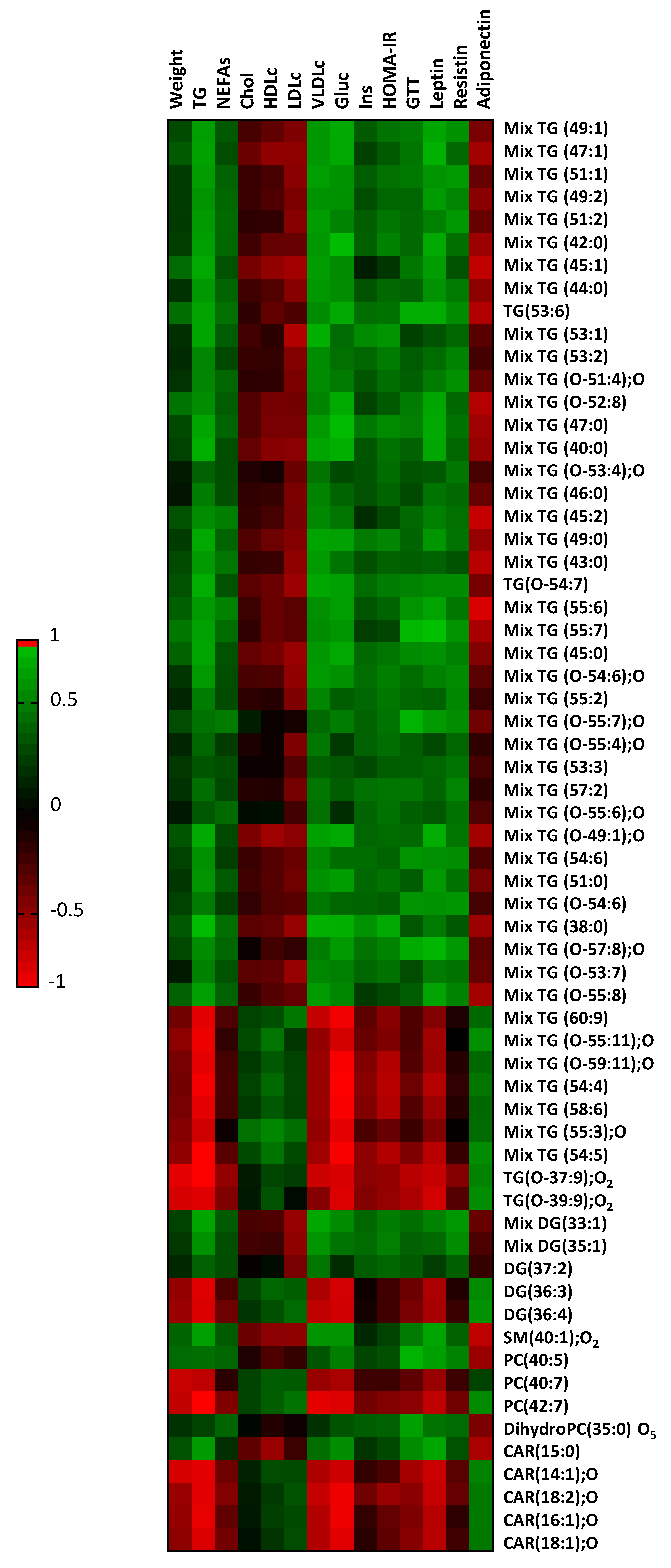
2.4. Myocardial Lipid Composition Correlates to Plasma Variables
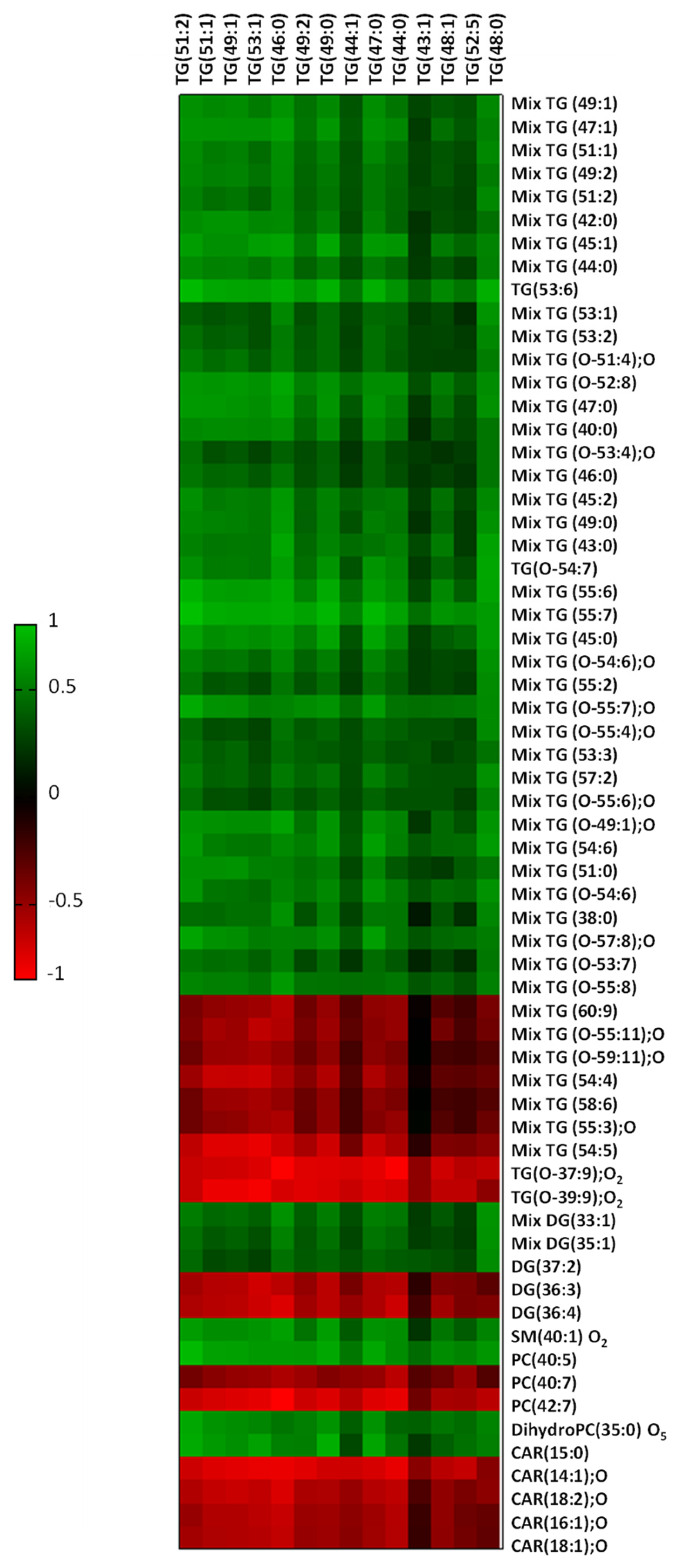
2.5. Liver Fat Content Is Related to Myocardial Lipid Composition
3. Discussion
4. Materials and Methods
4.1. Animal Model Experiments
4.2. Biochemical Plasma Profile
4.3. Lipid Staining
4.4. Lipidomics
4.5. Tissue Molecular Imaging
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Britton, K.A.; Fox, C.S. Ectopic fat depots and cardiovascular disease. Circulation 2011, 124, e837–e841. [Google Scholar] [CrossRef]
- Levelt, E.; Mahmod, M.; Piechnik, S.K.; Ariga, R.; Francis, J.M.; Rodgers, C.T.; Clarke, W.T.; Sabharwal, N.; Schneider, J.E.; Karamitsos, T.D.; et al. Relationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 Diabetes. Diabetes 2016, 65, 44–52. [Google Scholar] [CrossRef] [PubMed]
- McGavock, J.M.; Lingvay, I.; Zib, I.; Tillery, T.; Salas, N.; Unger, R.; Levine, B.D.; Raskin, P.; Victor, R.G.; Szczepaniak, L.S. Cardiac steatosis in diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Circulation 2007, 116, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.; Delgado, V.; Bertini, M.; van der Meer, R.W.; Rijzewijk, L.J.; Hooi Ewe, S.; Siebelink, H.M.; Smit, J.W.; Diamant, M.; Romijn, J.A.; et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 2010, 122, 2538–2544. [Google Scholar] [CrossRef]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef]
- Djaberi, R.; Schuijf, J.D.; van Werkhoven, J.M.; Nucifora, G.; Jukema, J.W.; Bax, J.J. Relation of epicardial adipose tissue to coronary atherosclerosis. Am. J. Cardiol. 2008, 102, 1602–1607. [Google Scholar] [CrossRef]
- Ouwens, D.M.; Sell, H.; Greulich, S.; Eckel, J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell. Mol. Med. 2010, 14, 2223–2234. [Google Scholar] [CrossRef]
- Gonzalez, N.; Moreno-Villegas, Z.; Gonzalez-Bris, A.; Egido, J.; Lorenzo, O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef]
- Gottlicher, M.; Widmark, E.; Li, Q.; Gustafsson, J.A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 4653–4657. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Wilcox, J.E.; Colangelo, L.A.; Lloyd-Jones, D.M.; Carr, J.J.; Lima, J.A.; Lewis, C.E.; Rinella, M.E.; Shah, S.J. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology 2015, 62, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzen, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef]
- Adams, L.A.; Sanderson, S.; Lindor, K.D.; Angulo, P. The histological course of nonalcoholic fatty liver disease: A longitudinal study of 103 patients with sequential liver biopsies. J. Hepatol. 2005, 42, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin. Liver Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef]
- Iozzo, P. Myocardial, perivascular, and epicardial fat. Diabetes Care 2011, 34 (Suppl. S2), S371–S379. [Google Scholar] [CrossRef]
- Drosatos, K.; Schulze, P.C. Cardiac lipotoxicity: Molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 2013, 10, 109–121. [Google Scholar] [CrossRef]
- Birse, R.T.; Bodmer, R. Lipotoxicity and cardiac dysfunction in mammals and Drosophila. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 376–385. [Google Scholar] [CrossRef]
- D’Souza, K.; Nzirorera, C.; Kienesberger, P.C. Lipid metabolism and signaling in cardiac lipotoxicity. Biochim. Biophys. Acta 2016, 1861, 1513–1524. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Bosquet, A.; Girona, J.; Guaita-Esteruelas, S.; Heras, M.; Saavedra-Garcia, P.; Martinez-Micaelo, N.; Masana, L.; Rodriguez-Calvo, R. FABP4 inhibitor BMS309403 decreases saturated-fatty-acid-induced endoplasmic reticulum stress-associated inflammation in skeletal muscle by reducing p38 MAPK activation. Biochim. Biophys. Acta 2018, 1863, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Calvo, R.; Samino, S.; Girona, J.; Martinez-Micaelo, N.; Rafols, P.; Garcia-Altares, M.; Guaita-Esteruelas, S.; Junza, A.; Heras, M.; Yanes, O.; et al. Hepatic Lipidomics and Molecular Imaging in a Murine Non-Alcoholic Fatty Liver Disease Model: Insights into Molecular Mechanisms. Biomolecules 2020, 10, 1275. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, R.; Girona, J.; Rodriguez, M.; Samino, S.; Barroso, E.; de Gonzalo-Calvo, D.; Guaita-Esteruelas, S.; Heras, M.; van der Meer, R.W.; Lamb, H.J.; et al. Fatty acid binding protein 4 (FABP4) as a potential biomarker reflecting myocardial lipid storage in type 2 diabetes. Metab. Clin. Exp. 2019, 96, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Calvo, R.; Samino, S.; Guaita-Esteruelas, S.; Martinez-Micaelo, N.; Heras, M.; Girona, J.; Yanes, O.; Correig, X.; Masana, L. Plasma glucose, triglycerides, VLDL, leptin and resistin levels as potential biomarkers for myocardial fat in mice. Clin. Investig. Arterioscler. 2020, 32, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Adrogue, J.V.; Golfman, L.; Uray, I.; Lemm, J.; Youker, K.; Noon, G.P.; Frazier, O.H.; Taegtmeyer, H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004, 18, 1692–1700. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Dobbins, R.L.; Metzger, G.J.; Sartoni-D’Ambrosia, G.; Arbique, D.; Vongpatanasin, W.; Unger, R.; Victor, R.G. Myocardial triglycerides and systolic function in humans: In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn. Reson. Med. 2003, 49, 417–423. [Google Scholar] [CrossRef]
- Reingold, J.S.; McGavock, J.M.; Kaka, S.; Tillery, T.; Victor, R.G.; Szczepaniak, L.S. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: Reproducibility and sensitivity of the method. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E935–E939. [Google Scholar] [CrossRef]
- Kankaanpaa, M.; Lehto, H.R.; Parkka, J.P.; Komu, M.; Viljanen, A.; Ferrannini, E.; Knuuti, J.; Nuutila, P.; Parkkola, R.; Iozzo, P. Myocardial triglyceride content and epicardial fat mass in human obesity: Relationship to left ventricular function and serum free fatty acid levels. J. Clin. Endocrinol. Metab. 2006, 91, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Utz, W.; Engeli, S.; Haufe, S.; Kast, P.; Bohnke, J.; Haas, V.; Hermsdorf, M.; Wiesner, S.; Pofahl, M.; Traber, J.; et al. Moderate dietary weight loss reduces myocardial steatosis in obese and overweight women. Int. J. Cardiol. 2013, 167, 905–909. [Google Scholar] [CrossRef]
- Denton, R.M.; Randle, P.J. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. Biochem. J. 1967, 104, 416–422. [Google Scholar] [CrossRef]
- Finck, B.N.; Han, X.; Courtois, M.; Aimond, F.; Nerbonne, J.M.; Kovacs, A.; Gross, R.W.; Kelly, D.P. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: Modulation by dietary fat content. Proc. Natl. Acad. Sci. USA 2003, 100, 1226–1231. [Google Scholar] [CrossRef]
- Coort, S.L.; Hasselbaink, D.M.; Koonen, D.P.; Willems, J.; Coumans, W.A.; Chabowski, A.; van der Vusse, G.J.; Bonen, A.; Glatz, J.F.; Luiken, J.J. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes 2004, 53, 1655–1663. [Google Scholar] [CrossRef]
- O’Donnell, J.M.; Zampino, M.; Alpert, N.M.; Fasano, M.J.; Geenen, D.L.; Lewandowski, E.D. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E448–E455. [Google Scholar] [CrossRef]
- Basu, R.; Oudit, G.Y.; Wang, X.; Zhang, L.; Ussher, J.R.; Lopaschuk, G.D.; Kassiri, Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2096–H2108. [Google Scholar] [CrossRef]
- Abdesselam, I.; Pepino, P.; Troalen, T.; Macia, M.; Ancel, P.; Masi, B.; Fourny, N.; Gaborit, B.; Giannesini, B.; Kober, F.; et al. Time course of cardiometabolic alterations in a high fat high sucrose diet mice model and improvement after GLP-1 analog treatment using multimodal cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2015, 17, 95. [Google Scholar] [CrossRef]
- Xu, X.; Luo, Z.; He, Y.; Shan, J.; Guo, J.; Li, J. Application of untargeted lipidomics based on UHPLC-high resolution tandem MS analysis to profile the lipid metabolic disturbances in the heart of diabetic cardiomyopathy mice. J. Pharm. Biomed. Anal. 2020, 190, 113525. [Google Scholar] [CrossRef]
- Marin-Royo, G.; Martinez-Martinez, E.; Gutierrez, B.; Jurado-Lopez, R.; Gallardo, I.; Montero, O.; Bartolome, M.V.; San Roman, J.A.; Salaices, M.; Nieto, M.L.; et al. The impact of obesity in the cardiac lipidome and its consequences in the cardiac damage observed in obese rats. Clin. Investig. Arterioscler. 2018, 30, 10–20. [Google Scholar] [CrossRef]
- Coen, P.M.; Goodpaster, B.H. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. TEM 2012, 23, 391–398. [Google Scholar] [CrossRef]
- Hammer, S.; van der Meer, R.W.; Lamb, H.J.; de Boer, H.H.; Bax, J.J.; de Roos, A.; Romijn, J.A.; Smit, J.W. Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E714–E718. [Google Scholar] [CrossRef]
- Hammer, S.; van der Meer, R.W.; Lamb, H.J.; Schar, M.; de Roos, A.; Smit, J.W.; Romijn, J.A. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J. Clin. Endocrinol. Metab. 2008, 93, 497–503. [Google Scholar] [CrossRef]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metab. Clin. Exp. 2016, 65, 1049–1061. [Google Scholar] [CrossRef]
- Sasson, S. Nutrient overload, lipid peroxidation and pancreatic beta cell function. Free Radic. Biol. Med. 2017, 111, 102–109. [Google Scholar] [CrossRef]
- Brons, C.; Grunnet, L.G. MECHANISMS IN ENDOCRINOLOGY: Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes: A causal mechanism or an innocent bystander? Eur. J. Endocrinol. 2017, 176, R67–R78. [Google Scholar] [CrossRef]
- Chokshi, A.; Drosatos, K.; Cheema, F.H.; Ji, R.; Khawaja, T.; Yu, S.; Kato, T.; Khan, R.; Takayama, H.; Knoll, R.; et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 2012, 125, 2844–2853. [Google Scholar] [CrossRef]
- Wang, P.; Lloyd, S.G.; Zeng, H.; Bonen, A.; Chatham, J.C. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2102–H2110. [Google Scholar] [CrossRef]
- Liu, L.; Yu, S.; Khan, R.S.; Ables, G.P.; Bharadwaj, K.G.; Hu, Y.; Huggins, L.A.; Eriksson, J.W.; Buckett, L.K.; Turnbull, A.V.; et al. DGAT1 deficiency decreases PPAR expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J. Lipid Res. 2011, 52, 732–744. [Google Scholar] [CrossRef]
- Gizurarson, S.; Stahlman, M.; Jeppsson, A.; Shao, Y.; Redfors, B.; Bergfeldt, L.; Boren, J.; Omerovic, E. Atrial fibrillation in patients admitted to coronary care units in western Sweden—Focus on obesity and lipotoxicity. J. Electrocardiol. 2015, 48, 853–860. [Google Scholar] [CrossRef]
- Wang, X.; West, J.A.; Murray, A.J.; Griffin, J.L. Comprehensive Metabolic Profiling of Age-Related Mitochondrial Dysfunction in the High-Fat-Fed ob/ob Mouse Heart. J. Proteome Res. 2015, 14, 2849–2862. [Google Scholar] [CrossRef]
- Li, Y.; Soos, T.J.; Li, X.; Wu, J.; Degennaro, M.; Sun, X.; Littman, D.R.; Birnbaum, M.J.; Polakiewicz, R.D. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J. Biol. Chem. 2004, 279, 45304–45307. [Google Scholar] [CrossRef]
- Szendroedi, J.; Yoshimura, T.; Phielix, E.; Koliaki, C.; Marcucci, M.; Zhang, D.; Jelenik, T.; Muller, J.; Herder, C.; Nowotny, P.; et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 9597–9602. [Google Scholar] [CrossRef]
- Nowotny, B.; Zahiragic, L.; Krog, D.; Nowotny, P.J.; Herder, C.; Carstensen, M.; Yoshimura, T.; Szendroedi, J.; Phielix, E.; Schadewaldt, P.; et al. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes 2013, 62, 2240–2248. [Google Scholar] [CrossRef]
- Petersen, M.C.; Madiraju, A.K.; Gassaway, B.M.; Marcel, M.; Nasiri, A.R.; Butrico, G.; Marcucci, M.J.; Zhang, D.; Abulizi, A.; Zhang, X.M.; et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Investig. 2016, 126, 4361–4371. [Google Scholar] [CrossRef]
- Zhang, L.; Ussher, J.R.; Oka, T.; Cadete, V.J.; Wagg, C.; Lopaschuk, G.D. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc. Res. 2011, 89, 148–156. [Google Scholar] [CrossRef]
- Song, X.; Qian, X.; Shen, M.; Jiang, R.; Wagner, M.B.; Ding, G.; Chen, G.; Shen, B. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim. Biophys. Acta 2015, 1853, 513–521. [Google Scholar] [CrossRef]
- Connelly, K.A.; Kelly, D.J.; Zhang, Y.; Prior, D.L.; Advani, A.; Cox, A.J.; Thai, K.; Krum, H.; Gilbert, R.E. Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy. Circ. Heart Fail. 2009, 2, 129–137. [Google Scholar] [CrossRef]
- Adebiyi, O.A.; Adebiyi, O.O.; Owira, P.M. Naringin Reduces Hyperglycemia-Induced Cardiac Fibrosis by Relieving Oxidative Stress. PLoS ONE 2016, 11, e0149890. [Google Scholar] [CrossRef]
- George, M.; Vijayakumar, A.; Dhanesh, S.B.; James, J.; Shivakumar, K. Molecular basis and functional significance of Angiotensin II-induced increase in Discoidin Domain Receptor 2 gene expression in cardiac fibroblasts. J. Mol. Cell. Cardiol. 2016, 90, 59–69. [Google Scholar] [CrossRef]
- Kolesnick, R. Signal transduction through the sphingomyelin pathway. Mol. Chem. Neuropathol. 1994, 21, 287–297. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Liu, J.; Liang, C.P.; Li, Y.; Li, Y.; Teitelman, G.; Beyer, T.; Bui, H.H.; Peake, D.A.; et al. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol. Cell. Biol. 2011, 31, 4205–4218. [Google Scholar] [CrossRef]
- Belay, B.; Esteban-Cruciani, N.; Walsh, C.A.; Kaskel, F.J. The use of levo-carnitine in children with renal disease: A review and a call for future studies. Pediatr. Nephrol. 2006, 21, 308–317. [Google Scholar] [CrossRef]
- Jakobsson, P.J.; Odlander, B.; Steinhilber, D.; Rosen, A.; Claesson, H.E. Human B lymphocytes possess 5-lipoxygenase activity and convert arachidonic acid to leukotriene B4. Biochem. Biophys. Res. Commun. 1991, 178, 302–308. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. TEM 2017, 28, 497–505. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, W606–W612. [Google Scholar] [CrossRef]
- Kind, T.; Liu, K.H.; Lee, D.Y.; DeFelice, B.; Meissen, J.K.; Fiehn, O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 2013, 10, 755–758. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Rafols, P.; Vilalta, D.; Torres, S.; Calavia, R.; Heijs, B.; McDonnell, L.A.; Brezmes, J.; Del Castillo, E.; Yanes, O.; Ramirez, N.; et al. Assessing the potential of sputtered gold nanolayers in mass spectrometry imaging for metabolomics applications. PLoS ONE 2018, 13, e0208908. [Google Scholar] [CrossRef]
- Rafols, P.; Castillo, E.D.; Yanes, O.; Brezmes, J.; Correig, X. Novel automated workflow for spectral alignment and mass calibration in MS imaging using a sputtered Ag nanolayer. Anal. Chim. Acta 2018, 1022, 61–69. [Google Scholar] [CrossRef]
- Rafols, P.; Torres, S.; Ramirez, N.; Del Castillo, E.; Yanes, O.; Brezmes, J.; Correig, X. rMSI: An R package for MS imaging data handling and visualization. Bioinformatics 2017, 33, 2427–2428. [Google Scholar] [CrossRef]


| HFD vs. STD | ||||||
|---|---|---|---|---|---|---|
| m/z | Metabolites | Ion | Formula | Fold | p-Value | |
| TG | ||||||
| 836.7700 | Mix TG (49:1) | NH4+ | C52H98O6 | 16.37 | 0.026 | |
| 808.7379 | Mix TG (47:1) | NH4+ | C50H94O6 | 12.94 | 0.030 | |
| 864.8008 | Mix TG (51:1) | NH4+ | C54H102O6 | 12.45 | 0.023 | |
| 834.7538 | Mix TG (49:2) | NH4+ | C52H96O6 | 12.37 | 0.032 | |
| 862.7855 | Mix TG (51:2) | NH4+ | C54H100O6 | 12.21 | 0.021 | |
| 740.6752 | Mix TG (42:0) | NH4+ | C45H86O6 | 9.94 | 0.042 | |
| 780.7065 | Mix TG (45:1) | NH4+ | C48H90O6 | 9.61 | 0.029 | |
| 768.7070 | Mix TG (44:0) | NH4+ | C47H90O6 | 8.15 | 0.037 | |
| 887.7088 | TG (53:6) | Na+ | C56H96O6 | 7.63 | 0.003 | |
| 892.8326 | Mix TG (53:1) | NH4+ | C56H106O6 | 7.53 | 0.026 | |
| 890.8169 | Mix TG (53:2) | NH4+ | C56H104O6 | 7.13 | 0.018 | |
| 860.7692 | Mix TG (O-51:4); O | NH4+ | C54H98O6 | 7.08 | 0.024 | |
| 855.6826 | Mix TG (O-52:8) | Na+ | C55H92O5 | 5.42 | 0.006 | |
| 810.7533 | Mix TG (47:0) | NH4+ | C50H96O6 | 5.33 | 0.014 | |
| 712.6440 | Mix TG (40:0) | NH4+ | C43H82O6 | 5.18 | 0.036 | |
| 888.8010 | Mix TG (O-53:4); O | NH4+ | C56H102O6 | 5.09 | 0.026 | |
| 796.7386 | Mix TG (46:0) | NH4+ | C49H94O6 | 4.36 | 0.041 | |
| 778.6904 | Mix TG (45:2) | NH4+ | C48H88O6 | 4.22 | 0.030 | |
| 838.7852 | Mix TG (49:0) | NH4+ | C52H100O6 | 4.03 | 0.018 | |
| 754.6908 | Mix TG (43:0) | NH4+ | C46H88O6 | 4.02 | 0.029 | |
| 885.7314 | TG(O-54:7) | Na+ | C57H98O5 | 3.98 | 0.005 | |
| 915.7406 | Mix TG (55:6) | Na+ | C58H100O6 | 3.96 | 0.001 | |
| 913.7245 | Mix TG (55:7) | Na+ | C58H98O6 | 3.83 | 0.004 | |
| 782.7220 | Mix TG (45:0) | NH4+ | C48H92O6 | 3.69 | 0.020 | |
| 898.7885 | Mix TG (O-54:6); O | NH4+ | C57H100O6 | 3.29 | 0.015 | |
| 923.8039 | Mix TG (55:2) | Na+ | C58H108O6 | 3.13 | 0.014 | |
| 910.7853 | Mix TG (O-55:7); O | NH4+ | C58H100O6 | 3.04 | 0.016 | |
| 916.8320 | Mix TG (O-55:4); O | NH4+ | C58H106O6 | 2.99 | 0.036 | |
| 893.7564 | Mix TG (53:3) | Na+ | C56H102O6 | 2.81 | 0.018 | |
| 951.8339 | Mix TG (57:2) | Na+ | C60H112O6 | 2.64 | 0.019 | |
| 912.7994 | Mix TG (O-55:6); O | NH4+ | C58H102O6 | 2.54 | 0.032 | |
| 843.7401 | Mix TG (O-49:1); O | Na+ | C52H100O6 | 2.53 | 0.007 | |
| 901.7247 | Mix TG (54:6) | Na+ | C57H98O6 | 2.50 | 0.016 | |
| 866.8155 | Mix TG (51:0) | NH4+ | C54H104O6 | 2.41 | 0.013 | |
| 887.7463 | Mix TG (O-54:6) | Na+ | C57H100O5 | 2.41 | 0.036 | |
| 689.5683 | Mix TG (38:0) | Na+ | C41H78O6 | 2.39 | 0.029 | |
| 936.8010 | Mix TG (O-57:8); O | NH4+ | C60H102O6 | 2.28 | 0.005 | |
| 871.7145 | Mix TG (O-53:7) | Na+ | C56H96O5 | 1.62 | 0.006 | |
| 897.7299 | Mix TG (O-55:8) | Na+ | C58H98O5 | 1.19 | 0.009 | |
| 979.7707 | Mix TG (60:9) | Na+ | C63H104O6 | 0.56 | 0.007 | |
| 902.7280 | Mix TG (O-55:11); O | NH4+ | C58H92O6 | 0.52 | 0.007 | |
| 958.7905 | Mix TG (O-59:11); O | NH4+ | C62H100O6 | 0.49 | 0.009 | |
| 905.7571 | Mix TG (54:4) | Na+ | C57H102O6 | 0.49 | 0.001 | |
| 957.7873 | Mix TG (58:6) | Na+ | C61H106O6 | 0.47 | 0.009 | |
| 953.7562 | Mix TG (55:3); O | K+ | C58H106O7 | 0.47 | 0.005 | |
| 903.7412 | Mix TG (54:5) | Na+ | C57H100O6 | 0.44 | 0.007 | |
| 653.4408 | TG(O-37:9); O2 | H+ | C40H60O7 | 0.41 | 0.001 | |
| 681.4719 | TG(O-39:9); O2 | H+ | C42H64O7 | 0.39 | 0.007 | |
| DG | ||||||
| 563.5029 | Mix DG (33:1) | -H2O+H+ | C36H68O5 | 8.01 | 0.019 | |
| 591.5337 | Mix DG (35:1) | -H2O+H+ | C38H72O5 | 6.76 | 0.018 | |
| 617.5495 | DG (37:2) | -H2O+H+ | C40H74O5 | 2.64 | 0.038 | |
| 641.5107 | DG (36:3) | Na+ | C39H70O5 | 0.42 | 0.004 | |
| 639.4951 | DG (36:4) | Na+ | C39H68O5 | 0.41 | 0.004 | |
| SM | ||||||
| 787.6681 | SM (40:1);O2 | H+ | C45H91N2O6P | 5.94 | 0.015 | |
| PC | ||||||
| 858.5976 | PC (40:5) | Na+ | C48H86NO8P | 2.85 | 0.003 | |
| 854.5668 | PC (40:7) | Na+ | C48H82NO8P | 0.43 | 0.003 | |
| 860.6144 | PC (42:7) | H+ | C50H86NO8P | 0.27 | 0.004 | |
| Dihydro-PC | ||||||
| 835.6033 | DihydroPC (35:0); O5 | NH4+ | C40H82NO13P | 2.46 | 0.020 | |
| Others | ||||||
| 386.3264 | CAR (15:0) | H+ | C22H43NO4 | 2.90 | 0.032 | |
| 386.2894 | CAR (14:1); O | H+ | C21H39NO5 | 0.25 | 0.006 | |
| 440.3369 | CAR (18:2); O | H+ | C25H45NO5 | 0.24 | 0.005 | |
| 414.3209 | CAR (16:1); O | H+ | C23H43NO5 | 0.21 | 0.019 | |
| 442.3521 | CAR (18:1); O | H+ | C25H47NO5 | 0.18 | 0.019 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girona, J.; Soler, O.; Samino, S.; Junza, A.; Martínez-Micaelo, N.; García-Altares, M.; Ràfols, P.; Esteban, Y.; Yanes, O.; Correig, X.; et al. Lipidomics Reveals Myocardial Lipid Composition in a Murine Model of Insulin Resistance Induced by a High-Fat Diet. Int. J. Mol. Sci. 2024, 25, 2702. https://doi.org/10.3390/ijms25052702
Girona J, Soler O, Samino S, Junza A, Martínez-Micaelo N, García-Altares M, Ràfols P, Esteban Y, Yanes O, Correig X, et al. Lipidomics Reveals Myocardial Lipid Composition in a Murine Model of Insulin Resistance Induced by a High-Fat Diet. International Journal of Molecular Sciences. 2024; 25(5):2702. https://doi.org/10.3390/ijms25052702
Chicago/Turabian StyleGirona, Josefa, Oria Soler, Sara Samino, Alexandra Junza, Neus Martínez-Micaelo, María García-Altares, Pere Ràfols, Yaiza Esteban, Oscar Yanes, Xavier Correig, and et al. 2024. "Lipidomics Reveals Myocardial Lipid Composition in a Murine Model of Insulin Resistance Induced by a High-Fat Diet" International Journal of Molecular Sciences 25, no. 5: 2702. https://doi.org/10.3390/ijms25052702
APA StyleGirona, J., Soler, O., Samino, S., Junza, A., Martínez-Micaelo, N., García-Altares, M., Ràfols, P., Esteban, Y., Yanes, O., Correig, X., Masana, L., & Rodríguez-Calvo, R. (2024). Lipidomics Reveals Myocardial Lipid Composition in a Murine Model of Insulin Resistance Induced by a High-Fat Diet. International Journal of Molecular Sciences, 25(5), 2702. https://doi.org/10.3390/ijms25052702






