Abstract
Acyclovir and ganciclovir comprise the prophylaxis and treatment of herpesvirus and cytomegalovirus infections occurring in immunocompromised patients. Their therapeutic drug monitoring is fundamental because of interindividual variability leading to side effects and drug resistance and is performed through several techniques, such as liquid chromatography coupled with UV spectrophotometry (HPLC-UV) or mass spectrometry (LC-MS/MS). Therefore, we developed and validated a low-cost, non-time-consuming, and low-sample-consuming HPLC-UV method. Briefly, 100 µL of sample was used for sample preparation, mainly consisting of precipitation through organic solvent. In total, 20 µL was injected into the instrument. Chromatographic separation was obtained eluting mobile phases A (10 mM ammonium formiate 0.01% formic acid) and B (acetonitrile) on a Poroshell 120 SB-C8 2.1 × 150 mm, 2.7 µm for 12 min isocratically (97:3; A:B) at a flow rate of 0.2 mL/min. The linearity range (0.5–40 mg/L) of the method allowed us to quantify both the Cmin and Cmax of acyclovir and ganciclovir. Plasma concentrations measured on a small cohort of patients undergoing acyclovir (31) and ganciclovir (9) treatment by the proposed method and the LC-MS/MS methods, already in use, were significantly correlated. The proposed HPLC-UV method may be implemented in diagnostics as an alternative method in case of the unavailability of the LC-MS/MS system.
1. Introduction
Herpesvirus (HSV) and cytomegalovirus (CMV) infections occur as major complications in immunocompromised patients, leading in some cases to fatal outcomes [1,2].
To date, the gold standard prophylaxis and treatment of HSV and CMV infections consist of the antivirals acyclovir and ganciclovir. These agents are nucleoside analogues that, once converted in their corresponding triphosphates by cellular kinases, are able to incorporate themselves into viral DNA and to disrupt DNA synthesis, thus inhibiting viral replication [3].
Despite the proven efficacy of these agents, there is growing evidence of high interindividual variability in patients undergoing acyclovir and ganciclovir therapy, especially if they are pediatric patients undergoing treatment with immunosuppressive drugs [4,5]. On one hand, the dose regimen of pediatric patients is based on adult population studies and adjusted based on simple algorithms, rather than pharmacokinetic data, even if it is well known that the pharmacokinetic profile of pediatric patients differs greatly from that of adults due to anatomical and physiological factors [6]. On the other hand, therapy with immunosuppressants, such as the calcineurin inhibitors, and pre-existent pathologies could cause renal impairment, influencing drug elimination [7]. Beyond an undefined dose regimen and co-treatment with immunosuppressive drugs, genetic variants such as the NUDT15 polymorphism could also be responsible for interindividual variability in patients undergoing acyclovir and ganciclovir treatment [8].
Acyclovir overdosing could lead to renal failure and to neuropsychiatric symptoms, such as confusion, somnolence, and hallucinations, due to the accumulation of its main metabolite 9-carboxymethoxymethylguanine; ganciclovir overdosing can cause neutropenia, thrombocytopenia, leukopenia, and elevated serum creatinine [9,10]. Notably, underdosing as an attempt to prevent the above-mentioned side effects can lead to drug resistance and therapy failure [10].
In this context, therapeutic drug monitoring is a potential solution to avoid toxicity and drug resistance and to optimize treatment in peculiar clinical scenarios. To date, target drug concentration ranges were determined for the trough concentration (Cmin) and peak concentration (Cmax) on the basis of previous studies. In particular, Maximova et al. investigated the population pharmacokinetics of intravenous acyclovir in oncologic children, defining Cmin (0.85 ± 1.3 mg/L) and Cmax (7.6 ± 5.4 mg/L) [4]. Instead, Richie and colleagues suggested 1–3 mg/L for Cmin and 3–12.5 mg/L for Cmax of ganciclovir, even if based on an adult population [5].
High-performance liquid chromatography (HPLC), coupled with several detectors, such as mass spectrometry, diode array detectors, UV spectrophotometry, and fluorescence detectors, has been applied for the quantification of acyclovir and ganciclovir in plasma and serum samples [4,10,11]. Generally, liquid chromatography coupled with mass spectrometry (LC-MS/MS) is the preferred technique to quantify these agents because of its high sensitivity, selectivity, and capability of providing quick results, even if such instrumentations are not always available and reagents employed in the analysis and sample preparation are expensive, in comparison with the conventional HPLC methods [12].
Clinical laboratories need more than one analytical method for the quantification of drugs whose therapeutic drug monitoring is necessary, so as not to discontinue the diagnostics routine and to provide rapid drug plasma concentration results to clinicians even in cases of instrumental damage. Therefore, we present a novel developed and validated method of HPLC coupled with UV spectrophotometry (HPLC-UV) for the simultaneous quantification of the antivirals acyclovir and ganciclovir, which provides comparable quantitative results to the LC-MS/MS methods used to date for diagnostics. The proposed HPLC-UV method may be implemented in clinical diagnostics routines as an alternative method in the case of the unavailability of an LC-MS/MS system, for instance as a result of instrumental damage.
2. Results
2.1. Method Development
An HPLC-UV method for the simultaneous quantification of the antivirals acyclovir and ganciclovir was compared with the LC-MS/MS methods for the quantification of each antiviral used to date for diagnostic purposes. A comparison between the features of the analytical methods is displayed in Table 1.

Table 1.
Comparison of the features of the HPLC-UV method developed with the LC-MS/MS methods, already in use for diagnostic purposes.
As shown in Figure 1, the proposed HPLC-UV method has demonstrated no interference of compounds belonging to the biological matrix at the same retention time of the analytes and internal standard (IS) at the wavelength used for the analysis In detail, the retention times of acyclovir, ganciclovir, and IS are 6.1, 4.5, and 4.9 min, respectively.
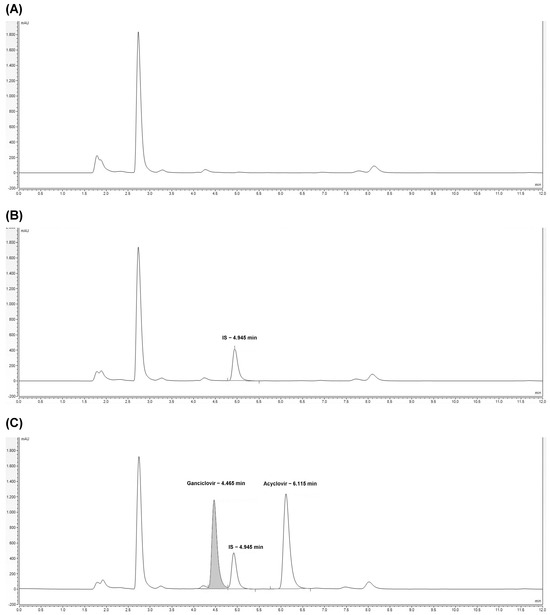
Figure 1.
Chromatograms related to HPLC-UV analysis of plasma free drug (blank) (A), plasma spiked with IS (calibrator 0) (B), and plasma spiked with ganciclovir and acyclovir (quality control at a concentration of 20 µg/mL) (C). The retention times of IS, ganciclovir, and acyclovir are reported.
Chromatograms related to the LC-MS/MS analysis showing the background noise, acyclovir, and ganciclovir are reported in Figure S1.
Sample purification using Phree Phospholipid Removal Products, capable of removing both proteins and phospholipids, was also tested without any benefit; on the contrary, the signals related to the analytes and the IS were reduced.
2.2. Method Validation
2.2.1. Linearity
The linear regression model confirmed the linearity of the HPLC-UV method in the range of concentrations tested (0.5–40 µg/mL). The coefficient of determination (R2) for all the three calibration curves was greater than 0.99 for both acyclovir and ganciclovir quantification. Furthermore, the within-run and between-run percentages of accuracy of the calibrators (CAL) were estimated: the calculated concentration resulted in accurate values (100 ± 15% of the nominal concentration) (Table 2).

Table 2.
Within-run (1 injection) and between-un accuracy (%) of CAL referring to acyclovir and ganciclovir quantification by HPLC-UV. RSD%: relative standard deviation %.
The within-run and between-run percentages of accuracy of the CAL analyzed by LC-MS/MS are shown in Table S1.
2.2.2. Sensitivity
Sensitivity was evaluated by analyzing dilutions of the calibrator with the lowest concentration and determining the lower limit of quantification (LLOQ) and detection (LOD) values, intended as the lowest concentration quantifiable and detectable. In particular, diluting CAL1 resulted in a loss of accuracy in quantification and a loss of signal for both acyclovir and ganciclovir; therefore, LLOQ and LOD correspond to CAL1 (0.5 µg/mL). Instead, LLOQ and LOD values for the quantification of both acyclovir and ganciclovir referred to the LC-MS/MS analyses resulted in values of 0.5 and 0.05 µg/mL, respectively.
2.2.3. Accuracy and Precision
The within-run and between-run accuracy and precision of three levels of quality controls (QC) were also estimated to assess an accurate and reproducible quantification of the antivirals. As shown in Table 3, the percentage of accuracy was 100 ± 15% of the nominal concentration, which in our opinion does not exceed the threshold value established by the ICH guidelines [13]. Also, CV% was far lower than 15%.

Table 3.
Within-run (3 injections) and between-run accuracy (%) and precision, expressed as the coefficient of variation (CV%) of the QC referring to acyclovir and ganciclovir quantification by HPLC-UV. SD: standard deviation.
The within-run and between-run percentages of accuracy and CV% of QCs analyzed by LC-MS/MS are shown in Table S2.
2.2.4. Matrix Effect and Recovery
In order to allow the reliability of results and efficiency of the analytical procedure, the matrix effect (ME), recovery of the extraction procedure (RE) and the overall process efficiency (PE) were evaluated (Table 4). Notably, there was no signal suppression due to the biological matrix in all of the three levels of concentration tested. Furthermore, the recovery of the analytes after sample preparation and analysis was >90%. Therefore, the process efficiency, the overall parameter obtained from the previous ones, resulted in optimal values.

Table 4.
Matrix effect (ME), recovery (RE), and process efficiency (PE) calculated on QC referring to the HPLC-UV method.
Indications for ME, RE, and PE related to the LC-MS/MS analyses were reported in Table S3.
2.2.5. Specificity
The interference of another immunosuppressive drug, mycophenolate, which is administered sometimes in pediatric oncologic patients and whose therapeutic drug monitoring is usually performed in plasma, was also tested by injecting a pure standard added with an IS. As shown in Figure S2, the retention time of mycophenolate is 1.8 min, different from the analytes and IS.
2.3. Method Applicability
Plasma concentrations of acyclovir and ganciclovir were obtained respectively pre and post-dose from 31 and 9 samples of pediatric patients undergoing intravenous therapy. Both the HPLC-UV and LC-MS/MS methods were used for quantification. In detail, the median and the interquartile range (IQR) related to acyclovir measurements was 1.42 mg/L (IQR: 0.81–5.50) for HPLC-UV analysis and 1.16 mg/L (IQR: 0.53–4.90) for LC-MS/MS analysis. Instead, the median and the IQR related to ganciclovir measurements was 4.26 mg/L (IQR: 0.35–6.46) for HPLC-UV analysis and 3.37 mg/L (IQR: 0.39–8.10) for LC-MS/MS analysis.
Bland–Altman plots describe the agreement between the quantitative measurements between the methods. In particular, Figure 2A,C display a scatter diagram of the differences plotted against the averages of the two measurements. Horizontal lines represent the mean difference and the limits of agreement (±1.96 SD of differences). Notably, the plasma concentration of drugs obtained from the different analyses showed no significant biases.
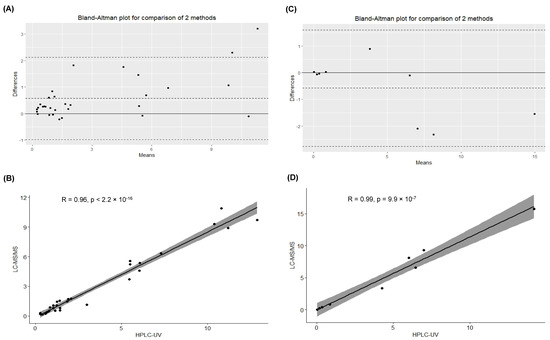
Figure 2.
(A) Bland–Altman plot evaluating the differences between the data obtained for the quantification of acyclovir by the analytical methods. (B) Spearman correlation test to evaluate the association between the calculated concentrations (mg/L) of acyclovir in the samples (n = 31) quantified by HPLC-UV and LC-MS/MS (p < 2.2 × 10−16). (C) Bland–Altman plot evaluating the differences between the data obtained for the quantification of ganciclovir by the analytical methods. (D) Pearson correlation test to evaluate the association between the calculated concentrations (mg/L) of ganciclovir in the samples (n = 9) quantified by HPLC-UV and LC-MS/MS (p < 9.9 × 10−7).
Furthermore, Spearman and Pearson correlation tests described a significant positive correlation for acyclovir and ganciclovir quantification, respectively (Spearman correlation test for acyclovir quantification: p < 2.2 × 10−16; Pearson correlation test for ganciclovir quantification: p < 9.9 × 10−7), as shown in Figure 2B,D.
3. Discussion
Since there is a need to have analytical methods which are not cost-, time-, and sample-consuming, with instrumentation easily available in every laboratory, and to overcome delays in providing results to clinicians in cases of instrumental damage, we have developed and validated an HPLC-UV method for the simultaneous quantification of the antivirals acyclovir and ganciclovir. Indeed, comparing the features of the HPLC-UV method with the LC-MS/MS methods already used for diagnostic purposes, we noticed several advantages consisting in the cost of reagents, the availability of the instrumentation, the volume of sample needed, the introduction of bromouracil as an IS, and the isocratic elution. In particular, the cost of reagents was greatly influenced by the choice of the stable isotope IS in LC-MS/MS, which have almost identical chemical properties [14].
Interestingly, since bromouracil was used in our work as an IS for the quantification of both acyclovir and ganciclovir in the proposed HPLC-UV method, the simultaneous quantification of these analytes was allowed by HPLC-UV. Instead, currently, the quantification of acyclovir and ganciclovir is performed by two LC-MS/MS methods, having the same instrumental conditions, but using each IS for the normalization of the response of the other. The introduction of bromouracil as an IS, and therefore the simultaneous quantification of both antivirals, could allow the use of the same calibrators and quality controls, allowing us to reduce the costs and the timing of the analysis. Techniques different from HPLC-UV are employed to obtain this purpose [15,16,17,18]; instead, to the authors’ knowledge, only a few papers have described HPLC-UV methods able to quantify both of these antivirals, as the chromatographic separation of these antivirals is hard to obtain due to their similar chemical structure [19,20,21]. Among the limitations of our study, there is the impossibility of quantifying 9-carboxymethoxymethylguanine, the main metabolite of acyclovir responsible for side effects encountered after antiviral treatment, as found in another study conducted previously [18].
Contrary to the manuscript from Shibata and colleagues, the proposed method was also validated in order to be implemented in clinical diagnostic routine [21]. Validation parameters were in line with those recommended by the ICH guidelines and by Matuszewski, thus suggesting the analytical method showed an appropriate sensitivity, linearity, accuracy, precision, and efficiency of the process [13,22,23]. Nonetheless, the analytical method could be improved to be more sensitive: other previous studies have described HPLC-UV methods with a lower LOD [19,20]. Anyway, our method is able to quantify both acyclovir and ganciclovir up to 0.5 mg/L, well below the Cmin reported in the scientific literature [4,5]. Notably, the linearity range assessed by our method was larger and covered the concentrations expected by Cmin and Cmax for acyclovir and ganciclovir, differently from the other studies [19,20].
Since the antivirals tested were administered in immunocompromised patients undergoing treatment with immunosuppressive drugs, we considered the interference of one of these agents in the optimization of the method. Interestingly, several immunosuppressive drugs, such as cyclosporine A, tacrolimus, sirolimus, and everolimus, were measured in whole blood and were not present in plasma after deproteinization, thus they were not potentially interfering with the analysis [24]. On the contrary, therapeutic drug monitoring of mycophenolate is generally performed in plasma and, for this reason, we decided to test the drug interference of this agent [25]. Notably, mycophenolate elutes at a different retention time from the analytes of interest, indicating that the proposed HPLC-UV method could also be performed in cases of co-treatment with this immunosuppressant.
As previously conducted [15], a small cohort of patients undergoing intravenous acyclovir (31) or ganciclovir (9) treatment was also investigated to assess the applicability of the novel HPLC-UV method. Notably, the quantitative results obtained with the proposed HPLC-UV method were significantly correlated and comparable with the ones obtained from the LC-MS/MS analyses actually used for diagnostic purposes.
Our study presents some limitations. In particular, based on the ICH Q2(R2) guideline on the validation of analytical procedures, the evaluation of accuracy and precision was performed by testing three levels of concentrations across the reportable range in triplicate, differently from what was suggested by the ICH guideline M10 on bioanalytical method validation and study sample analysis, Step 5 (four concentration levels and five replicates per level) [13,23].
In conclusion, beyond developing and validating a novel HPLC-UV method for the simultaneous quantification of acyclovir and ganciclovir, we tested the agreement between the novel analytical method and the LC-MS/MS methods actually used in our laboratory. Based on the results obtained from validation but particularly from quantitative data on real samples, the novel HPLC-UV may be implemented as an alternative method in the case of the unavailability of the LC-MS/MS system, for instance following instrumental damage.
Interesting new research insights would be gained from analyzing the plasma concentrations of the antivirals acyclovir and ganciclovir administered intravenously in cohorts of pediatric patients, taking into consideration their age, genotype, and dose.
4. Materials and Methods
4.1. Chemicals and Reagents
All chemicals and reagents used were of analytical grade. Pure water was obtained from a Milli-Q system (Millipore, Darmstadt, Germany). Acyclovir, ganciclovir, bromouracil, methanol, acetonitrile, trifluoracetic acid, ammonium formiate, and formic acid were purchased by Sigma-Aldrich (Milan, Italy). Phree Phospholipid Removal Products were purchased from Phenomenex (Bologna, Italy).
4.2. Biological Samples
Peripheral blood samples (3 mL) were collected in EDTA tubes from pediatric patients (0–18 years) undergoing intravenous acyclovir (n = 31) and ganciclovir (n = 9) therapeutic drug monitoring and were left over from routine clinical analysis. The use of leftover samples for the validation of analytical methods for the improvement of therapeutic drug monitoring was approved by IRCCS Burlo Garofolo (RC 56/22). Peripheral blood samples were collected after at least 5 days of antiviral treatment before the morning dose (trough concentration) or 30 min after the end of the infusion (peak concentration). Plasma was obtained by centrifugation at 3000× g for 5 min and stored at −80 °C until the following analysis for up to 1 month.
4.3. Stocking and Working Solutions
Free-drug plasma samples were obtained from healthy subjects and stored at −80 °C. Stock solutions, comprising acyclovir and ganciclovir dissolved in MilliQ water (1 mg/mL), were prepared and aliquoted at −80 °C for up to 12 months, a period in which they were certified to be stable. Working solutions A (500 µg/mL acyclovir) and B (500 µg/mL ganciclovir) were prepared by diluting the stock solutions 1:2 in MilliQ water on the day of the analysis. Moreover, CAL and QC were prepared by spiking the working solutions in free-drug plasma to achieve the final concentrations. In detail, a calibration curve comprising 6 calibration points (0.5, 1, 5, 10, 25, and 40 µg/mL) and 3 levels of QC (2.5, 12.5, 20 µg/mL) was constructed using different working solutions. A solution of bromouracil in methanol (100 µg/mL) was used as an IS for the quantification by HPLC-UV. Instead, regarding the LC-MS/MS analysis for the quantification of acyclovir, the working solution B was used as an IS; instead, regarding the LC-MS/MS analysis for the quantification of ganciclovir, the working solution A was used as an IS.
4.4. HPLC-UV Analysis
Regarding the sample preparation for HPLC-UV analysis, 25 µL of IS and 175 µL of methanol were added to 100 µL of CAL/QC/sample to allow deproteinization. After centrifugation for 5 min at 12,100× g, 250 µL of supernatant was collected, dried under a gentle stream of nitrogen, and subsequently resuspended in 50 µL of MilliQ water. Sample purification using Phree Phospholipid Removal Products was also tested. In total, 20 µL was injected into the instrument for the analysis.
The HPLC-UV method for the quantification of the antivirals acyclovir and ganciclovir was developed on a UPLC UltiMate™ 3000 coupled with the Dionex™ UltiMate™ 3000 VWD-3000 (Thermo Fischer Scientific, Milan, Italy). Separation was obtained by eluting mobile phases A (10 mM ammonium formiate 0.01% formic acid) and B (acetonitrile) on a Poroshell 120 SB-C8 2.1 × 150 mm, 2.7 µm (Agilent Technologies, Milan, Italy) for 12 min isocratically (97:3; A:B) at a flow rate of 0.2 mL/min. The column temperature was set at 25 °C. The wavelength set up for the analysis was 250 nm. Data acquisition and processing was performed using the Chromeleon™ Chromatography Data System version 7.0 (Thermo Fischer Scientific, Milan, Italy).
4.5. LC-MS/MS Analyses
Regarding the sample preparation for LC-MS/MS analyses, 10 µL of IS and 50 µL of a solution of 20% trifluoracetic acid were added to 150 µL of CAL/QC/sample. After centrifugation for 5 min at 121,00× g, 10 µL of supernatant was diluted with 90 µL of 0.1% formic acid in acetonitrile, and subsequently 5 µL was injected into the instrument.
The LC-MS/MS method for the quantification of the antivirals acyclovir and ganciclovir, used for the data comparation with the HPLC-UV method, was developed on a Shimadzu LC-40D XR coupled with a triple quad Sciex 3200. Separation for each molecule was obtained likewise, eluting mobile phases A (2 mM ammonium formiate 0.2% formic acid) and B (2 mM ammonium formiate 0.2% formic acid in acetonitrile) on an Allure PFPP 2.1 × 50 mm, 5 µm (Restek srl, Milan, Italy) at a flow rate of 0.4 mL/min with a gradient. In particular, the gradient used was 0–0.5 min, 5% B; 1 min, 10% B; 1.6 min, 98% B; 1.9–2.2 min, 2% B; 4.0 min, stop. The column temperature was set at 25 °C. The electrospray ionization was set to positive mode. The mass spectrometer operated in multiple reaction monitoring (MRM) mode controlled by Analyst operating software version 1.4 (Sciex, Milan, Italy). The ion spray voltage was set to 5000 V and the source temperature to 500 °C. Nitrogen was used as the collision gas. The nebulizer (GS1), curtain, and turbo gas (GS2) were set to 50, 25, and 60 psi, respectively. MRM parameters of analytes were optimized as described in Table 5. Dwell times were set to 75 ms for each transition.

Table 5.
Optimized compound parameters for MRM detection of quantifier (_1) and qualifier ions (_2/_3) including mass selected in the first quadrupole (Q1 mass) and in the third quadrupole (Q3 mass), declustering potential (DP), entrance potential (EP), collision energy (CE), and cell exit potential (CXP).
4.6. Analytical Validation
Validation of the analytical method was performed using the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines [13,23]. Briefly, the selectivity and specificity of the analytical method were assessed testing blank samples; linearity was determined through the construction of a 6-point calibration curve within a run in 3 different runs; sensitivity was assessed through the dilution of the calibrator at the lowest concentration and the evaluation of LLOQ and LOD. The within-run and between-run accuracy, measured as the percentage of accuracy, and precision, measured as CV%, were estimated by the use of 3 levels of QC. Interferences in the analytical method due to co-treatment with immunosuppressants (mycophenolate) were also investigated. Instead, the ME, RE, and process efficiency were determined using a pool of plasma samples obtained from 6 individuals and performing experiments in triplicate, as previously described by Matuszewski [22].
4.7. Statistical Analysis
Concentration was calculated by normalizing the response ratio of analytes to that of IS; calibration curves were fit by linear regression with 1/ꭕ 2) weighting. In order to compare the concentrations of samples quantified by the analytical methods, Pearson and Spearman correlation tests, depending on data distribution, were performed using R software version 3.0.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25052685/s1.
Author Contributions
Conceptualization, R.A. and M.F.; methodology, R.R.; software, R.R.; validation, M.F., R.R. and R.D.S.; formal analysis, M.F.; investigation, R.R. and R.D.S.; resources, R.A.; data curation, M.F.; writing—original draft preparation, M.F.; writing—review and editing, R.A.; visualization, M.F.; supervision, R.A.; project administration, M.F.; funding acquisition, R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health, through the contribution given to the Institute for Maternal and Child Health IRCCS “Burlo Garofolo”, Trieste, Italy (RC 56/22).
Institutional Review Board Statement
This study was approved by IRCCS Burlo Garofolo (RC 56/22).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Beyar-Katz, O.; Bitterman, R.; Zuckerman, T.; Ofran, Y.; Yahav, D.; Paul, M. Anti-herpesvirus prophylaxis, pre-emptive treatment or no treatment in adults undergoing allogeneic transplant for haematological disease: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N. CMV: Prevention, Diagnosis and Therapy. Am. J. Transplant. 2013, 13 (Suppl. S3), 24–40, quiz 40. [Google Scholar] [CrossRef] [PubMed]
- Huntjens, D.W.; Dijkstra, J.A.; Verwiel, L.N.; Slijkhuis, M.; Elbers, P.; Welkers, M.R.A.; Veldkamp, A.I.; Kuijvenhoven, M.A.; de Leeuw, D.C.; Abdullah-Koolmees, H.; et al. Optimizing Antiviral Dosing for HSV and CMV Treatment in Immunocompromised Patients. Pharmaceutics 2023, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Maximova, N.; Nisticò, D.; Luci, G.; Simeone, R.; Piscianz, E.; Segat, L.; Barbi, E.; Di Paolo, A. Population Pharmacokinetics of Intravenous Acyclovir in Oncologic Pediatric Patients. Front. Pharmacol. 2022, 13, 865871. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, B.M.; Barreto, J.N.; Barreto, E.F.; Crow, S.A.; Dierkhising, R.A.; Jannetto, P.J.; Tosh, P.K.; Razonable, R.R. Relationship of Ganciclovir Therapeutic Drug Monitoring with Clinical Efficacy and Patient Safety. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Stockmann, C.; Roberts, J.K.; Knackstedt, E.D.; Spigarelli, M.G.; Sherwin, C.M. Clinical pharmacokinetics and pharmacodynamics of ganciclovir and valganciclovir in children with cytomegalovirus infection. Expert Opin. Drug Metab. Toxicol. 2015, 11, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Pazhayattil, G.S.; Shirali, A.C. Drug-induced impairment of renal function. Int. J. Nephrol. Renov. Dis. 2014, 7, 457–468. [Google Scholar]
- Nishii, R.; Mizuno, T.; Rehling, D.; Smith, C.; Clark, B.L.; Zhao, X.; Brown, S.A.; Smart, B.; Moriyama, T.; Yamada, Y.; et al. NUDT15 polymorphism influences the metabolism and therapeutic effects of acyclovir and ganciclovir. Nat. Commun. 2021, 12, 4181. [Google Scholar] [CrossRef]
- Kacirova, I.; Urinovska, R.; Sagan, J. Therapeutic monitoring of serum concentrations of acyclovir and its metabolite 9-(carboxymethoxymethyl) guanine in routine clinical practice. Biomed. Pharmacother. 2022, 156, 113852. [Google Scholar] [CrossRef]
- Märtson, A.G.; Edwina, A.E.; Kim, H.Y.; Knoester, M.; Touw, D.J.; Sturkenboom, M.G.G.; Alffenaar, J.C. Therapeutic Drug Monitoring of Ganciclovir: Where Are We? Ther. Drug Monit. 2022, 44, 138–147. [Google Scholar] [CrossRef]
- Zendelovska, D.; Simeska, S.; Atanasovska, E.; Georgievska, K.; Kikerkov, I.; Labachevski, N.; Jakovski, K.; Balkanov, T. Determination of Acyclovir in Human Plasma Samples by HPLC Method with UV Detection: Application to Single-Dose Pharmacokinetic Study. Open Access Maced. J. Med. Sci. 2015, 3, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Grebe, S.K.; Singh, R.J. LC-MS/MS in the Clinical Laboratory—Where to From Here? Clin. Biochem. Rev. 2011, 32, 5–31. [Google Scholar] [PubMed]
- ICH Q2(R2) Guideline on Validation of Analytical Procedures. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guideline-validation-analytical-procedures-step-5-revision-1_en.pdf (accessed on 1 January 2023).
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19–34. [Google Scholar] [PubMed]
- Pigliasco, F.; Cafaro, A.; Simeoli, R.; Barco, S.; Magnasco, A.; Faraci, M.; Tripodi, G.; Goffredo, B.M.; Cangemi, G. A UHPLC-MS/MS Method for Therapeutic Drug Monitoring of Aciclovir and Ganciclovir in Plasma and Dried Plasma Spots. Biomedicines 2021, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Dao, Y.J.; Jiao, Z.; Zhong, M.K. Simultaneous determination of aciclovir, ganciclovir, and penciclovir in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 867, 270–276. [Google Scholar] [CrossRef]
- Alffenaar, J.; van Hateren, K.; Martson, A.; van den Bosch, G.; van der Werf, T.; Touw, D.; Alffenaar, J. Determination of ganciclovir and acyclovir in human serum using liquid chromatography-tandem mass spectrometry. J. Appl. Bioanal. 2018, 4, 175–186. [Google Scholar] [CrossRef]
- Ärlemalm, A.; Helldén, A.; Karlsson, L.; Carlsson, B.J.B.C. Rapid determination of acyclovir, its main metabolite 9-carboxymethoxymethylguanine, ganciclovir, and penciclovir in human serum using LC–MS/MS. Biomed. Chromatogr. 2022, 36, e5315. [Google Scholar] [CrossRef]
- Weller, D.R.; Balfour, H.H., Jr.; Vezina, H.E. Simultaneous determination of acyclovir, ganciclovir, and (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine in human plasma using high-performance liquid chromatography. Biomed. Chromatogr. 2009, 23, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Teshima, D.; Otsubo, K.; Yoshida, T.; Itoh, Y.; Oishi, R. A simple and simultaneous determination of acyclovir and ganciclovir in human plasma by high-performance liquid chromatography. Biomed. Chromatogr. 2003, 17, 500–503. [Google Scholar] [CrossRef]
- Shibata, N.; Kitamura, A.; Yoshikawa, Y.; Inoue, T.; Bamba, T.; Takada, K.J.P.; Communications, P. Simultaneous determination of aciclovir and ganciclovir in plasma by HPLC and pharmacokinetic interactions. Pharm. Pharmacol. Commun. 2000, 6, 501–506. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 1 January 2023).
- Pablo, A.H.; Breaud, A.R.; Clarke, W. Analysis of Immunosuppressant Drugs in Whole Blood by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Curr. Protoc. Toxicol. 2020, 84, e92. [Google Scholar] [CrossRef] [PubMed]
- Bunch, D.R.; Wang, S. Measurement of mycophenolic acid in plasma or serum by a commercial enzyme inhibition technique in comparison with a high performance liquid chromatography method. Clin. Chem. Lab. Med. 2008, 46, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).