1. Introduction
Sudden death (SD) is the sudden and nonviolent death of an apparently healthy individual, and it represents one of the most significant challenges in modern cardiology due to its wide incidence and significant social impact [
1]. Most cases of SD are typically cardiovascular in nature, and 20% are secondary to ischemic heart disease, mainly due to ST-elevation acute myocardial infarction (STEAMI), especially in patients with ventricular fibrillation (VF) [
1,
2].
VF rarely spontaneously reverts to non-lethal rhythms, with only 10% to 25% of patients [
3] surviving out-of-hospital cardiac arrest. Although this survival rate is increasing significantly, a high mortality rate still remains [
4]. Furthermore, in patients who reach the hospital and receive treatment, the presence of VF indicates a short-term unfavorable prognosis, as it has been described that these patients have higher in-hospital mortality [
2].
The risk of SD due to ventricular arrhythmias in STEAMI, with a special focus on VF, has been proposed to result from the interaction between genetic factors and environmental factors [
1,
5,
6]. While various genetic variations, such as single nucleotide polymorphisms (SNPs), have been extensively studied in VF associated with STEAMI [
7,
8,
9], there is a notable gap in the exploration of copy number variants (CNVs).
The frequency of CNVs in the human genome is approximately 12% [
10,
11], making them a common component of the human genome. They play a significant role in evolution, contributing to population diversity [
11], but also in the development of certain diseases [
12,
13] such as cancer [
14], autoimmune diseases [
15], neurodegenerative diseases [
16], and cardiovascular diseases [
17] such as tetralogy of Fallot, aortic stenosis, coarctation of the aorta, and ventricular septal defect.
Previous studies have focused on CNVs’ associations with SD [
18,
19,
20,
21], but none have been directly related to VF in STEAMI. This study aims to investigate CNVs in the context of STEAMI and VF using NGS technology. The detection of CNVs through NGS is anticipated to provide valuable insights into risk assessment and prognosis, holding significant implications for the prevention and treatment of SD associated with STEAMI.
2. Hypothesis and Objectives
The hypothesis is that CNVs significantly influence the predisposition to VF in patients with STEAMI.
Thus, the main objective of this study is to detect and characterize genes within CNVs identified in a cohort of patients with VF in the context of STEAMI. To achieve this objective, we will pursue a dual approach. Firstly, we will examine genes exhibiting CNVs in a significant population of STEAMI patients with VF, and concurrently, we will assess the presence of recurrent CNV patterns within these specific genes. Secondly, we will investigate the average gene copy number within the STEAMI and VF patient population, conducting a comprehensive analysis to identify the specific genes involved.
3. Results
The principal characteristics of the 39 patients analyzed with VF during AMI and of the 41 patients without VF during AMI used as control included in the study are summarized in
Table 1. There was not a significant difference between the groups in any of these variables.
To ensure the quality of the alignments, a preliminary assessment was performed by analyzing the bioinformatic quality report. This analysis allowed for the verification of the integrity and reliability of the aligned data before proceeding with subsequent stages of the analysis. It is noteworthy that everything in the data was found to be correct during this review.
The complete pipeline was then executed using a single-node configuration with 24 processes, which took approximately 80 min. This execution benefited from parallelization capability through a tool parameter that utilized all available cores on the node, resulting in significant time savings compared to sequential execution.
As a result of this process, a table containing a total of 4688 CNVs in the 41 VF patients was obtained. A preliminary inspection was then conducted to assess the data’s integrity. During this initial review, it was identified that two patients exhibited a significantly higher number of CNVs compared to the rest of the samples. Consequently, a scatter plot was generated to examine the nature of CNVs in these two particular patients. The scatterplots revealed the presence of considerable noise in the CNVs of these individuals, making it challenging to distinguish them from the true CNVs. Although antitarget BED files were calculated to mitigate this issue, the decision was made to exclude these two patients from further analysis to ensure the integrity and reliability of the remaining data. Subsequently, a new analysis was conducted, detecting a total of 1589 CNVs in the 39 VF patients.
3.1. CNVs in a Significant Number of Patients
Using the data obtained after the filtering, tables of genes with CNVs in the form of deletions and duplications that occur in a significant number of patients were obtained (see
Table 2 and
Table 3). It is essential to note that, by what was mentioned in
Section 5.5 of this study, a significant minimum threshold of 6 VF patients with specific CNVs has been established. Therefore, the tables presented only include genes in patients equal to or exceeding this threshold.
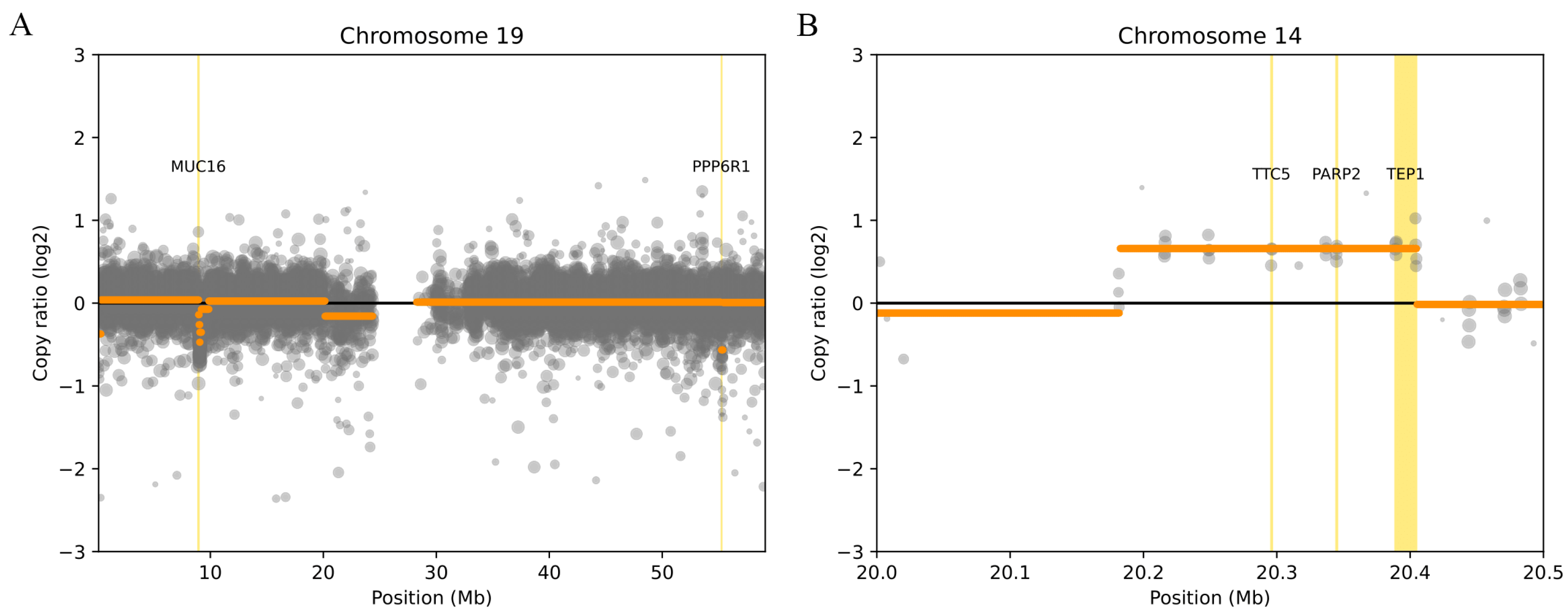
Regarding the results of the deletions presented in
Table 2, the presence of two genes,
MUC16 and
PPP6R1 (
Figure 1A), was observed in a significant number of patients, both located on chromosome 19. It is important to note that DisGeNET has associated
MUC16 with heart failure, suggesting greater relevance in the context of this study.
The results of CNVs in the form of duplications in a significant number of patients can be found in
Table 3. Duplications were observed in the
PARP2,
TEP1, and
TTC5 genes (
Figure 1B). It should be noted that all three genes have been associated with heart disease by DisGeNET.
PARP2 has been associated with cardiac hypertrophy,
TEP1 has been associated with myocardial infarction (MI) and ischemic stroke, and
TTC5 has been associated with cardiovascular disease.
3.2. Average Copy Number
To achieve the results for the second sub-objective, the average copy number for each CNV associated with different genes was calculated. It is worth noting that the copy number reflects the total number of copies the gene in question has in relation to the control reference, initially estimated at two. The results are detailed in
Table 4.
In
Table 4, the gene exhibiting the greatest mean copy number is
DEFB134, with a value of 4.5 copies. The other genes,
FCGR2C,
GREM1,
PARM1,
SCG5, and
UNC79, have an average copy number of 4 copies. It is important to note that DisGeNET has associated
DEFB134 with defects in the atrioventricular septum, while the rest of the genes have not been associated with any cardiac diseases by DisGeNET. This underscores the potential relevance of
DEFB134 in the context of this study.
4. Discussion
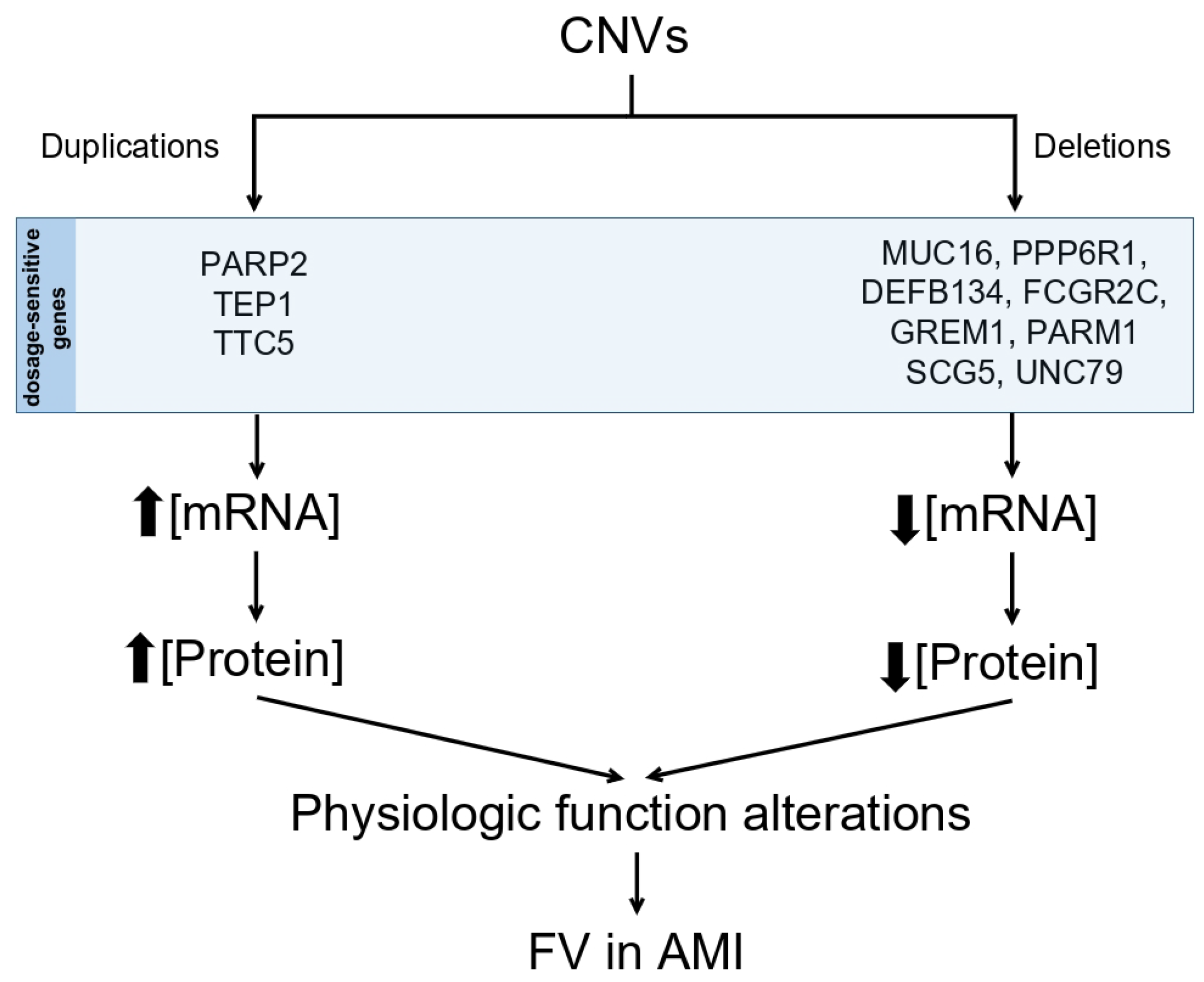
In this study, a comprehensive analysis was conducted to detect CNVs using whole exome sequencing (WES) data from a cohort of 39 patients previously diagnosed with VF in STEAMI. During the investigation, CNVs in the form of deletions were identified within the genes MUC16 and PPP6R1, along with CNVs in the form of duplications in the genes PARP2, TEP1, and TTC5. Furthermore, it was observed that the genes DEFB134, FCGR2C, GREM1, PARM1, SCG5, and UNC79 exhibited an average copy number greater than or equal to 4. These 11 genes show significant CNVs that may play a key role in the predisposition and development of VF.
4.1. CNV Deletions
It is worth noting that the genes
MUC16 and
PPP6R1 exhibited CNVs in the form of deletions in a significant number of patients, proposing
MUC16 and
PPP6R1 as genes of particular interest in the context of this study (see
Figure 2).
In the gene
MUC16, mucin 16, an association between increased
MUC16 expression and heart failure (HF) has been observed in previous studies [
22,
23,
24]. It is important to note that HF is a factor that can lead to STEAMI and potentially contribute to the development of VF. However, in the present study, a depletion in the copy number of the
MUC16 gene suggests a possible decrease in its expression. Although a reduction in
MUC16 has previously been associated with certain types of cancer [
25,
26], further studies are needed to elucidate the role of
MUC16 on VF.
In the case of PPP6R1, Protein Phosphatase 6 Regulatory Subunit 1, no previous information related to CNVs has been found in the context of cardiac diseases or any other context.
4.2. CNV Duplications
It is important to note that
PARP2,
TEP1, and
TTC5 were identified as duplications in a significant portion of the patient population (see
Figure 2).
The gene
PARP2, poly(ADP-ribose) polymerase 2, has been associated with cardiac hypertrophy in both mouse and human [
27] research studies. These studies have demonstrated that reducing gene expression (knockdown, KO) has a protective effect on cardiomyocytes against cardiac hypertrophy. In the case of
PARP2, the KO of this gene results in decreased gene expression and, consequently, a reduction in the amount of
PARP2 protein produced, which has a beneficial effect in preventing excessive cardiomyocyte growth [
27]. However, when considering duplications of the
PARP2 gene, we may expect the opposite effect. Duplication may lead to an increase in the number of gene copies in the genome, which could result in increased
PARP2 transcription and protein synthesis. Furthermore, there is a well-documented relationship between cardiac hypertrophy and VF [
28,
29]. Unfortunately, it was not possible to perform an echocardiogram in these patients to determine the degree of hypertrophy in patients with this duplication. However, the effects of this duplication on cardiac function and its association with cardiac hypertrophy may play a crucial role in the development of VF. This reinforces the potential involvement of the
PARP2 gene CNV as a predisposing factor to VF in the context of STEAMI.
Regarding the
TTC5 gene, tetratricopeptide repeat domain 5, a previous study [
30] has described that inhibiting
TTC5 expression leads to a significant attenuation of the damage suffered by cardiomyocytes due to oxygen and glucose deprivation, such as in ischemia. Furthermore, overexpression of
TTC5 has been observed to increase oxidative stress and levels of inflammatory factors in cardiomyocytes [
30]. These findings suggest that
TTC5 may play an adverse role in the cellular stress response, and its inhibition could confer protective effects at the cardiac cell level. The presence of CNVs in the form of duplications in
TTC5 in this study suggests that this gene may be overexpressed, increasing stress and causing cardiomyocyte injury, which could lead to STEAMI and subsequently VF. Taken together, these findings suggest that
TTC5 could be involved in the underlying mechanisms of VF in the context of STEAMI.
No direct relationship with CNV has been found related to
TEP1, telomerase-associated protein 1. Instead, associations with SNPs have been reported in cardiovascular diseases. In fact, rs1760898, rs8022805, rs1760897, rs2228041, and rs3093926 were specifically associated with myocardial infarction, while rs2228041, rs2184282, rs2104978, rs8022805, rs1713456, rs1713456, rs1713434, and rs7145318 were linked to ischemic stroke [
31].
4.3. Average Gene Copy Number
Finally, it is essential to highlight the presence of CNVs with an average gene copy number equal to or greater than four in
DEFB134,
FCGR2C,
GREM1,
PARM1,
SCG5, and
UNC79 (see
Figure 2).
The gene
DEFB134, defensin beta 134, has been associated with an atrioventricular septal defect in fetuses [
32], and this association has been established in cases involving a complete deletion of
DEFB134. To date, no reports in the scientific literature have linked the
DEFB134 gene to cardiovascular diseases in adults. This discovery and further research have the potential to elucidate the role of
DEFB134 in VF.
In relation to the genes FCGR2C, GREM1, PARM1, SCG5, and UNC79, no evidence supporting an association between CNVs and cardiovascular diseases has been identified in the scientific literature. It is important to note that the absence of information in this context does not rule out the possibility that these genes may play a role in cardiovascular health. Additional research is required to provide clarity on the influence of FCGR2C, GREM1, PARM1, SCG5, and UNC79 in the context of VF.
Although some of the statements of this discussion are mainly speculative but based in previous studies, we think that our data open gates to further research in this little-explored field, particularly the study of mechanisms through which the genes described in this study may contribute to develop FV in AMI. One promising option may be the use of CRISPR technology to inhibit the action of the described genes.
5. Materials and Methods
5.1. Study Population
The study was conducted in patients aged 18 years and older at the Complejo Hospitalario Universitario de A Coruña (CHUAC) who presented with ST-segment elevation myocardial infarction. Two main groups were included in the study population: cases and controls.
The case group consists of 41 patients who experienced SD due to VF in the context of acute coronary syndrome. The control group consisted of 40 patients with STEAMI who did not experience VF.
Exclusion criteria were applied to ensure the homogeneity of the study population. Patients with other known causes of SD, such as cardiomyopathies and channelopathies, were excluded. Additionally, patients whose SD had a cause different from ischemia, such as the abuse of toxic substances that could induce Brugada-like syndrome (like cocaine), were also excluded.
In total, 81 patients diagnosed with STEAMI at CHUAC between August 2017 and January 2021 were included in this study.
5.2. Next-Generation Sequencing
Exome sequencing was performed using NGS using a specific panel designed to capture the coding regions. For all 81 patients, the IDT xGen™ Exome Hybridization Panel v2 was utilized. This panel allowed the generation of paired-end reads in which DNA sequences were sequenced from both ends of DNA fragments, thus enhancing the quality and reliability of the obtained results. These sequencing procedures were carried out using the Illumina NextSeq500 platform.
The alignment of the sequences was conducted against the human genome GRCh37/hg19 using BWA-MEM software (Burrows–Wheeler Aligner-Maximal Exact Matches) version 0.7.15.
5.3. Storage and Processing of Data
Both data management and a substantial part of the analysis process were performed on the Finisterrae-III supercomputer, located at the facilities of the Galician Supercomputing Center (CESGA,
https://www.cesga.es/, accessed on 18 May 2023). This supercomputer provided the computational power and storage capacity required to perform the computationally intensive tasks needed for this study.
5.4. CNVkit
To perform the analysis of the CNVs present in the WES data, the CNVkit bioinformatics tool (0.9.10) [
33] was implemented. Among the majority of WES CNV detection tools that rely on the RD approach, CNVkit is one of the most comprehensive tools for CNV analysis [
13]. The pipeline designed for CNV analysis using CNVkit followed the guidelines suggested in the CNVkit documentation with some modifications.
5.4.1. Calculation of Accessible Coordinates
To optimize and expedite the analysis process, the calculation of accessible genomic regions was performed. These accessible regions are crucial to avoid unnecessary computations, as in most fully sequenced genomes, such as the human genome, there are extensive areas of DNA considered inaccessible for the sequencing process, such as centromeres, telomeres, and highly repetitive regions.
Identifying the coordinates of accessible regions was carried out using the reference genome GRCh37/hg19 and the command --annotate, generating a BED format file that stores only these locations.
5.4.2. Preparation of BED Files for the Target and Antitarget
The BED file for the target contains the genomic regions that are the target for the CNV analysis. The company of the panel provides this file, although it had to be modified to adapt it for CNVkit analysis.
Since exon regions can vary, the --split option was used to split the larger regions so that the average size of the resulting bins approximated the predefined average size of 267 bp. Additionally, because the provided BED file lacked gene labels for each genomic region, the option --annotate was added to include these labels. This involved incorporating the refFlat file obtained from the UCSC Genome Browser for annotation purposes.
The genomic regions for the antitarget were calculated using the
antitarget command based on the genomic positions of the target regions. By using the
-g option along with the BED file of accessible coordinates from
Section 5.4.1, the calculation process was expedited by avoiding read depth measurements in inaccessible areas. The BED file for the antitarget regions provides a background context for CNV analysis in the target regions. When calculating read depth metrics in the antitarget regions, it yields a measure of read depth variability in regions of no interest. This can help differentiate true CNVs from read depth fluctuations throughout the genome.
5.4.3. Read Depth
Using the coverage command, the
of the mean read depth was calculated using reads from BAM (Binary Alignment Map) files for each patient and the target and antitarget regions. The result is a table that provides the average read depths corresponding to each of the bins defined in
Section 5.4.2, centered around the mean read depth of all chromosomes.
5.4.4. Reference
CNVkit requires a control sample group to compare the mean read depths. Control samples should be generated using the same technical protocol to ensure that they exhibit a similar background noise pattern. Due to the experimental design’s nature, control samples from the same patients were not available to create this control group. Instead, genomes from the group without VF were selected as control samples.
For these samples, the reference command was used with the files obtained in
Section 5.4.3, along with the reference genome GRCh37/hg19, to create a reference file containing the mean read depths from all control samples. This reference file allows comparison with the remaining samples that present VF.
5.4.5. Normalization and Correction
When the fix command is used, the read depths of the targets and antitargets from the samples are combined, and any bin that does not meet the pre-established standards (in this case, the tool’s default values) is removed. It also corrects systematic biases in bin coverage, subtracts the read depths from the reference file, and then centers the median of the ratios to ensure that positive and negative biases are balanced around zero. This step is crucial as it allows real differences to stand out more clearly while reducing the influence of any unwanted noise or variability in the data.
The read depth itself is not a sufficient indicator of copy number due to systematic biases introduced during library preparation and sequencing. For example, read depth is affected by GC content [
34], sequence complexity, and interval size.
The fix command can remove most systematic errors by normalizing to a reference. However, even after normalization to a reference, coverage biases often persist compared to individual samples and need to be corrected. To resolve this, three additional correction steps were performed together with the fix command. Here is an explanation of what each command aims to correct:
GC bias correction: GC-rich fragments are underrepresented because DNA regions with a high GC content are less accessible for hybridization and susceptible to amplification during library preparation [
35].
Repeated sequences: Repetitive sequences in the genome can complicate read depth calculations because these regions often exhibit high coverage variability from one sample to another [
33]. In reference genome sequences, repetitive regions are masked using RepeatMasker (
http://repeatmasker.org, accessed on 18 May 2023). CNVkit calculates the proportion of each bin that is masked and uses this information for bias correction.
Target density: There are two systematic read depth distortions that occur at the edges of each target. The boundaries of each target display reduced read depth due to incomplete sequence matching with the probe, creating a negative bias in the observed read depth within the interval near each boundary. Additionally, some captures occur outside the target on the flanks of the baited interval due to the same mechanism. When targets are closely spaced or adjacent, this read depth in the flanks can overlap with a neighboring target, creating a positive bias in its read depth.
5.4.6. Segmentation and Calling
Once all sample bins have been corrected, they can be segmented into regions with a discrete number of copies using the
segment command. Before segmentation, the
values for each bin are automatically filtered to prevent outliers. The segmentation algorithm used, among all options available, is circular binary segmentation [
36], as it produced the best results in comparative evaluations conducted by CNVkit creators with exome panels. The segmentation results are presented in BED format.
Finally, the call command directly maps the ratios of the segment to absolute copy numbers for genes, based on a set of numerical thresholds.
5.4.7. Genemetrics
The final step of the pipeline involves the identification of specific genes that exhibit alterations in genomic copy number, which exceed a critical threshold. In the context of a diploid genome, gaining a single copy in a sample translates to a copy ratio of 3/2. This relationship can be expressed on a logarithmic base 2 scale, where (3/2) equals 0.585. Similarly, losing a single copy is represented as (1/2), which is equal to −1.0 and so on.
To carry out this process, the genemetrics command has been used with a specific threshold, following the tool’s documentation recommendations, set at −t 0.4. Additionally, to mitigate the presence of false positives in the results, a filter based on the number of probes per bin has been implemented. Following the documentation, a value of −m 5 has been chosen. Although this choice may exclude some true positives, it significantly reduces the presence of false positives in the analysis, improving the reliability of the final results.
5.5. Statistical Analysis
To analyze the demographic characteristics, a test was performed for the following variables: sex; dyslipidemia; hypertension; diabetes; and tobacco, as these variables were considered dichotomous. For age and BMI, an ANOVA test was performed.
To determine the minimum threshold of VF patients required to consider the frequency of a significant CNV, Fisher’s exact test was used. This test is particularly suitable in situations where the sample size is small and applying tests is not feasible. The objective is to assess whether the distribution of CNVs presence or absence significantly departs from what would be expected by chance. A contingency table was constructed and a significance level alpha of 0.05 was established. Using R, we computed the minimum sample size required to achieve a significance level below 0.05. A sensitivity analysis indicated that an N ≥ 6 VF patients exhibiting CNVs was indispensable to achieve statistical significance within the context of this study.
In the context of evaluating the average copy number of relevant genes, no specific threshold has been universally established. Previous studies focusing on cancer-related genes [
37,
38,
39] have consistently demonstrated significant variations when gene copy numbers reached or exceeded 4 copies. Consequently, we have opted to implement an average copy number filtration threshold of 4 or greater.
5.6. Filtering
Based on the results generated by the pipeline described in
Section 5.4, an extra filtering step was implemented to enhance the selection of CNVs. This refinement process was conducted in R, employing the dplyr library (version 1.1.2).
Initially, CNVs with a copy ratio equal to 2, as elaborated in
Section 5.4.7, were excluded. This exclusion is rooted in the assumption that CNVs with a copy ratio of 2 do not represent a significant variation when compared to the diploid reference. Additionally, a filtration step was applied to the
p-values assigned to each CNV, discarding those exceeding the threshold of 0.01. This
p-value filtration is essential to concentrate the analysis on the most pertinent and statistically significant CNVs. Lastly, CNVs lacking an association with any specific gene were eliminated.
5.7. Enrichment through DisGeNET
DisGeNET [
40] is a research platform that contains one of the largest public collections of genes and variants associated with human diseases. It was used, along with its R library “disgenet2r” (version 0.99.3), to enrich the analysis by incorporating information about diseases directly related to each gene. It is essential to emphasize that the diseases associated with “disgenet2r” are not limited exclusively to those linked to a particular gene’s CNVs. They encompass a wider spectrum of medical conditions that have previously been related to each gene in earlier research. The purpose of using this library is to identify any previously established link between the gene under study and a disease.
6. Limitations
A significant limitation of the study lies in the sample size. However, it is essential to note that obtaining a significant number of samples is a challenge in this context, as VF occurs in less than 3% of MI cases [
41]. Another limitation of the study is that the CNVs on the X and Y chromosomes were not analyzed, as it was not the main objective of the study.
7. Conclusions
This study has addressed the relationship between CNVs and predisposition to VF in patients who have experienced an MI. We can conclude that: The presence of 3 CNVs in the form of duplications in the PARP2, TEP1, and TTC5 genes has been confirmed in 6 patients with VF. The presence of 2 CNVs in the form of deletions in the genes MUC16 and PPP6R1 has been confirmed in 10 patients with VF. An association has been observed between the mean copy number of genes DEFB134, FCGR2C, GREM1, PARM1, SCG5, and UNC79 and VF in the population of patients with STEAMI. Literature supports the involvement of the PARP2 and TTC5 genes as potential candidates of relevance in the pathogenesis of VF in the context of MI.
Author Contributions
Conceptualization, L.N., J.M.V.-R. and M.H.-P.; methodology, R.L.-B., R.P.-L., F.R.-L., and D.L.-V.; software, R.L.-B., R.P.-L., and L.N.; investigation, R.L.-B., R.P.-L., L.N., F.R.-L., and D.L.-V.; data curation, F.R.-L., D.L.-V., and J.M.V.-R.; writing, R.L.-B., R.P.-L., L.N., J.M.V.-R. and M.H.-P.; funding acquisition, J.M.V.-R. and M.H.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from “Instituto de Salud Carlos III” (PI18/01737) and a non-conditional grant from Abbott Vascular.
Institutional Review Board Statement
The protocol of this study was in accordance with the principles of the Declaration of Helsinki, and the “Comité de Ética de la Investigación de Galicia” (ref: 2016/299) had approved it. All the samples were included in the biobank of the National Biobank Network of “Instituto de Salud Carlos III” (C.0002483, 2013/109).
Informed Consent Statement
Written informed consent was obtained from every patient included in the study.
Data Availability Statement
The data that support the findings of this study are available on justified request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Glinge, C.; Sattler, S.; Jabbari, R.; Tfelt-Hansen, J. Epidemiology and genetics of ventricular fibrillation during acute myocardial infarction. J. Geriatr. Cardiol. JGC 2016, 13, 789–797. [Google Scholar] [CrossRef]
- Demidova, M.M.; Smith, J.G.; Höijer, C.J.; Holmqvist, F.; Erlinge, D.; Platonov, P.G. Prognostic impact of early ventricular fibrillation in patients with ST-elevation myocardial infarction treated with primary PCI. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 302–311. [Google Scholar] [CrossRef]
- Hundahl, L.A.; Tfelt-Hansen, J.; Jespersen, T. Rat Models of Ventricular Fibrillation Following Acute Myocardial Infarction. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 514–528. [Google Scholar] [CrossRef]
- Thompson, C.A.; Yarzebski, J.; Goldberg, R.J.; Lessard, D.; Gore, J.M.; Dalen, J.E. Changes over time in the incidence and case-fatality rates of primary ventricular fibrillation complicating acute myocardial infarction: Perspectives from the Worcester Heart Attack Study. Am. Heart J. 2000, 139, 1014–1021. [Google Scholar] [CrossRef]
- Myocardial Infarction Genetics Consortium; Kathiresan, S.; Voight, B.F.; Purcell, S.; Musunuru, K.; Ardissino, D.; Mannucci, P.M.; Anand, S.; Engert, J.C.; Samani, N.J.; et al. Genome-wide association of early-onset myocardial infarction with common single nucleotide polymorphisms, common copy number variants, and rare copy number variants. Nat. Genet. 2009, 41, 334–341. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Pazoki, R.; Bardai, A.; Marsman, R.F.; de Jong, J.S.S.G.; Blom, M.T.; Scicluna, B.P.; Jukema, J.W.; Bindraban, N.R.; Lichtner, P.; et al. Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat. Genet. 2010, 42, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wang, P.; Yu, D.; Zhang, X.; Gou, D.; Liang, L.; Bai, X.; Xie, W.; Li, H.; Pu, J.; et al. Genome-Wide Association Study for Idiopathic Ventricular Tachyarrhythmias Identifies Key Role of CCR7 and PKN2 in Calcium Homeostasis and Cardiac Rhythm Maintenance. Circ. Genom. Precis. Med. 2022, 15, e003603. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Chang, S.N.; Chiu, F.C.; Huang, P.S.; Wu, C.K.; Wang, Y.C.; Hwang, J.J.; Tsai, C.T. Identification of Genetic Mutations in Patients with Idiopathic Ventricular Fibrillation Implying Underlying Mechanism—An Asian Sudden Death Longitudinal Cohort. 2022. Available online: https://ssrn.com/abstract=4305294 (accessed on 3 September 2023).
- Stranger, B.E.; Forrest, M.S.; Dunning, M.; Ingle, C.E.; Beazley, C.; Thorne, N.; Redon, R.; Bird, C.P.; de Grassi, A.; Lee, C.; et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 2007, 315, 848–853. [Google Scholar] [CrossRef]
- Redon, R.; Ishikawa, S.; Fitch, K.R.; Feuk, L.; Perry, G.H.; Andrews, T.D.; Fiegler, H.; Shapero, M.H.; Carson, A.R.; Chen, W.; et al. Global variation in copy number in the human genome. Nature 2006, 444, 444–454. [Google Scholar] [CrossRef]
- Pös, O.; Radvanszky, J.; Buglyó, G.; Pös, Z.; Rusnakova, D.; Nagy, B.; Szemes, T. DNA copy number variation: Main characteristics, evolutionary significance, and pathological aspects. Biomed. J. 2021, 44, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, H.; Yuan, X.; Gao, K.; Duan, J. Comparative study of whole exome sequencing-based copy number variation detection tools. BMC Bioinform. 2020, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.M.; Holmdahl, R. Copy number variation in autoimmunity–importance hidden in complexity? Eur. J. Immunol. 2012, 42, 1969–1976. [Google Scholar] [CrossRef]
- Lee, J.A.; Lupski, J.R. Genomic rearrangements and gene copy-number alterations as a cause of nervous system disorders. Neuron 2006, 52, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.G.; Seidman, C.E. Genetics of Congenital Heart Disease: The Glass Half Empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.; Mademont-Soler, I.; Fernandez-Falgueras, A.; Sarquella-Brugada, G.; Cesar, S.; Arbelo, E.; García-Álvarez, A.; Jordà, P.; Toro, R.; Coll, M.; et al. Sudden Cardiac Death and Copy Number Variants: What Do We Know after 10 Years of Genetic Analysis? Forensic Sci. Int. Genet. 2020, 47, 102281. [Google Scholar] [CrossRef]
- Singer, E.S.; Ross, S.B.; Skinner, J.R.; Weintraub, R.G.; Ingles, J.; Semsarian, C.; Bagnall, R.D. Characterization of clinically relevant copy-number variants from exomes of patients with inherited heart disease and unexplained sudden cardiac death. Genet. Med. 2021, 23, 86–93. [Google Scholar] [CrossRef]
- Brownstein, C.A.; Douard, E.; Haynes, R.L.; Koh, H.Y.; Haghighi, A.; Keywan, C.; Martin, B.; Alexandrescu, S.; Haas, E.A.; Vargas, S.O.; et al. Copy Number Variation and Structural Genomic Findings in 116 Cases of Sudden Unexplained Death between 1 and 28 Months of Age. Adv. Genet. 2023, 4, 2200012. [Google Scholar] [CrossRef]
- Ehrlich, L.; Prakash, S.K. Copy-number variation in congenital heart disease. Curr. Opin. Genet. Dev. 2022, 77, 101986. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Miñana, G.; Santas, E.; Llácer, P.; Núñez, E.; Palau, P.; Bodí, V.; Chorro, F.J.; Sanchis, J.; et al. Antigen carbohydrate 125 as a biomarker in heart failure: A narrative review. Eur. J. Heart Fail. 2021, 23, 1445–1457. [Google Scholar] [CrossRef]
- Xu, K.; Wu, M.; Huang, M.; Zhuo, X.; Weng, Y.; Chen, X. Carbohydrate antigen 125 combined with N-terminal pro-B-type natriuretic peptide in the prediction of acute heart failure following ST-elevation myocardial infarction. Medicine 2022, 101, e32129. [Google Scholar] [CrossRef]
- Wu, H.; Cao, G.; Wang, Y.; Tian, H.; Du, R. Increased Serum CA125 and Brain-Derived Neurotrophic Factor (BDNF) Levels on Acute Myocardial Infarction: A Predictor for Acute Heart Failure. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 913–919. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Zeng, J.; Lin, J. Knockdown of MUC16 (CA125) Enhances the Migration and Invasion of Hepatocellular Carcinoma Cells. Front. Oncol. 2021, 11, 667669. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S.; Failer, S.; Schuell, T.; Wagner, U. CA125 (MUC16) gene silencing suppresses growth properties of ovarian and breast cancer cells. Eur. J. Cancer 2012, 48, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Cai, Y.; Gao, S.; Lu, J.; Zhang, L.; Zou, J.; Liu, M.; Yu, S.; Ye, J.; Liu, P. PARP-2 knockdown protects cardiomyocytes from hypertrophy via activation of SIRT1. Biochem. Biophys. Res. Commun. 2013, 430, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Jang, A.Y.; Chung, W.J.; Han, S.H.; Semsarian, C.; Choi, I.S. Ventricular fibrillation and sudden cardiac arrest in apical hypertrophic cardiomyopathy: Two case reports. World J. Clin. Cases 2021, 9, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Saumarez, R.C.; Camm, A.J.; Panagos, A.; Gill, J.S.; Stewart, J.T.; de Belder, M.A.; Simpson, I.A.; McKenna, W.J. Ventricular fibrillation in hypertrophic cardiomyopathy is associated with increased fractionation of paced right ventricular electrograms. Circulation 1992, 86, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, Y.; Long, X.; Chen, H.; Zheng, Z.; Pan, H.; Peng, J.; Liu, Y.; Wang, H.; Hu, Y. Ultrasound-targeted microbubble destruction (UTMD)-mediated miR-150-5p attenuates oxygen and glucose deprivation-induced cardiomyocyte injury by inhibiting TTC5 expression. Mol. Biol. Rep. 2022, 49, 6041–6052. [Google Scholar] [CrossRef] [PubMed]
- Zee, R.Y.; Ridker, P.M.; Chasman, D.I. Genetic Variants in Eleven Telomere-Associated Genes and the Risk of Incident Cardio/Cerebrovascular Disease: The Women’s Genome Health Study. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Keitges, E.A.; Pasion, R.; Burnside, R.D.; Mason, C.; Gonzalez-Ruiz, A.; Dunn, T.; Masiello, M.; Gebbia, J.A.; Fernandez, C.O.; Risheg, H. Prenatal diagnosis of two fetuses with deletions of 8p23.1, critical region for congenital diaphragmatic hernia and heart defects. Am. J. Med. Genet. Part A 2013, 161A, 1755–1758. [Google Scholar] [CrossRef]
- Talevich, E.; Shain, A.H.; Botton, T.; Bastian, B.C. CNVkit: Genome-Wide Copy Number Detection and Visualization from Targeted DNA Sequencing. PLoS Comput. Biol. 2016, 12, e1004873. [Google Scholar] [CrossRef] [PubMed]
- Boeva, V.; Zinovyev, A.; Bleakley, K.; Vert, J.P.; Janoueix-Lerosey, I.; Delattre, O.; Barillot, E. Control-free calling of copy number alterations in deep-sequencing data using GC-content normalization. Bioinformatics 2011, 27, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Speed, T.P. Summarizing and correcting the GC content bias in high-throughput sequencing. Nucleic Acids Res. 2012, 40, e72. [Google Scholar] [CrossRef]
- Olshen, A.B.; Venkatraman, E.S.; Lucito, R.; Wigler, M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics 2004, 5, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.W.; Pearson, J.; Gearry, R.B.; Barclay, M.L.; McKinney, C.; Merriman, T.R.; Roberts, R.L. Association of higher DEFB4 genomic copy number with Crohn’s disease. Am. J. Gastroenterol. 2010, 105, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Bofin, A.M.; Ytterhus, B.; Klæstad, E.; Valla, M. FGFR1 copy number in breast cancer: Associations with proliferation, histopathological grade and molecular subtypes. J. Clin. Pathol. 2022, 75, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Valla, M.; Opdahl, S.; Ytterhus, B.; Bofin, A.M. DTX3 copy number increase in breast cancer: A study of associations to molecular subtype, proliferation and prognosis. Breast Cancer Res. Treat. 2021, 187, 57–67. [Google Scholar] [CrossRef]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, À.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database J. Biol. Databases Curation 2015, 2015, bav028. [Google Scholar] [CrossRef]
- Weizman, O.; Marijon, E.; Narayanan, K.; Boveda, S.; Defaye, P.; Martins, R.; Deharo, J.; Laurent, G.; Klug, D.; Sadoul, N.; et al. Incidence, Characteristics, and Outcomes of Ventricular Fibrillation Complicating Acute Myocardial Infarction in Women Admitted Alive in the Hospital. J. Am. Heart Assoc. 2022, 11, e025959. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).