Research into New Molecular Mechanisms in Thrombotic Diseases Paves the Way for Innovative Therapeutic Approaches
Abstract
1. Introduction
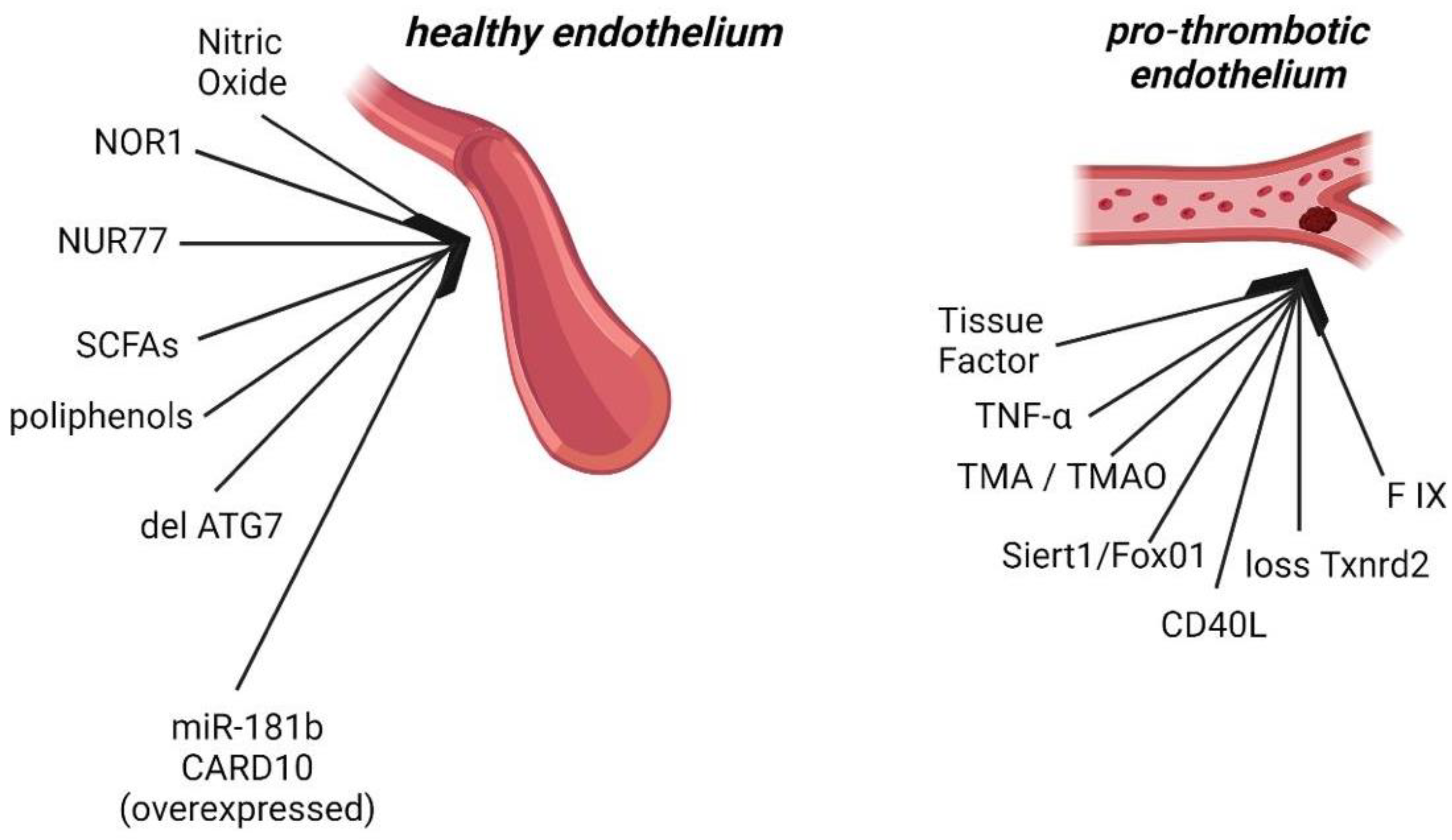
2. Endothelial Dysfunction
3. Platelets and Endothelial Inflammation
4. Role of Coagulation Cascade and Anticoagulant System
5. Genetic Studies in Thrombosis
6. Role of Extracellular Vesicles in Thrombosis
Evidence for the Involvement of EVs in Arterial and Venous Thrombosis
7. Thrombotic Diseases and New Therapies
7.1. Atrial Fibrillation and Venous Thrombosis
Life-Threatening Clot
7.2. Therapeutic Implications of Extracellular Vesicles in Thrombosis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Fang, J.; Liu, Z.; Shi, Y.; Agostini, M.; Bernassola, F.; Bove, P.; Candi, E.; Rovella, V.; Sica, G.; et al. Macrophage Polarization and Metabolism in Atherosclerosis. Cell Death Dis. 2023, 14, 691. [Google Scholar] [CrossRef]
- Davì, G.; Patrono, C. Platelet Activation and Atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Bhatt, D.L.; Wilson, P.W.F.; D’Agostino, R.; Ohman, E.M.; Röther, J.; Liau, C.-S.; Hirsch, A.T.; Mas, J.-L.; Ikeda, Y.; et al. One-Year Cardiovascular Event Rates in Outpatients with Atherothrombosis. JAMA 2007, 297, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; Arcelus, J.I.; Bergqvist, D.; Brecht, J.G.; Greer, I.A.; Heit, J.A.; Hutchinson, J.L.; Kakkar, A.K.; et al. Venous Thromboembolism (VTE) in Europe. The Number of VTE Events and Associated Morbidity and Mortality. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar]
- Anton, A.; Campreciós, G.; Pérez-Campuzano, V.; Orts, L.; García-Pagán, J.C.; Hernández-Gea, V. The Pathophysiology of Portal Vein Thrombosis in Cirrhosis: Getting Deeper into Virchow’s Triad. J. Clin. Med. 2022, 11, 800. [Google Scholar] [CrossRef]
- Turner, B.R.H.; Machin, M.; Salih, M.; Jasionowska, S.; Lawton, R.; Siracusa, F.; Gwozdz, A.M.; Shalhoub, J.; Davies, A.H. An Updated Systematic Review and Meta-Analysis of the Impact of Graduated Compression Stockings in Addition to Pharmacological Thromboprophylaxis for Prevention of Venous Thromboembolism in Surgical Inpatients. Ann. Surg. 2024, 279, 29–36. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial Function in Cardiovascular Medicine: A Consensus Paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, J.; Schneider, H.; Pagel, J.-I.; Rehberg, M.; Singer, M.; Hellfritsch, J.; Chillo, O.; Schubert, K.M.; Qiu, J.; Pogoda, K.; et al. Endothelial Dysfunction, and A Prothrombotic, Proinflammatory Phenotype Is Caused by Loss of Mitochondrial Thioredoxin Reductase in Endothelium. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Singh, K.K.; Hou, Y.; Lei, X.; Ramadan, A.; Quan, A.; Teoh, H.; Kuebler, W.M.; Al-Omran, M.; Yanagawa, B.; et al. Endothelial-Specific Deletion of Autophagy-Related 7 (ATG7) Attenuates Arterial Thrombosis in Mice. J. Thorac. Cardiovasc. Surg. 2017, 154, 978–988.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, Y.; Jiang, M.; Wang, F.; Gong, G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4132. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, S.; Sun, X.; Franck, G.; Deng, Y.; Yang, D.; Haemmig, S.; Wara, A.K.M.; Icli, B.; Li, D.; et al. MicroRNA-181b Inhibits Thrombin-Mediated Endothelial Activation and Arterial Thrombosis by Targeting Caspase Recruitment Domain Family Member 10. FASEB J. 2016, 30, 3216–3226. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Dhanesha, N.; Prakash, P.; Doddapattar, P.; Khanna, I.; Pollpeter, M.J.; Nayak, M.K.; Staber, J.M.; Chauhan, A.K. Endothelial Cell-Derived von Willebrand Factor Is the Major Determinant That Mediates von Willebrand Factor-Dependent Acute Ischemic Stroke by Promoting Postischemic Thrombo-Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1829–1837. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Gresele, P.; Momi, S.; Guglielmini, G. Nitric Oxide-Enhancing or -Releasing Agents as Antithrombotic Drugs. Biochem. Pharmacol. 2019, 166, 300–312. [Google Scholar] [CrossRef]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Mollace, R.; Scarano, F.; Scicchitano, M.; Macrì, R.; Nucera, S.; Bosco, F.; Oppedisano, F.; et al. The Contribution of Gut Microbiota and Endothelial Dysfunction in the Development of Arterial Hypertension in Animal Models and in Humans. Int. J. Mol. Sci. 2022, 23, 3698. [Google Scholar] [CrossRef]
- Amedei, A.; Morbidelli, L. Circulating Metabolites Originating from Gut Microbiota Control Endothelial Cell Function. Molecules 2019, 24, 3992. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Migliori, M.; Cantaluppi, V.; Mannari, C.; Bertelli, A.A.E.; Medica, D.; Quercia, A.D.; Navarro, V.; Scatena, A.; Giovannini, L.; Biancone, L.; et al. Caffeic Acid, a Phenol Found in White Wine, Modulates Endothelial Nitric Oxide Production and Protects from Oxidative Stress-Associated Endothelial Cell Injury. PLoS ONE 2015, 10, e0117530. [Google Scholar] [CrossRef] [PubMed]
- Rolla, R.; Puricelli, C.; Bertoni, A.; Boggio, E.; Gigliotti, C.L.; Chiocchetti, A.; Cappellano, G.; Dianzani, U. Platelets: “Multiple Choice” Effectors in the Immune Response and Their Implication in COVID-19 Thromboinflammatory Process. Int. J. Lab. Hematol. 2021, 43, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, C.; Boggio, E.; Gigliotti, C.L.; Stoppa, I.; Sutti, S.; Giordano, M.; Dianzani, U.; Rolla, R. Platelets, Protean Cells with All-Around Functions and Multifaceted Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 4565. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.D.; Jackson, D.E. Platelets: Pleiotropic Roles in Atherogenesis and Atherothrombosis. Int. J. Biochem. Cell Biol. 2010, 42, 1762–1766. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.; Skendros, P.; Konstantinides, S.; Ritis, K. Traps N’ Clots: NET-Mediated Thrombosis and Related Diseases. Thromb. Haemost. 2020, 120, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New Fundamentals in Hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Bouwens, E.A.M.; Stavenuiter, F.; Mosnier, L.O. Mechanisms of Anticoagulant and Cytoprotective Actions of the Protein C Pathway. J. Thromb. Haemost. 2013, 11 (Suppl. S1), 242–253. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How It All Starts: Initiation of the Clotting Cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef]
- Thaler, J.; Ay, C.; Mackman, N.; Bertina, R.M.; Kaider, A.; Marosi, C.; Key, N.S.; Barcel, D.A.; Scheithauer, W.; Kornek, G.; et al. Microparticle-Associated Tissue Factor Activity, Venous Thromboembolism and Mortality in Pancreatic, Gastric, Colorectal and Brain Cancer Patients. J. Thromb. Haemost. 2012, 10, 1363–1370. [Google Scholar] [CrossRef]
- Ten Cate, H.; Hackeng, T.M.; García de Frutos, P. Coagulation Factor and Protease Pathways in Thrombosis and Cardiovascular Disease. Thromb. Haemost. 2017, 117, 1265–1271. [Google Scholar] [CrossRef]
- Morange, P.-E.; Suchon, P.; Trégouët, D.-A. Genetics of Venous Thrombosis: Update in 2015. Thromb Haemost 2015, 114, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Al-Koussa, H.; AlZaim, I.; El-Sabban, M.E. Pathophysiology of Coagulation and Emerging Roles for Extracellular Vesicles in Coagulation Cascades and Disorders. J. Clin. Med. 2022, 11, 4932. [Google Scholar] [CrossRef] [PubMed]
- Renné, T.; Schmaier, A.H.; Nickel, K.F.; Blombäck, M.; Maas, C. In Vivo Roles of Factor XII. Blood 2012, 120, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Memtsas, V.P.; Arachchillage, D.R.J.; Gorog, D.A. Role, Laboratory Assessment and Clinical Relevance of Fibrin, Factor XIII and Endogenous Fibrinolysis in Arterial and Venous Thrombosis. Int. J. Mol. Sci. 2021, 22, 1472. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.M.; Macrae, F.L.; Duval, C.; McPherson, H.R.; Bridge, K.I.; Ajjan, R.A.; Ridger, V.C.; Connell, S.D.; Philippou, H.; Ariëns, R.A.S. Thrombin and Fibrinogen γ’ Impact Clot Structure by Marked Effects on Intrafibrillar Structure and Protofibril Packing. Blood 2016, 127, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Ariëns, R.A.S. Fibrin Clot Structure and Function: A Role in the Pathophysiology of Arterial and Venous Thromboembolic Diseases. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef] [PubMed]
- Korte, W.; Poon, M.-C.; Iorio, A.; Makris, M. Thrombosis in Inherited Fibrinogen Disorders. Transfus. Med. Hemother 2017, 44, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Zalewski, J.; Krochin, M.; Siudak, Z.; Sadowski, M.; Pregowski, J.; Dudek, D.; Janion, M.; Witkowski, A.; Zmudka, K. Altered Plasma Fibrin Clot Properties Are Associated with In-Stent Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Rooth, E.; Wallen, N.H.; Blombäck, M.; He, S. Decreased Fibrin Network Permeability and Impaired Fibrinolysis in the Acute and Convalescent Phase of Ischemic Stroke. Thromb. Res. 2011, 127, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Natorska, J. Improving Fibrinolysis in Venous Thromboembolism: Impact of Fibrin Structure. Expert Rev. Hematol. 2019, 12, 597–607. [Google Scholar] [CrossRef]
- Bagoly, Z.; Koncz, Z.; Hársfalvi, J.; Muszbek, L. Factor XIII, Clot Structure, Thrombosis. Thromb. Res. 2012, 129, 382–387. [Google Scholar] [CrossRef]
- Jung, J.H.; Song, G.G.; Kim, J.-H.; Seo, Y.H.; Choi, S.J. Association of Factor XIII Val34Leu Polymorphism and Coronary Artery Disease: A Meta-Analysis. Cardiol. J. 2017, 24, 74–84. [Google Scholar] [CrossRef]
- Stepień, E.; Plicner, D.; Kapelak, B.; Wypasek, E.; Sadowski, J.; Undas, A. Factor XIII Val34Leu Polymorphism as a Modulator of Fibrin Clot Permeability and Resistance to Lysis in Patients with Severe Coronary Artery Disease. Kardiol. Pol. 2009, 67, 947–955. [Google Scholar]
- Siegerink, B.; Maino, A.; Algra, A.; Rosendaal, F.R. Hypercoagulability and the Risk of Myocardial Infarction and Ischemic Stroke in Young Women. J. Thromb. Haemost. 2015, 13, 1568–1575. [Google Scholar] [CrossRef]
- Landau, M.B.; Renni, M.S.; Zalis, M.G.; Spector, N.; Gadelha, T. Coagulation Factor XIII Tyr204Phe Gene Variant and the Risk of Ischemic Stroke. J. Thromb. Haemost. 2013, 11, 1426–1427. [Google Scholar] [CrossRef]
- Mezei, Z.A.; Katona, É.; Kállai, J.; Bereczky, Z.; Somodi, L.; Molnár, É.; Kovács, B.; Miklós, T.; Ajzner, É.; Muszbek, L. Factor XIII Levels and Factor XIII B Subunit Polymorphisms in Patients with Venous Thromboembolism. Thromb. Res. 2017, 158, 93–97. [Google Scholar] [CrossRef]
- Saraf, S.; Christopoulos, C.; Salha, I.B.; Stott, D.J.; Gorog, D.A. Impaired Endogenous Thrombolysis in Acute Coronary Syndrome Patients Predicts Cardiovascular Death and Nonfatal Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Spinthakis, N.; Gue, Y.X.; Srinivasan, M.; Sullivan, K.; Wellsted, D.; Gorog, D.A. Impaired Endogenous Fibrinolysis in ST-Segment Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention Is a Predictor of Recurrent Cardiovascular Events: The RISK PPCI Study. Eur. Heart J. 2019, 40, 295–305. [Google Scholar] [CrossRef]
- Karasu, A.; Baglin, T.P.; Luddington, R.; Baglin, C.A.; van Hylckama Vlieg, A. Prolonged Clot Lysis Time Increases the Risk of a First but Not Recurrent Venous Thrombosis. Br. J. Haematol. 2016, 172, 947–953. [Google Scholar] [CrossRef]
- Traby, L.; Kollars, M.; Eischer, L.; Eichinger, S.; Kyrle, P.A. Prediction of Recurrent Venous Thromboembolism by Clot Lysis Time: A Prospective Cohort Study. PLoS ONE 2012, 7, e51447. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Barbero, M.; Arnaez, J.; Martín-Ancel, A.; González, H.; García-Alix, A. Thrombin, Protein C, and Protein S Values in Mother-Infant Dyads in the Postpartum Period. An. Pediatr. 2023, 98, 338–343. [Google Scholar] [CrossRef]
- Hackeng, T.M.; Rosing, J. Protein S as Cofactor for TFPI. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2015–2020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zöller, B.; Svensson, P.J.; Dahlbäck, B.; Lind-Hallden, C.; Hallden, C.; Elf, J. Genetic Risk Factors for Venous Thromboembolism. Expert Rev. Hematol. 2020, 13, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Moser, K.A. Protein C Deficiency. Arch. Pathol. Lab. Med. 2019, 143, 1281–1285. [Google Scholar] [CrossRef]
- Gupta, A.; Tun, A.M.; Gupta, K.; Tuma, F. Protein S Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bravo-Pérez, C.; Vicente, V.; Corral, J. Management of Antithrombin Deficiency: An Update for Clinicians. Expert Rev. Hematol. 2019, 12, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, R.; Zaręba, L.; Iwaniec, T.; Kubicka-Trząska, A.; Romanowska-Dixon, B.; Bazan-Socha, S.; Dropiński, J. High Prevalence of Thrombophilic Risk Factors in Patients with Central Retinal Artery Occlusion. Thromb. J. 2023, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Brouns, S.L.N.; Tullemans, B.M.E.; Bulato, C.; Perrella, G.; Campello, E.; Spiezia, L.; van Geffen, J.P.; Kuijpers, M.J.E.; van Oerle, R.; Spronk, H.M.H.; et al. Protein C or Protein S Deficiency Associates with Paradoxically Impaired Platelet-Dependent Thrombus and Fibrin Formation under Flow. Res. Pract. Thromb. Haemost. 2022, 6, e12678. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Gu, Y.; Hu, S.; Ji, X.; Wang, Y.; Duan, J. An Exploration of Anti-Inflammatory Therapy in Acute/Subacute Severe Cerebral Venous Thrombosis with Hereditary Protein C/S Deficiency: Case Series. J. Inflamm. Res. 2023, 16, 5403–5415. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Nguyen, T. Protein S: Function, Regulation, and Clinical Perspectives. Curr. Opin. Hematol. 2021, 28, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Rezende, S.M.; Simmonds, R.E.; Lane, D.A. Coagulation, Inflammation, and Apoptosis: Different Roles for Protein S and the Protein S-C4b Binding Protein Complex. Blood 2004, 103, 1192–1201. [Google Scholar] [CrossRef]
- Lemke, G.; Silverman, G.J. Blood Clots and TAM Receptor Signalling in COVID-19 Pathogenesis. Nat. Rev. Immunol. 2020, 20, 395–396. [Google Scholar] [CrossRef]
- Voicu, S.; Delrue, M.; Chousterman, B.G.; Stépanian, A.; Bonnin, P.; Malissin, I.; Deye, N.; Neuwirth, M.; Ketfi, C.; Mebazaa, A.; et al. Imbalance between Procoagulant Factors and Natural Coagulation Inhibitors Contributes to Hypercoagulability in the Critically Ill COVID-19 Patient: Clinical Implications. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9161–9168. [Google Scholar] [CrossRef]
- Englisch, C.; Moik, F.; Thaler, J.; Koder, S.; Mackman, N.; Preusser, M.; Pabinger, I.; Ay, C. Tissue Factor Pathway Inhibitor Is Associated with Risk of Venous Thromboembolism and All-Cause Mortality in Patients with Cancer. Haematologica, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Manderstedt, E.; Lind-Halldén, C.; Halldén, C.; Elf, J.; Svensson, P.J.; Engström, G.; Melander, O.; Baras, A.; Lotta, L.A.; Zöller, B.; et al. Genetic Variation of the Blood Coagulation Regulator Tissue Factor Pathway Inhibitor and Venous Thromboembolism among Middle-Aged and Older Adults: A Population-Based Cohort Study. Res. Pract. Thromb. Haemost. 2022, 6, e12842. [Google Scholar] [CrossRef] [PubMed]
- Baylis, R.A.; Smith, N.L.; Klarin, D.; Fukaya, E. Epidemiology and Genetics of Venous Thromboembolism and Chronic Venous Disease. Circ. Res. 2021, 128, 1988–2002. [Google Scholar] [CrossRef] [PubMed]
- Chiasakul, T.; De Jesus, E.; Tong, J.; Chen, Y.; Crowther, M.; Garcia, D.; Chai-Adisaksopha, C.; Messé, S.R.; Cuker, A. Inherited Thrombophilia and the Risk of Arterial Ischemic Stroke: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012877. [Google Scholar] [CrossRef]
- Clark, P.; Wu, O. ABO Blood Groups and Thrombosis: A Causal Association, but Is There Value in Screening? Future Cardiol. 2011, 7, 191–201. [Google Scholar] [CrossRef]
- Wu, W.; Wang, R.; Xu, Q.; Wang, G.; Jiang, S.; Lou, C.; Wu, X.; Shao, Y.; Lu, Y.; Liu, Y.; et al. Thrombosis Caused By a Novel Coagulation Factor IX Mutation (FIX Shanghai II). Blood 2023, 142, 1233. [Google Scholar] [CrossRef]
- Lindström, S.; Wang, L.; Smith, E.N.; Gordon, W.; van Hylckama Vlieg, A.; de Andrade, M.; Brody, J.A.; Pattee, J.W.; Haessler, J.; Brumpton, B.M.; et al. Genomic and Transcriptomic Association Studies Identify 16 Novel Susceptibility Loci for Venous Thromboembolism. Blood 2019, 134, 1645–1657. [Google Scholar] [CrossRef]
- Constantinescu, A.-E.; Bull, C.J.; Goudswaard, L.J.; Zheng, J.; Elsworth, B.; Timpson, N.J.; Moore, S.F.; Hers, I.; Vincent, E.E. A Phenome-Wide Approach to Identify Causal Risk Factors for Deep Vein Thrombosis. BMC Med. Genom. 2023, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Thibord, F.; Klarin, D.; Brody, J.A.; Chen, M.-H.; Levin, M.G.; Chasman, D.I.; Goode, E.L.; Hveem, K.; Teder-Laving, M.; Martinez-Perez, A.; et al. Cross-Ancestry Investigation of Venous Thromboembolism Genomic Predictors. Circulation 2022, 146, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, J.; Tragante, V.; Ahlberg, G.; Rand, S.A.; Jespersen, J.B.; Leinøe, E.B.; Vissing, C.R.; Trudsø, L.; Jonsdottir, I.; Banasik, K.; et al. Genome-Wide Meta-Analysis Identifies 93 Risk Loci and Enables Risk Prediction Equivalent to Monogenic Forms of Venous Thromboembolism. Nat. Genet. 2023, 55, 399–409. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- He, Y.; Wu, Q. The Effect of Extracellular Vesicles on Thrombosis. J. Cardiovasc. Transl. Res. 2023, 16, 682–697. [Google Scholar] [CrossRef]
- Witwer, K.W.; Théry, C. Extracellular Vesicles or Exosomes? On Primacy, Precision, and Popularity Influencing a Choice of Nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells Release Subpopulations of Exosomes with Distinct Molecular and Biological Properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma Membrane Changes during Programmed Cell Deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liao, L.; Tian, W. Extracellular Vesicles Derived From Apoptotic Cells: An Essential Link Between Death and Regeneration. Front. Cell Dev. Biol. 2020, 8, 573511. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, A.; Koenen, R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Zifkos, K.; Dubois, C.; Schäfer, K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 9317. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and Phenotyping of Cellular Vesicles Using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Böing, A.N.; Romijn, F.P.; Hack, C.E.; Sturk, A. Cell-Derived Microparticles Circulate in Healthy Humans and Support Low Grade Thrombin Generation. Thromb. Haemost. 2001, 85, 639–646. [Google Scholar]
- Deng, F.; Wang, S.; Zhang, L. Endothelial Microparticles Act as Novel Diagnostic and Therapeutic Biomarkers of Diabetes and Its Complications: A Literature Review. Biomed. Res. Int. 2016, 2016, 9802026. [Google Scholar] [CrossRef]
- Akbar, N.; Azzimato, V.; Choudhury, R.P.; Aouadi, M. Extracellular Vesicles in Metabolic Disease. Diabetologia 2019, 62, 2179–2187. [Google Scholar] [CrossRef]
- Stenz, K.T.; Just, J.; Blauenfeldt, R.A.; Drasbek, K.R. Extracellular Vesicles in Acute Stroke Diagnostics. Biomedicines 2020, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.M.; Tedgui, A. Elevated Levels of Shed Membrane Microparticles with Procoagulant Potential in the Peripheral Circulating Blood of Patients with Acute Coronary Syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Biró, E.; Sturk-Maquelin, K.N.; Vogel, G.M.T.; Meuleman, D.G.; Smit, M.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. Human Cell-Derived Microparticles Promote Thrombus Formation in Vivo in a Tissue Factor-Dependent Manner. J. Thromb. Haemost. 2003, 1, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Reddy, E.C.; Rand, M.L. Procoagulant Phosphatidylserine-Exposing Platelets in Vitro and in Vivo. Front. Cardiovasc. Med. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; López, J.A. Tissue-Factor-Bearing Microvesicles Arise from Lipid Rafts and Fuse with Activated Platelets to Initiate Coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet Microparticle Membranes Have 50- to 100-Fold Higher Specific Procoagulant Activity than Activated Platelets. Thromb. Haemost. 2007, 97, 425–434. [Google Scholar] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- von Brühl, M.-L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, Neutrophils, and Platelets Cooperate to Initiate and Propagate Venous Thrombosis in Mice in Vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Kuckleburg, C.J.; Yates, C.M.; Kalia, N.; Zhao, Y.; Nash, G.B.; Watson, S.P.; Rainger, G.E. Endothelial Cell-Borne Platelet Bridges Selectively Recruit Monocytes in Human and Mouse Models of Vascular Inflammation. Cardiovasc. Res. 2011, 91, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Rada, B. Neutrophil Extracellular Traps. Methods Mol. Biol. 2019, 1982, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, T.; Jin, J.; Liu, Y.; Li, B.; Sun, Q.; Tian, J.; Zhao, H.; Liu, Z.; Ma, S.; et al. Interactions between Neutrophil Extracellular Traps and Activated Platelets Enhance Procoagulant Activity in Acute Stroke Patients with ICA Occlusion. EBioMedicine 2020, 53, 102671. [Google Scholar] [CrossRef] [PubMed]
- Vallier, L.; Cointe, S.; Lacroix, R.; Bonifay, A.; Judicone, C.; Dignat-George, F.; Kwaan, H.C. Microparticles and Fibrinolysis. Semin. Thromb. Hemost. 2017, 43, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lupu, C.; Goodwin, C.A.; Westmuckett, A.D.; Emeis, J.J.; Scully, M.F.; Kakkar, V.V.; Lupu, F. Tissue Factor Pathway Inhibitor in Endothelial Cells Colocalizes with Glycolipid Microdomains/Caveolae. Regulatory Mechanism(s) of the Anticoagulant Properties of the Endothelium. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Shustova, O.N.; Antonova, O.A.; Golubeva, N.V.; Khaspekova, S.G.; Yakushkin, V.V.; Aksuk, S.A.; Alchinova, I.B.; Karganov, M.Y.; Mazurov, A.V. Differential Procoagulant Activity of Microparticles Derived from Monocytes, Granulocytes, Platelets and Endothelial Cells: Impact of Active Tissue Factor. Blood Coagul. Fibrinolysis 2017, 28, 373–382. [Google Scholar] [CrossRef]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 6522. [Google Scholar] [CrossRef]
- Liu, M.-L.; Reilly, M.P.; Casasanto, P.; McKenzie, S.E.; Williams, K.J. Cholesterol Enrichment of Human Monocyte/Macrophages Induces Surface Exposure of Phosphatidylserine and the Release of Biologically-Active Tissue Factor-Positive Microvesicles. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 430–435. [Google Scholar] [CrossRef]
- Holme, P.A.; Orvim, U.; Hamers, M.J.; Solum, N.O.; Brosstad, F.R.; Barstad, R.M.; Sakariassen, K.S. Shear-Induced Platelet Activation and Platelet Microparticle Formation at Blood Flow Conditions as in Arteries with a Severe Stenosis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 646–653. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Nomura, S.; Miyake, T.; Kagawa, H.; Kitada, C.; Taniguchi, H.; Komiyama, Y.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. High Shear Stress Can Initiate Both Platelet Aggregation and Shedding of Procoagulant Containing Microparticles. Blood 1996, 88, 3456–3464. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Han, X.; Li, Y.; Cheng, W.; Wang, L.; Lu, Z.; Yang, J.; Zhao, M. The Level of Circulating Microparticles in Patients with Coronary Heart Disease: A Systematic Review and Meta-Analysis. J. Cardiovasc. Transl. Res. 2020, 13, 702–712. [Google Scholar] [CrossRef]
- Leroyer, A.S.; Isobe, H.; Lesèche, G.; Castier, Y.; Wassef, M.; Mallat, Z.; Binder, B.R.; Tedgui, A.; Boulanger, C.M. Cellular Origins and Thrombogenic Activity of Microparticles Isolated from Human Atherosclerotic Plaques. J. Am. Coll. Cardiol. 2007, 49, 772–777. [Google Scholar] [CrossRef]
- Vélez, P.; Parguiña, A.F.; Ocaranza-Sánchez, R.; Grigorian-Shamagian, L.; Rosa, I.; Alonso-Orgaz, S.; de la Cuesta, F.; Guitián, E.; Moreu, J.; Barderas, M.G.; et al. Identification of a Circulating Microvesicle Protein Network Involved in ST-Elevation Myocardial Infarction. Thromb. Haemost. 2014, 112, 716–726. [Google Scholar] [CrossRef]
- Ye, R.; Ye, C.; Huang, Y.; Liu, L.; Wang, S. Circulating Tissue Factor Positive Microparticles in Patients with Acute Recurrent Deep Venous Thrombosis. Thromb. Res. 2012, 130, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Spiezia, L.; Radu, C.M.; Bon, M.; Gavasso, S.; Zerbinati, P.; Woodhams, B.; Tormene, D.; Prandoni, P.; Simioni, P. Circulating Microparticles in Carriers of Factor V Leiden with and without a History of Venous Thrombosis. Thromb. Haemost. 2012, 108, 633–639. [Google Scholar] [CrossRef]
- Campello, E.; Spiezia, L.; Radu, C.M.; Gavasso, S.; Zerbinati, P.; Woodhams, B.; Simioni, P. Circulating Microparticles in Carriers of Prothrombin G20210A Mutation. Thromb. Haemost. 2014, 112, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.M.; Artoni, A.; Carrabba, G.; Merati, G.; Abbattista, M.; Capecchi, M.; Castellani, M.; Marenghi, C.; Trombetta, E.; Giammattei, L.; et al. Plasma Levels of Extracellular Vesicles and the Risk of Post-Operative Pulmonary Embolism in Patients with Primary Brain Tumors: A Prospective Study. J. Thromb. Thrombolysis 2021, 52, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Varbella, F.; Gaggiano, A.; Mennuni, M.; Annibali, G.; Celentani, D.; Del Nevo, F.; Piazza, S.; Musumeci, G. Proposal for a diagnostic and therapeutic care pathway of the Piedmont Region on lipid-lowering and antithrombotic treatment in patients with peripheral arterial disease. G. Ital. Cardiol. 2023, 24, 344–354. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity Arteries, Endorsed by: The European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; De Filippo, O.; Gallone, G.; Mittone, G.; Deriu, M.A.; Iannaccone, M.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; Quadri, G.; et al. Machine Learning-Based Prediction of Adverse Events Following an Acute Coronary Syndrome (PRAISE): A Modelling Study of Pooled Datasets. Lancet 2021, 397, 199–207. [Google Scholar] [CrossRef]
- Mennuni, M.G.; Pagnotta, P.A.; Stefanini, G.G. Coronary Stents: The Impact of Technological Advances on Clinical Outcomes. Ann. Biomed. Eng. 2016, 44, 488–496. [Google Scholar] [CrossRef]
- Mennuni, M.G.; Rolla, R.; Grisafi, L.; Spinoni, E.G.; Rognoni, A.; Lio, V.; Castello, L.M.; Sainaghi, P.P.; Pirisi, M.; Avanzi, G.C.; et al. Interaction between Thrombin Potential and Age on Early Clinical Outcome in Patients Hospitalized for COVID-19. J. Thromb. Thrombolysis 2021, 52, 746–753. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Hutt, E.; Bloomfield, D.M.; Gailani, D.; Weitz, J.I. Factor XI Inhibition to Uncouple Thrombosis From Hemostasis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Disharoon, D.; Marr, D.W.M.; Neeves, K.B. Engineered Microparticles and Nanoparticles for Fibrinolysis. J. Thromb. Haemost. 2019, 17, 2004–2015. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.L.; Li, W.; Sun, M.; Ravichandran, K.; Hickman, D.; Kos, C.; Kaur, G.; Sen Gupta, A. Platelet Microparticle-Inspired Clot-Responsive Nanomedicine for Targeted Fibrinolysis. Biomaterials 2017, 128, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Li, J.-A.; Zhu, S.-J.; Cao, C.; Tang, J.-N.; Zhang, J.-Y.; Guan, S.-K. Tailoring of Cardiovascular Stent Material Surface by Immobilizing Exosomes for Better Pro-Endothelialization Function. Colloids Surf. B Biointerfaces 2020, 189, 110831. [Google Scholar] [CrossRef] [PubMed]


| Gene | Product | SNP | Position in the Gene | Risk Allele Frequency a | OR/RR b |
|---|---|---|---|---|---|
| ABO | Glycosyltransferases, responsible for the formation of antigens in blood type A and/or B | rs579459 | Intronic (T/C *) | 0.196 | 1.50 |
| rs8176749 | Leu310Leu * | 0.128 | 1.50 | ||
| F2 | Protrombin | rs1799963 | 3′UTR (G/A *) | 0.0084 | 2.50 |
| rs3136516 | Intronic (G/A *) | 0.388 | 1.12 | ||
| F5 | Factor V | rs6025 | Arg506Gln (G/A *) (FV Leiden) | 0.017 | 3.00 |
| rs4524 | Lys858Arg (A/G *) | 0.75 | 1.20 | ||
| F8 | Factor VIII | rs114209171 | Upstream (T */C) | 0.80 | 1.11 |
| FGG | Gamma component of fibrinogen | rs2066865 | 3′UTR (C/T *) | 1.50 | 1.25 |
| KNG1 | Kininogen 1 | rs7100446 | Ile581Thr * | 0.40 | 1.20 |
| PROCR | Protein C receptor | rs6088735 | Upstream (C/T) | 0.23 | 1.11 |
| rs867186 | Ser219Gly * | 0.10 | 1.22 | ||
| PROS1 | Protein S | rs121918472 | Ser501Pro * | 0.002 | 6.57 |
| rs121918474 | Lys196Glu * | 0.009 | 5.00 | ||
| SERPINC1 | Serpin family C member 1 | rs2227624 | Val30Glu * | 0.004 | 1.30 |
| SLC44A2 | Solute carrier family 44 member 2 | rs2288904 | Arg * 154Gln | 0.76 | 1.28 |
| STXBP5 | Syntaxin binding Protein 5 | rs1039084 | Asn * 436Ser | 0.55 | 1.11 |
| THBD | Thrombomodulin | rs16984852 | 5′UTR (G/T *) | 0.005 | 2.80 |
| TSPAN15 | Tetraspanin 15 | rs78707713 | Intronic (T */C) | 0.90 | 1.42 |
| VWF | von Willebrand factor | rs1063856 | Thr789Ala * | 0.40 | 1.15 |
| Cells of Origin | Markers |
|---|---|
| Endothelial cells | CD31 (PECAM-1), CD51 (integrin αv), CD61 (integrin β3, CD54 (ICAM-1), CD62-E (E-selectin), CD105 (endoglin), CD144 (VE-cadherin), CD146 (S-endo-1), CD106 (V-CAM1) *, CD142 (TF) * |
| Platelets | CD31 (PECAM-1), CD41 (integrin αIIb), CD42b (integrin Ibα), CD61 (integrin β3), CD62P (P-selectin) *, AnxA5, CD142 (TF) *, if derived from megakaryocytes: full-length filamin-A, LAMP-1 |
| Red blood cells | CD235a (glycophorin A), CD108a (semaphorin 7A), AchE-E, AnxA5 |
| Neutrophils | CD11b (integrin αM), CD66b (CEACAM8), CD142 (TF) *, AnxA5, CD35, MPO |
| Monocytes | CD11b (integrin αM), CD14, CD16 (FcγR III), CD18 (integrin β2), CD64 (FcγRI), CD142(TF) *, MPO, CD31, CD142, AnxA5 |
| Lymphocyte | CD3, CD45 |
| Name | Molecule Type | Mechanism | Half-Life | Duration of Action | Administration | Indications | ||
|---|---|---|---|---|---|---|---|---|
| Platelet Target | Oxygenase | Aspirin | Acetylsalicylic acid | Irreversible acetylation and inhibition of COX enzyme | Dose dependent | 10 days | Oral, once daily | Secondary prevention of CVD; ACS ± PCI |
| Triflusal | Acetoxy-trifluoromethylbenzoic acid | Irreversible acetylation and inhibition of COX enzyme | 34 h | 10 days | Oral | Prevention of ACSa dn stroke, but not EMA and FDA approved | ||
| ML355 * | Small molecule | 12-LOX Inhibition | 2.5 h (murine) | - | Oral | - | ||
| ADP receptor | Clopidogrel | Thienopyridine, P2Y12 antagonist | Competitive, irreversible, P2Y12 receptor blockade | 6 h | 5–7 days | Oral, once daily | ACS ± PCI; PCI (elective); symptomatic, high risk, CVD | |
| Prasugrel | Thienopyridine, P2Y12 antagonist | Competitive, irreversible, P2Y12 receptor blockade | 7 h | 7–10 days | Oral, once daily | ACS + PCI only | ||
| Ticagrelor | Triazolopyrimidine, P2Y12 antagonist | Noncompetitive, reversible, P2Y12 receptor blockade | 8–12 h | 3–5 days | Oral, twice daily | ACS ± PCI, long term with history of ACS | ||
| Cangrelor | Nonthienopyridine, ATP analogue | Noncompetitive, reversible, P2Y12 receptor blockade | 3–5 min | 30–60 min | Intravenous | PCI | ||
| Glycorotein IIb-IIIa | Abciximab | Humanized mouse monoclonal ab | Fab antibody fragment that binds to IIb/IIIa receptor with high affinity and low dissociation | 4 h | 24–48 h | Intravenous | ACS + PCI, PCI only | |
| Eptifibatide | KGD-containing heptapeptide | Competitive, reversible IIb/IIIa receptor blockade | 2.5 h | 4–8 h | Intravenous | ACS ± PCI | ||
| Tirofiban | Nonpeptide RGD mimetic | Competitive, reversible IIb/IIIa receptor blockade | 2 h | 4–8 h | Intravenous | ACS ± PCI, PCI only | ||
| Thrombin Receptor | Vorapaxar | Tricyclic 3-phenylpyridine | Reversible, PAR-1 receptor blockade | 5–13 days | 4 weeks | Oral, once daily | History of MI or PAD | |
| Atopaxar * | Bicyclic amidine | Reversible, PAR-1 receptor blockade | 22–26 h | 3–5 days | Oral | - | ||
| BMS-986120 * | Small molecule | Reversible, PAR-4 receptor blockade | 4 h | 24 h | Oral | - | ||
| Collagen Recptor | Revacept * | Soluble dimeric GP VI-Fc fusion protein | - | 67–137 h | 7 days | Intravenous | - | |
| PDE | Dipyridamole | Pyrimido-pyrimidine derivative | Reversible, PDE and adenosine deaminase inhibition | 10 h | - | Oral | Prevention of postoperative thromboembolic complications | |
| Cilostazol | Quinoline derivative | Reversible, PDE3 inhibition | 10 h | 12–16 h | Oral | Claudication in PAD |
| Name | Molecule Type | Mechanism | Half-Life | Activity | Administration | ||
|---|---|---|---|---|---|---|---|
| Target | Factor XI | IONIS-FXIRx | Antisense oligonucleotide of FXI | FXI messenger RNA inhibition | 20 days | Slow and long | Sub-cutaneous (weekly) |
| Fesomersen | Antisense oligonucleotide of FXI | FXI messenger RNA inhibition | 1–122 h | Slow and long | Sub-cutaneous (weekly) | ||
| Osocimab | Monoclonal antibody to FXIa | FXIa inhibition | 30–40 days | Fast and long | Intravenous, sub-cutaneous (monthly) | ||
| Abelacimab | Monoclonal antibody to FXI/FXIa | FXI and FXIa inhibition | 25–30 days | Fast and long | Sub-cutaneous (monthly) | ||
| Xisomab 3G3 | Monoclonal antibody to FXI | Binds FXI and blocks activation by FXIIa | 20–28 days | Fast and long | Intravenous (monthly) | ||
| Milvexian | Small molecule | FXIa inhibition | 11–18 h | Fast and short | Oral | ||
| Asundexian | Small molecule | FXIa inhibition | 16–18 h | Fast and short | Oral | ||
| Factor XI and Factor XII | Ixodes ricinus contact phase inhibitor | Serine protease inhibitor | Interacts with FXIIa, FXIa, and kallikrein | - | - | - | |
| Factor XII | Garadacimab CSL312 | Monoclonal antibody to FXIIa | FXIa inhibition | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchetti, S.; Puricelli, C.; Mennuni, M.; Zanotti, V.; Giacomini, L.; Giordano, M.; Dianzani, U.; Patti, G.; Rolla, R. Research into New Molecular Mechanisms in Thrombotic Diseases Paves the Way for Innovative Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 2523. https://doi.org/10.3390/ijms25052523
Sacchetti S, Puricelli C, Mennuni M, Zanotti V, Giacomini L, Giordano M, Dianzani U, Patti G, Rolla R. Research into New Molecular Mechanisms in Thrombotic Diseases Paves the Way for Innovative Therapeutic Approaches. International Journal of Molecular Sciences. 2024; 25(5):2523. https://doi.org/10.3390/ijms25052523
Chicago/Turabian StyleSacchetti, Sara, Chiara Puricelli, Marco Mennuni, Valentina Zanotti, Luca Giacomini, Mara Giordano, Umberto Dianzani, Giuseppe Patti, and Roberta Rolla. 2024. "Research into New Molecular Mechanisms in Thrombotic Diseases Paves the Way for Innovative Therapeutic Approaches" International Journal of Molecular Sciences 25, no. 5: 2523. https://doi.org/10.3390/ijms25052523
APA StyleSacchetti, S., Puricelli, C., Mennuni, M., Zanotti, V., Giacomini, L., Giordano, M., Dianzani, U., Patti, G., & Rolla, R. (2024). Research into New Molecular Mechanisms in Thrombotic Diseases Paves the Way for Innovative Therapeutic Approaches. International Journal of Molecular Sciences, 25(5), 2523. https://doi.org/10.3390/ijms25052523






