Stereoselective Analysis of the Antiseizure Activity of Fenfluramine and Norfenfluramine in Mice: Is l-Norfenfluramine a Better Follow-Up Compound to Racemic-Fenfluramine?
Abstract
1. Introduction
2. Results
2.1. Antiseizure Activity and Protective Index in the MES Model in Mice
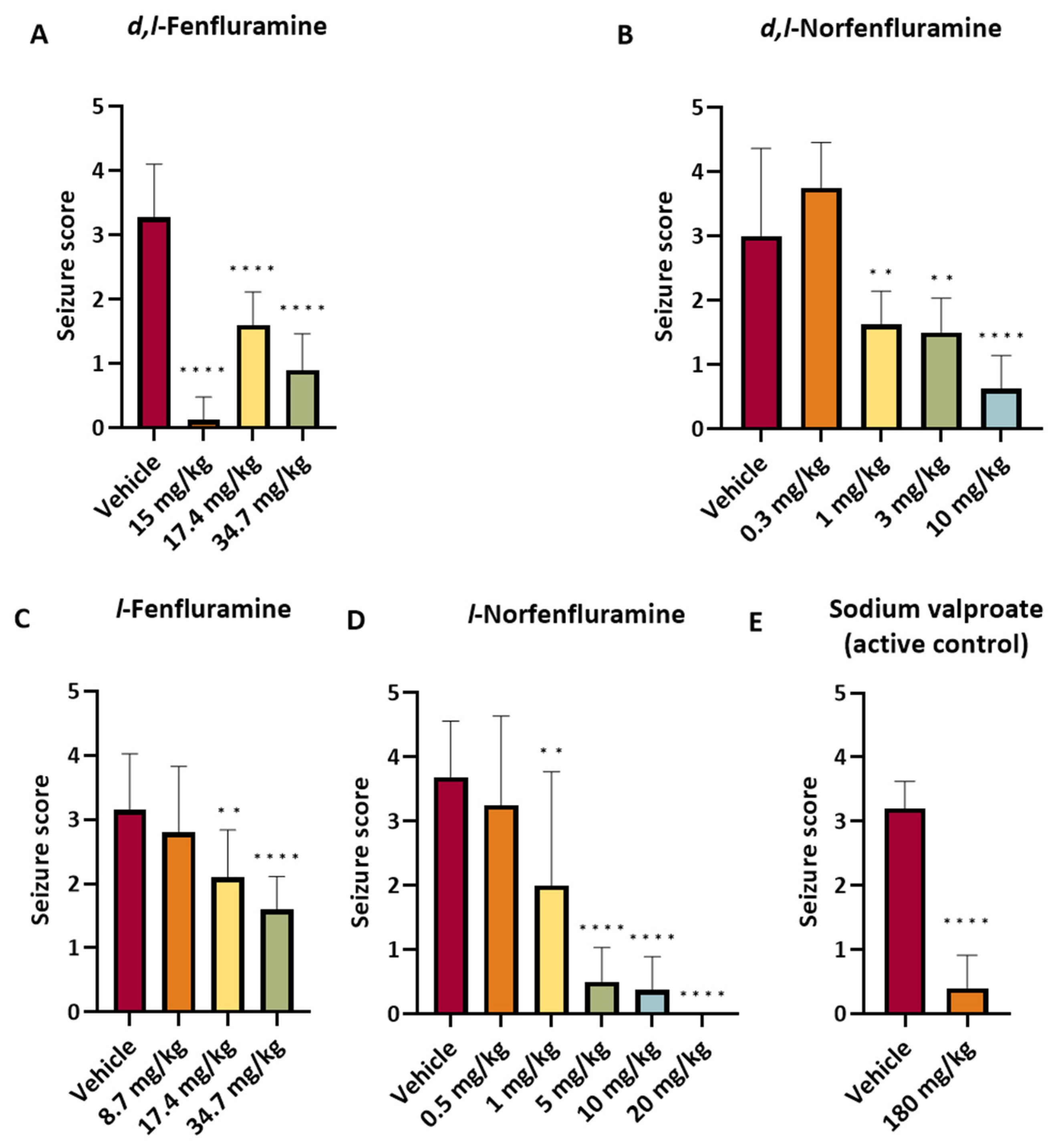
2.2. Studies in the DBA/2 Mouse Model of Audiogenic Seizures
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Assessment of Antiseizure Activity and Neurotoxicity in the Mouse MES Model
4.3. Assessment of Antiseizure Activity in the DBA/2 Mouse Model of Audiogenic Seizures
4.4. Assay of Fenfluramine and Norfenfluramine in Biological Samples
4.4.1. Sample Collection and Preparation
4.4.2. Quantification of Norfenfluramine and L-norfenfluramine in Plasma
4.4.3. Quantification of Fenfluramine, Norfenfluramine and Their l-Enantiomers in Brain
4.5. Assessment of Concentration-Response Relationships and Statistical Analysis
- E = response
- B = bottom of the maximal effect (Emax) set as 0.
- T = top of the maximal effect (Emax) set as 4.
- C = concentration
- N = the Hill coefficient or steepness of the concentration-response relationship
- IC50 = concentration that reduces the response by half.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fintepla (Fenfluramine) Oral Solution. Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212102s003lbl.pdf (accessed on 26 October 2023).
- Fintepla (2.2 mg/mL Oral Solution) (2022). Summary of Product Characteristics (Last Updated 26 July 2022). Available online: https://www.ema.europa.eu/en/documents/product-information/fintepla-epar-product-information_en.pdf (accessed on 26 October 2023).
- Odi, R.; Invernizzi, R.W.; Gallily, T.; Bialer, M.; Perucca, E. Fenfluramine repurposing from weight loss to epilepsy: What we do and do not know. Pharmacol. Ther. 2021, 226, 107866. [Google Scholar] [CrossRef] [PubMed]
- Caccia, S.; Ballabio, M.; De Ponte, P. Pharmacokinetics of fenfluramine enantiomers in man. Eur. J. Drug Metab. Pharmacokinet. 1979, 4, 129–132. [Google Scholar] [CrossRef]
- Garattini, S.; Caccia, S.; Mennini, T.; Samanin, R.; Consolo, S.; Ladinski, H. Biochemical pharmacology of the anorectic drug fenfluramine; a review. Curr. Med. Res. Opin. 1979, 1 (Suppl. S6), 15–27. [Google Scholar] [CrossRef]
- Caccia, S.; Conforti, I.; Duchier, J.; Garattini, S. Pharmacokinetics of fenfluramine and norfenfluramine in volunteers given D-and DL-fenfluramine for 15 days. Eur. J. Clin. Pharmacol. 1985, 9, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Mennini, T.; Garattini, S.; Caccia, S. Anorectic effect of fenfluramine isomers and metabolites: Relationship between brain levels and in vitro potencies on serotonergic mechanisms. Psychopharmacology 1985, 85, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, R.; Berettera, C.; Garattini, S.; Samanin, R. D- and L-isomers of fenfluramine differ markedly in their interaction with brain serotonin and catecholamines in the rat. Eur. J. Pharmacol. 1986, 120, 9–15. [Google Scholar] [CrossRef]
- Hirsch, J.A.; Goldberg, S.; Wurtman, R.J. Effect of (+)- or (−)-enantiomers of fenfluramine or norfenfluramine on nutrient selection by rats. J. Pharm. Pharmacol. 1982, 34, 18–21. [Google Scholar] [CrossRef]
- Spinelli, R.; Fracasso, C.; Guiso, G.; Garattini, S.; Caccia, S. Disposition of (−)-fenfluramine and its active metabolite, (−)-norfenfluramine in rat: A single dose-proportionality study. Xenobiotica 1988, 18, 573–584. [Google Scholar] [CrossRef]
- Schoonjans, A.S.; Roosens, L.; Dewals, W.; Paelinck, B.P.; Ceulemans, B. Therapeutic drug monitoring of fenfluramine in clinical practice: Pharmacokinetic variability and impact of concomitant antiseizure medications. Epilepsia 2022, 63, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Czerwiński, M.; Limaye, P.B.; Muranjan, S.; Ogilvie, B.W.; Smith, S.; Boyd, B. In vitro evaluation of fenfluramine and norfenfluramine as victims of drug interactions. Pharmacol. Res. Perspect. 2022, 10, e00958. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Czerwiński, M.; Limaye, P.B.; Ogilvie, B.W.; Smith, S.; Boyd, B. In vitro evaluation suggests fenfluramine and norfenfluramine are unlikely to act as perpetrators of drug interactions. Pharmacol. Res. Perspect. 2022, 10, e00959. [Google Scholar] [CrossRef]
- Frampton, J.E. Fenfluramine: A review in Dravet and Lennox-Gastaut Syndromes. Drugs 2023, 83, 923–934. [Google Scholar] [CrossRef]
- Sourbron, J.; Lagae, L. Fenfluramine: A plethora of mechanisms. Front. Pharmacol. 2023, 14, 1192022. [Google Scholar] [CrossRef]
- Fitzgerald, L.W.; Burn, T.C.; Brown, B.S.; Patterson, J.P.; Corjay, M.H.; Valentine, P.A.; Sun, J.H.; Link, J.R.; Abbaszade, I.; Hollis, J.M.; et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol. 2000, 57, 75–81. [Google Scholar] [PubMed]
- Hutcheson, J.D.; Setola, V.; Roth, B.L.; Merryman, W.D. Serotonin receptors and heart valve disease—It was meant 2B. Pharmacol. Ther. 2011, 132, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, C.; Andrejak, M.; Peltier, M.; Marchaux, S.; Tribouilloy, C. Adverse effects of benfluorex on heart valves and pulmonary circulation. Pharmacoepidem. Drug. Saf. 2014, 23, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Savale, L.; Chamais, M.C.; Cottin, V.; Bergot, E.; Frachon, I.; Prevot, G.; Pison, C.; Dromer, C.; Poubeau, P.; Lamblin, N.; et al. Pulmonary hypertension associated with benfluorex exposure. Eur. Respir. J. 2012, 40, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Goldner, V.; Karst, U. Benfluorex metabolism complemented by electrochemistry-mass spectrometry. J. Pharm. Biomed. Anal. 2023, 235, 115626. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; White, H.S.; Barker-Haliski, M.L. Evaluation of the acute anticonvulsant efficacy of fenfluramine in mouse models of acute and chronic seizures. In Proceedings of the 73rd Annual Meeting of the American Epilepsy Society, Baltimore, MD, USA, 6–10 December 2019; Available online: https://zogenix.com/wp-content/uploads/2019/12/08.-FINAL-52352-AES-Martin-Mouse-Poster-2019-12-03v5.pdf (accessed on 21 December 2022).
- Silenieks, L.B.; Carroll, N.K.; Van Niekerk, A.; Van Niekerk, E.; Taylor, C.; Upton, N.; Higgins, G.A. Evaluation of selective 5-HT2C agonists in acute seizure models. ACS Chem. Neurosci. 2019, 10, 3284–3295. [Google Scholar] [CrossRef] [PubMed]
- Tupal, S.; Faingold, C.L. Fenfluramine, a serotonin-releasing drug, prevents seizure-induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia 2019, 60, 485–494. [Google Scholar] [CrossRef]
- Li, J.; Nelis, M.; Sourbron, J.; Copmans, D.; Lagae, L.; Cabooter, D.; de Witte, P.A.M. Efficacy of fenfluramine and norfenfluramine enantiomers and various antiepileptic drugs in a zebrafish model of Dravet syndrome. Neurochem. Res. 2021, 46, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Erenburg, N.; Hamed, R.; Shaul, C.; Perucca, E.; Bialer, M. Comparative activity of the enantiomers of fenfluramine and norfenfluramine in rodent seizure models, and relationship with their concentrations in plasma and brain. Epilepsia 2023, 64, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.T. Chiral switches. Lancet 2000, 355, 1085–1087. [Google Scholar] [CrossRef]
- Agranat, I.; Caner, H.; Caldwell, J. Putting chirality to work: The strategy of chiral switches. Nat. Rev. Drug. Discov. 2002, 1, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Erenburg, N.; Hamed, R.; Shaul, C.; Barasch, D.; Perucca, E.; Bialer, M. Pharmacokinetics of d- and l-norfenfluramine following their administration as individual enantiomers in rats. Epilepsia 2024, 65, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- D’Acquarica, I.; Agranat, I. The quest for secondary pharmaceuticals: Drug repurposing/chiral–switches combination strategy. ACS Pharmacol. Trans. Sci. 2023, 6, 201–219. [Google Scholar] [CrossRef]
- De Sarro, G.; Russo, E.; Citraro, R.; Meldrum, B.S. Genetically epilepsy-prone rats (GEPRs) and DBA/2 mice: Two animal models of audiogenic reflex epilepsy for the evaluation of new generation AEDs. Epilepsy Behav. 2017, 71 Pt. B, 165–173. [Google Scholar] [CrossRef]
- Castel-Branco, M.M.; Alves, G.L.; Figueiredo, I.V.; Falcão, A.C.; Caramona, M.M. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find Exp. Clin. Pharmacol. 2009, 31, 101–106. [Google Scholar] [CrossRef]
- Setola, V.; Dukat, M.; Glennon, R.A.; Roth, B.L. Molecular determinants for the interaction of the valvulopathic anorexigen norfenfluramine with the 5-HT2B receptor. Mol. Pharmacol. 2005, 68, 20–33. [Google Scholar] [CrossRef]
- Rothman, R.B.; Baumann, M.H. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol. Biochem. Behav. 2002, 71, 825–836. [Google Scholar] [CrossRef]
- Kelly, C.R.; Sharif, N.A. Pharmacological evidence for a functional serotonin-2B receptor in a human uterine smooth muscle cell line. J. Pharmacol. Exp. Ther. 2006, 317, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Bialer, M.; Perucca, E. Lorcaserin for Dravet Syndrome: A Potential Advance Over Fenfluramine? CNS Drugs 2022, 36, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Xenon Pharmaceuticals. Method for Treating Epilepsy. Communication under Rule 71(3). EPC Application No. 19750214.9-1112, ref N421012EP, 28 June 2023. [Google Scholar]
- Gross, A.S.; Philips, A.C.; Rieutord, A.; Shenfield, G.M. The influence of sparteine/debrisoquine genetic polymorphism on the disposition of dexfenfluramine. Brit. J. Clin. Pharmacol. 1996, 41, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Barker-Haliski, M.L.; Johnson, K.; Billingsley, P.; Huff, J.; Handy, L.J.; Khaleel, R.; Lu, Z.; Mau, M.J.; Pruess, T.H.; Rueda, C.; et al. Validation of a Preclinical Drug Screening Platform for Pharmacoresistant Epilepsy. Neurochem. Res. 2017, 42, 1904–1918. [Google Scholar] [CrossRef] [PubMed]
- Kehne, J.H.; Klein, B.D.; Raeissi, S.; Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) epilepsy therapy screening program (ETSP). Neurochem. Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef]
- Wilcox, K.S.; West, P.J.; Metcalf, C.S. The current approach of the Epilepsy Therapy Screening Program contract site for identifying improved therapies for the treatment of pharmacoresistant seizures in epilepsy. Neuropharmacology 2020, 166, 107811. [Google Scholar] [CrossRef]
- Dürmüller, N.; Smith, S.E.; Meldrum, B.S. Proconvulsant and anticonvulsant effects of Evans blue dye in rodents. Neuroreport 1993, 4, 683–686. [Google Scholar] [CrossRef]
- Derendorf, H.; Schmidt, S. Rowland and Tozer’s Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020; pp. 34–43. [Google Scholar]


| Compounds and Doses Tested | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d,l-Fenfluramine | d,l-Norfenfluramine | d-Fenfluramine | l-Fenfluramine | d-Norfenfluramine | l-Norfenfluramine | |||||||
| Test | Dose (mg/kg) | N/T | Dose (mg/kg) | N/T | Dose (mg/kg) | N/T | Dose (mg/kg) | N/T | Dose (mg/kg) | N/T | Dose (mg/kg) | N/T |
| Maximal electro-shock (MES) | 2.5 | 0/8 1 | 1 | 0/8 | 2.5 | 0/8 | 4 | 2/8 | 0.25 | 0/8 1 | 5 | 1/8 |
| 5 | 3/8 2 | 2.5 | 3/8 | 5 | 3/8 | 8 | 2/8 12 | 1 | 0/8 1 | 10 | 3/8 | |
| 10 | 4/8 | 5 | 3/8 5 | 10 | 1/8 | 16 | 6/8 | 5 | 7/8 | 15 | 6/8 | |
| 15 | 7/8 | 10 | 6/8 6 | 15 | 5/8 | 25 | 7/8 | 10 | 7/8 | 20 | 3/8 | |
| 20 | 4/8 6,7 | 30 | 8/8 | 30 | 7/8 | 18 | 5/8 | 30 | 5/8 | |||
| 30 | 8/8 | 25 | 7/8 12 | 40 | 8/8 | |||||||
| 30 | 6/8 2,19 | |||||||||||
| Minimal motor impair-ment (MMI) | 30 | 0/8 | 10 | 0/8 | 15 | 0/8 | 50 | 2/16 13 | 2 | 1/8 | 5 | 0/8 |
| 40 | 3/8 | 15 | 1/8 | 30 | 4/8 | 75 | 13/16 14,15 | 5 | 4/8 20 | 10 | 0/8 | |
| 50 | 5/8 | 20 | 5/8 | 45 | 6/8 8,9,10 | 100 | 16/16 4,16,17,18 | 10 | 6/8 | 20 | 3/8 | |
| 60 | 8/8 3,4 | 25 | 4/8 | 60 | 8/8 4,11 | 20 | 8/8 11,21,22 | 25 | 6/8 | |||
| 30 | 8/8 | 30 | 8/8 4,11 | 50 | 8/8 4,14 | |||||||
| 45 | 8/8 11,21,23,24,25 | |||||||||||
| ED50 (mg/kg) * | TD50 (mg/kg) * | PI | |
|---|---|---|---|
| Mice | |||
| d,l-Fenfluramine | 8.1 (5.3–12.4) | 44.4 (38.5–49.8) | 5.5 |
| d,l-Norfenfluramine | 7.0 (3.52–12.6) | 20.7 (17.2–24.2) | 2.9 |
| d-Fenfluramine | 11.4 (7.4–17.6) | 32.3 (22.8–39.7) | 2.8 |
| l-Fenfluramine | 10.0 (5.0–15.0) | 62.7 (55.7–69.5) | 6.3 |
| d-Norfenfluramine | 5.1 (2.1–8.8) | 5.1 (2.9–7.5) | 1.0 |
| l-Norfenfluramine | 14.8 (8.3–22.1) * | 21.6 (10–50) | 1.5 |
| Rats | |||
| d,l-Fenfluramine | 10.7 (6.4–15.4) | 27.8 (22.3–33) | 2.6 |
| d,l-Norfenfluramine | 8.7 (7.2–10.4) | 10–15 ** | not assessed |
| d-Fenfluramine | 8.4 (6.6–10.6) | 26.7 (21.0–34.8) | 3.2 |
| l-Fenfluramine | 13.4 (10.1–16.3) | 38.5 (34.6–42) | 2.9 |
| d-Norfenfluramine | ≈5 ** | ≈5 ** | not assessed |
| l-Norfenfluramine | 10.2 (6.8–13.0) | 12.5 (8.7–16.1) | 1.2 |
| Experiment | Compound | Dose (mg/kg) | N. of Animals | Mean Seizure Score ± SD * | Mean Plasma Concentration ± SD (ng/mL) | Mean Brain Concentration ± SD (ng/g) | Brain/Plasma Concentration Ratio |
|---|---|---|---|---|---|---|---|
| 1 | Vehicle | N/A | 8 | 4.00 ± 0.00 | N/A | N/A | N/A |
| l- Norfenfluramine | 5 | 8 | 0.50 ± 0.54 | 442 ± 40.0 | 7780 ± 629 | 17.6 | |
| 10 | 8 | 0.38 ± 0.52 | 898 ± 62.5 | 16,900 ± 1680 | 18.7 | ||
| 20 | 8 | 0.00 ± 0.00 | 1950 ± 297 | 34,400 ± 3790 | 17.6 | ||
| 2 | Vehicle | N/A | 8 | 3.38 ± 1.19 | N/A | N/A | N/A |
| l-Norfenfluramine | 0.5 | 8 | 3.25 ± 1.39 | 43.5 ± 9.9 | 798 ± 91 | 18.4 | |
| 1 | 8 | 2.00 ± 1.77 | 83.7 ± 18.8 | 1770 ± 127 | 21.2 | ||
| d,l-Fenfluramine | 15 | 8 | 0.13 ± 0.35 | 1080 ± 98.4 * (131 ± 18.7) | 23,400 ± 1630 * (3016 ± 325) | 21.6 | |
| 3 | Vehicle | N/A | 7 | 2.57 ± 1.81 | N/A | N/A | N/A |
| d,l-Norfenfluramine | 0.3 | 8 | 3.75 ± 0.71 | 22.6 ± 2.1 | 299 ± 24 | 13.2 | |
| 1 | 8 | 1.63 ± 0.52 | 60.3 ± 17.6 | 981 ± 130 | 16.3 | ||
| 3 | 8 | 1.50 ± 0.54 | 184 ± 42.7 | 3450 ± 429 | 18.8 | ||
| 4 | Vehicle | N/A | 8 | 3.38 ± 0.74 | N/A | N/A | N/A |
| d,l-Norfenfluramine | 10 | 8 | 0.63 ± 0.52 | 1090 ± 194 | 22,400 ± 4740 | 20.5 | |
| 5 | Vehicle | N/A | 10 | 3.20 ± 0.42 | N/A | N/A | N/A |
| d,l-Fenfluramine | 17.4 | 10 | 1.60 ± 0.52 | N/A | 21,300 ± 3760 * (2459 ± 437) | N/A | |
| 34.7 | 10 | 0.90 ± 0.57 | N/A | 40,200 ± 6860 * (3094 ± 380) | N/A | ||
| l-Fenfluramine | 34.7 | 10 | 1.60 ± 0.52 | N/A | 52,200 ± 8550 | N/A | |
| * (3036 ± 400) | |||||||
| Sodium valproate | 180 | 10 | 0.40 ± 0.52 | N/A | N/A | N/A | |
| 6 | Vehicle | N/A | 10 | 3.10 ± 1.20 | N/A | N/A | N/A |
| l-Fenfluramine | 8.7 | 10 | 2.80 ± 1.03 | N/A | 9300 ± 2690 * (1274 ± 219) | N/A | |
| 17.4 | 10 | 2.10 ± 0.74 | N/A | 19,700 ± 2470 * (1976 ± 459) | N/A |
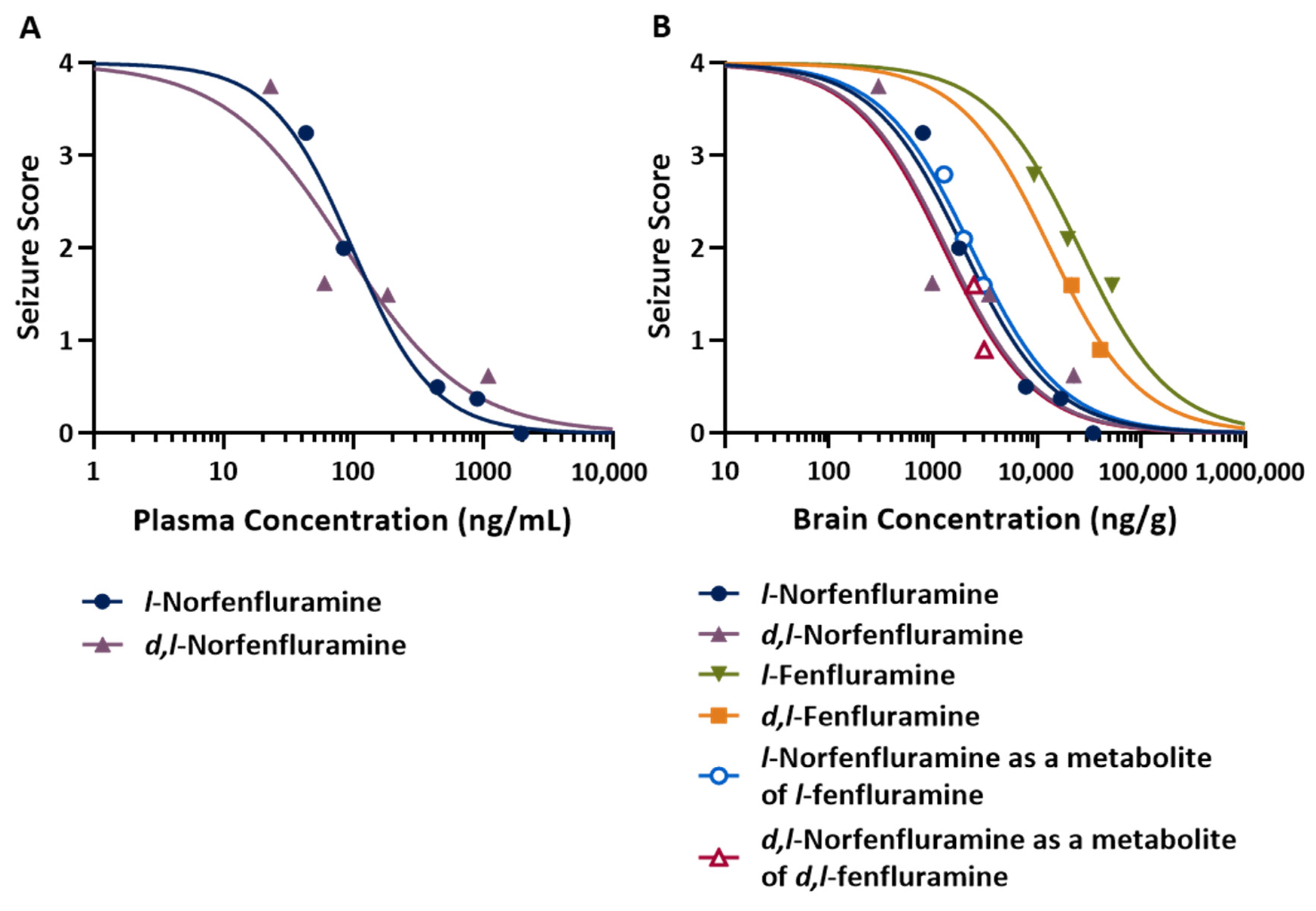
| Compound | ED50 (mg/kg) | EC50 | |
|---|---|---|---|
| Plasma (ng/mL) | Brain (ng/g) | ||
| l-Norfenfluramine | 1.18 | 101 | 1940 |
| d,l-Norfenfluramine | 1.28 | 81 | 1350 |
| l-Fenfluramine | 20.5 | N/A | 25,400 |
| d,l-Fenfluramine | 11.8 | N/A | 13,200 |
| l-Norfenfluramine (as metabolite of l-fenfluramine) | N/A | N/A | 2330 |
| d,l-Norfenfluramine (as metabolite of d,l-fenfluramine) | N/A | N/A | 1270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erenburg, N.; Perucca, E.; Bechard, J.; Dube, C.; Weishaupt, N.; Sherrington, R.; Bialer, M. Stereoselective Analysis of the Antiseizure Activity of Fenfluramine and Norfenfluramine in Mice: Is l-Norfenfluramine a Better Follow-Up Compound to Racemic-Fenfluramine? Int. J. Mol. Sci. 2024, 25, 2522. https://doi.org/10.3390/ijms25052522
Erenburg N, Perucca E, Bechard J, Dube C, Weishaupt N, Sherrington R, Bialer M. Stereoselective Analysis of the Antiseizure Activity of Fenfluramine and Norfenfluramine in Mice: Is l-Norfenfluramine a Better Follow-Up Compound to Racemic-Fenfluramine? International Journal of Molecular Sciences. 2024; 25(5):2522. https://doi.org/10.3390/ijms25052522
Chicago/Turabian StyleErenburg, Natalia, Emilio Perucca, Jeff Bechard, Celine Dube, Nina Weishaupt, Robin Sherrington, and Meir Bialer. 2024. "Stereoselective Analysis of the Antiseizure Activity of Fenfluramine and Norfenfluramine in Mice: Is l-Norfenfluramine a Better Follow-Up Compound to Racemic-Fenfluramine?" International Journal of Molecular Sciences 25, no. 5: 2522. https://doi.org/10.3390/ijms25052522
APA StyleErenburg, N., Perucca, E., Bechard, J., Dube, C., Weishaupt, N., Sherrington, R., & Bialer, M. (2024). Stereoselective Analysis of the Antiseizure Activity of Fenfluramine and Norfenfluramine in Mice: Is l-Norfenfluramine a Better Follow-Up Compound to Racemic-Fenfluramine? International Journal of Molecular Sciences, 25(5), 2522. https://doi.org/10.3390/ijms25052522




