Potential Mechanisms of the Protective Effects of the Cardiometabolic Drugs Type-2 Sodium–Glucose Transporter Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Heart Failure
Abstract
1. Introduction
2. Mechanisms of SGLT2i Benefits in Heart Failure
3. Mechanisms of GLP1-RA Benefits in Cardiovascular Diseases
4. Comparison of SGLT2i vs. GLP1-RA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B.; et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007, 50, 768–777. [Google Scholar] [CrossRef]
- Dauriz, M.; Targher, G.; Laroche, C.; Temporelli, P.L.; Ferrari, R.; Anker, S.; Coats, A.; Filippatos, G.; Crespo-Leiro, M.; Mebazaa, A.; et al. ESC-HFA Heart Failure Long-Term Registry. Association between diabetes and 1-year adverse clinical outcomes in a multinational cohort of ambulatory patients with chronic heart failure: Results from the ESC-HFA heart failure long-term registry. Diabetes Care 2017, 40, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- De Bellis, A.; De Angelis, G.; Fabris, E.; Cannatà, A.; Merlo, M.; Sinagra, G. Gender-related differences in heart failure: Beyond the “one-size-fits-all” paradigm. Heart Fail. Rev. 2020, 25, 245–255. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Damman, K.; Verhaar, M.C.; Paulus, W.J.; Duncker, D.J.; Cheng, C.; van Heerebeek, L.; Hillege, H.L.; Lam, C.S.; Navis, G.; et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur. J. Heart Fail. 2016, 18, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; LaMonte, M.; Klein, L.; Ayers, C.; Psaty, B.M.; Eaton, C.B.; Allen, N.B.; de Lemos, J.A.; Carnethon, M.; Greenland, P.; et al. Relationship between Physical Activity, Body Mass Index, and Risk of Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1129–1142. [Google Scholar] [CrossRef]
- Cave, A.C.; Brewer, A.C.; Narayanapanicker, A.; Ray, R.; Grieve, D.J.; Walker, S.; Shah, A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 2006, 8, 691–728. [Google Scholar] [CrossRef]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition from Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Kasiakogias, A.; Rosei, E.A.; Camafort, M.; Ehret, G.; Faconti, L.; Ferreira, J.P.; Brguljan, J.; Januszewicz, A.; Kahan, T.; Manolis, A.; et al. Hypertension and heart failure with preserved ejection fraction: Position paper by the European Society of Hypertension. J. Hypertens. 2021, 39, 1522–1545. [Google Scholar] [CrossRef] [PubMed]
- 1Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circulation 2019, 139, 1435–1450. [Google Scholar]
- Piatek, K.; Feuerstein, A.; Zach, V.; Rozados da Conceicao, C.; Beblo, A.; Belyavskiy, E.; Pieske-Kraigher, E.; Krannich, A.; Schwedhelm, E.; Hinz, S.; et al. Nitric oxide metabolites: Associations with cardiovascular biomarkers and clinical parameters in patients with HFpEF. ESC Heart Fail. 2022, 9, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Ryan, J.J. Nitric Oxide Signaling in Heart Failure with Preserved Ejection Fraction. JACC Basic Transl. Sci. 2017, 2, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Stokke, T.M.; Hasselberg, N.E.; Smedsrud, M.K.; Sarvari, S.I.; Haugaa, K.H.; Smiseth, O.A.; Edvardsen, T.; Remme, E.W. Geometry as a Confounder When Assessing Ventricular Systolic Function: Comparison between Ejection Fraction and Strain. J. Am. Coll. Cardiol. 2017, 70, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Okin, P.M.; Wachtell, K.; Gerdts, E.; Dahlöf, B.; Devereux, R.B. Relationship of left ventricular systolic function to persistence or development of electrocardiographic left ventricular hypertrophy in hypertensive patients: Implications for the development of new heart failure. J. Hypertens. 2014, 32, 2472–2478. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar]
- Gallo, G.; Volpe, M.; Rubattu, S. Angiotensin Receptor Blockers in the Management of Hypertension: A Real-World Perspective and Current Recommendations. Vasc. Health Risk Manag. 2022, 18, 507–515. [Google Scholar] [CrossRef]
- Castiglione, V.; Gentile, F.; Ghionzoli, N.; Chiriacò, M.; Panichella, G.; Aimo, A.; Vergaro, G.; Giannoni, A.; Passino, C.; Emdin, M. Pathophysiological Rationale and Clinical Evidence for Neurohormonal Modulation in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2023, 9, e09. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar]
- Gallo, G.; Tocci, G.; Fogacci, F.; Battistoni, A.; Rubattu, S.; Volpe, M. Blockade of the neurohormonal systems in heart failure with preserved ejection fraction: A contemporary meta-analysis. Int. J. Cardiol. 2020, 316, 172–179. [Google Scholar] [CrossRef]
- Solomon, S.D.; Vaduganathan, M.; Claggett, L.B.; Packer, M.; Zile, M.; Swedberg, K.; Rouleau, J.; Pfeffer, A.M.; Desai, A.; Lund, L.H.; et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 2020, 141, 352–361. [Google Scholar] [CrossRef]
- Rohde, L.E.; Claggett, B.L.; Wolsk, E.; Packer, M.; Zile, M.; Swedberg, K.; Rouleau, J.; Pfeffer, M.A.; Desai, A.S.; Lund, L.H.; et al. Cardiac and Noncardiac Disease Burden and Treatment Effect of Sacubitril/Valsartan: Insights from a Combined PARAGON-HF and PARADIGM-HF Analysis. Circ. Heart Fail. 2021, 14, e008052. [Google Scholar] [CrossRef] [PubMed]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Packer, M.; Senni, M.; Gori, M.; Jhund, P.S.; McGrath, M.M.; Rouleau, J.L.; Shi, V.; et al. Angiotensin-neprilysin inhibition and renal outcomes across the spectrum of ejection fraction in heart failure. Eur. J. Heart Fail. 2022, 24, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Jensen, M.D.; Kitzman, D.W.; Lam, C.S.P.; Obokata, M.; Rider, O.J. Obesity and heart failure with preserved ejection fraction: New insights and pathophysiological targets. Cardiovasc. Res. 2023, 118, 3434–3450. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. STEP-HFpEF Trial Committees and Investigators. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Busui, R.P.; Deanfield, J.; Inzucchi, S.E.; Mann, J.F.E.; Marx, N.; Mulvagh, S.L.; Poulter, N.; Engelmann, M.D.M.; Hovingh, G.K.; et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes. Metab. 2023, 25, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–64429. [Google Scholar] [CrossRef]
- Butler, J.; Handelsman, Y.; Bakris, G.; Verma, S. Use of sodium-glucose co-transporter-2 inhibitors in patients with and without type 2 diabetes: Implications for incident and prevalent heart failure. Eur. J. Heart Fail. 2020, 22, 604–617. [Google Scholar] [CrossRef]
- AbouEzzeddine, O.F.; Kemp, B.J.; Borlaug, B.A.; Mullan, B.P.; Behfar, A.; Pislaru, S.V.; Fudim, M.; Redfield, M.M.; Chareonthaitawee, P. Myocardial Energetics in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e006240. [Google Scholar] [CrossRef] [PubMed]
- Nespoux, J.; Vallon, V. SGLT2 inhibition and kidney protection. Clin. Sci. 2018, 132, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Kimura, K.; Peter, N.; Lai, V.; Tse, J.; Cham, L.; Perkins, B.A.; Soleymanlou, N.; Cherney, D.Z.I. Renal and Vascular Effects of Combined SGLT2 and Angiotensin-Converting Enzyme Inhibition. Circulation 2022, 146, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Choi, H.; Jeong, J.Y.; Na, K.R.; Lee, K.W.; Lim, B.J.; Choi, D.E. Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia-Reperfusion Injury. PLoS ONE 2016, 11, e0158810. [Google Scholar]
- Lim, V.G.; Bell, R.M.; Arjun, S.; Kolatsi-Joannou, M.; Long, D.A.; Yellon, D.M. SGLT2 Inhibitor, Canagliflozin, Attenuates Myocardial Infarction in the Diabetic and Nondiabetic Heart. JACC Basic Transl. Sci. 2019, 4, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Dyck, J.R.B.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: Evidence for potential off-target effects. J. Mol. Cell. Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Gao, H.K.; Kengne, A.P. Effect of Sodium-Glucose Cotransport-2 Inhibitors on Blood Pressure in People with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 43 Randomized Control Trials with 22,528 Patients. J. Am. Heart Assoc. 2017, 6, e004007. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Adamopoulou, E.; Pyrpyris, N.; Sakalidis, A.; Leontsinis, I.; Manta, E.; Mantzouranis, E.; Beneki, E.; Soulaidopoulos, S.; Konstantinidis, D.; et al. The Effect of SGLT2 Inhibitors on the Endothelium and the Microcirculation: From Bench to Bedside and Beyond. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 741–757. [Google Scholar] [CrossRef]
- Tikkanen, I.; Narko, K.; Zeller, C.; Green, A.; Salsali, A.; Broedl, U.C.; Woerle, H.J.; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015, 38, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Okada, K.; Kato, M.; Nishizawa, M.; Yoshida, T.; Asano, T.; Uchiyama, K.; Niijima, Y.; Katsuya, T.; Urata, H.; et al. Twenty-Four-Hour Blood Pressure-Lowering Effect of a Sodium-Glucose Cotransporter 2 Inhibitor in Patients with Diabetes and Uncontrolled Nocturnal Hypertension: Results from the Randomized, Placebo-Controlled SACRA Study. Circulation 2019, 139, 2089–2097. [Google Scholar] [CrossRef]
- Weber, M.A.; Mansfield, T.A.; Cain, V.A.; Iqbal, N.; Parikh, S.; Ptaszynska, A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016, 4, 211–220. [Google Scholar] [CrossRef]
- Pfeifer, M.; Townsend, R.R.; Davies, M.J.; Vijapurkar, U.; Ren, J. Effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: A post hoc analysis. Cardiovasc. Diabetol. 2017, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Neuen, B.L.; Li, J.; Perkovic, V.; Charytan, D.M.; de Zeeuw, D.; Edwards, R.; Greene, T.; Levin, A.; Mahaffey, K.W.; et al. Early Change in Albuminuria with Canagliflozin Predicts Kidney and Cardiovascular Outcomes: A PostHoc Analysis from the CREDENCE Trial. J. Am. Soc. Nephrol. 2020, 31, 2925–2936. [Google Scholar] [CrossRef]
- Herring, R.A.; Shojaee-Moradie, F.; Stevenage, M.; Parsons, I.; Jackson, N.; Mendis, J.; Middleton, B.; Umpleby, A.M.; Fielding, B.A.; Davies, M.; et al. The SGLT2 Inhibitor Dapagliflozin Increases the Oxidation of Ingested Fatty Acids to Ketones in Type 2 Diabetes. Diabetes Care 2022, 45, 1408–1415. [Google Scholar] [CrossRef]
- Byrne, N.J.; Matsumura, N.; Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Darwesh, A.M.; Levasseur, J.L.; Jahng, J.W.S.; Vos, D.; Parajuli, N.; et al. Empagliflozin Blunts Worsening Cardiac Dysfunction Associated with Reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) Inflammasome Activation in Heart Failure. Circ. Heart Fail. 2020, 13, e006277. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Liu, T.; Cai, L.; Yang, X.; Mou, C. The effects of Sodium-glucose cotransporter 2 inhibitors on adipose tissue in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1115321. [Google Scholar] [CrossRef]
- Mazer, C.D.; Hare, G.M.T.; Connelly, P.W.; Gilbert, R.E.; Shehata, N.; Quan, A.; Teoh, H.; Leiter, L.A.; Zinman, B.; Jüni, P.; et al. Effect of Empagliflozin on Erythropoietin Levels, Iron Stores, and Red Blood Cell Morphology in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease. Circulation 2020, 141, 704–707. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients with Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Cardoso, R.; Graffunder, F.P.; Ternes, C.M.P.; Fernandes, A.; Rocha, A.V.; Fernandes, G.; Bhatt, D.L. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100933. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Fonarow, G.C.; McGuire, D.K.; Hernandez, A.F.; Vaduganathan, M.; Rosenstock, J.; Handelsman, Y.; Verma, S.; Anker, S.D.; McMurray, J.J.V.; et al. Glucagon-Like Peptide 1 Receptor Agonists and Heart Failure: The Need for Further Evidence Generation and Practice Guidelines Optimization. Circulation 2020, 142, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Gallo, G.; Modena, M.G.; Ferri, C.; Desideri, G.; Tocci, G.; Members of the Board of the Italian Society of Cardiovascular Prevention. Updated Recommendations on Cardiovascular Prevention in 2022: An Executive Document of the Italian Society of Cardiovascular Prevention. High Blood Press. Cardiovasc. Prev. 2022, 29, 91–102, Erratum in High Blood Press. Cardiovasc. Prev. 2022, 29, 103. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Gallo, G. Obesity and cardiovascular disease: An executive document on pathophysiological and clinical links promoted by the Italian Society of Cardiovascular Prevention (SIPREC). Front. Cardiovasc. Med. 2023, 10, 1136340. [Google Scholar] [CrossRef] [PubMed]
- Lavalle-Cobo, A.; Masson, W.; Lobo, M.; Masson, G.; Molinero, G. Glucagon-like Peptide-1 Receptor Agonists and Cardioprotective Benefit in Patients with Type 2 Diabetes without Baseline Metformin: A Systematic Review and Update Meta-analysis. High Blood Press. Cardiovasc. Prev. 2021, 28, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jia, H.; Jiang, Y.; Wang, L.; Zhang, Y.; Mu, Y.; Liu, Y. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 Diabetes Mellitus: A meta-analysis. Sci. Rep. 2015, 5, 1020. [Google Scholar] [CrossRef] [PubMed]
- Pahud de Mortanges, A.; Sinaci, E.; Salvador, D., Jr.; Bally, L.; Muka, T.; Wilhelm, M.; Bano, A. GLP-1 Receptor Agonists and Coronary Arteries: From Mechanisms to Events. Front. Pharmacol. 2022, 13, 856111. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients with Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.; Abdelati, T.; Rizk, M.; Youssef, E.; Gaber, N.; Moghazy, K.; Yafei, S. Association of fasting glucagon-like peptide-1 with oxidative stress and subclinical atherosclerosis in type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Luna-Marco, C.; de Marañon, A.M.; Hermo-Argibay, A.; Rodriguez-Hernandez, Y.; Hermenejildo, J.; Fernandez-Reyes, M.; Apostolova, N.; Vila, J.; Sola, E.; Morillas, C.; et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023, 66, 102849. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Buracco, S.; Cavalot, F.; Frascaroli, C.; Guerrasio, A.; Russo, I. Glucagon-like peptide 1-related peptides increase nitric oxide effects to reduce platelet activation. Thromb. Haemost. 2017, 117, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B.; Duvillard, L.; Pais de Barros, J.P.; Bouillet, B.; Baillot-Rudoni, S.; Rouland, A.; Petit, J.M.; Degrace, P.; Demizieux, L. Liraglutide Increases the Catabolism of Apolipoprotein B100-Containing Lipoproteins in Patients with Type 2 Diabetes and Reduces Proprotein Convertase Subtilisin/Kexin Type 9 Expression. Diabetes Care 2021, 44, 1027–1037. [Google Scholar] [CrossRef]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef]
- Kahles, F.; Rückbeil, M.V.; Mertens, R.W.; Foldenauer, A.C.; Arrivas, M.C.; Moellmann, J.; Lebherz, C.; Biener, M.; Giannitsis, E.; Katus, H.A.; et al. Glucagon-like peptide 1 levels predict cardiovascular risk in patients with acute myocardial infarction. Eur. Heart J. 2020, 41, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.L.; Babkowski, M.C.; Miramontes González, J.P. GLP-1 and the renin-angiotensin-aldosterone system. Lancet Diabetes Endocrinol. 2019, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide In Mice with Experimental Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Baeres, F.M.M.; Bain, S.C.; Goldman, B.; Husain, M.; Nauck, M.A.; Poulter, N.R.; Pratley, R.E.; Thomsen, A.B.; Buse, J.B.; et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients with Diabetes with or without Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of Liraglutide on Clinical Stability among Patients with Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Lepore, J.J.; Olson, E.; Demopoulos, L.; Haws, T.; Fang, Z.; Barbour, A.M.; Fossler, M.; Davila-Roman, V.G.; Russell, S.D.; Gropler, R.J. Effects of the Novel Long-Acting GLP-1 Agonist, Albiglutide, on Cardiac Function, Cardiac Metabolism, and Exercise Capacity in Patients with Chronic Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2016, 4, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Stewart, M.W.; Perkins, C.; Jones-Leone, A.; Yang, F.; Perry, C.; Reinhardt, R.R.; Rendell, M. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 2016, 59, 266–274. [Google Scholar] [CrossRef]
- Kumarathurai, P.; Sajadieh, A.; Anholm, C.; Kristiansen, O.P.; Haugaard, S.B.; Nielsen, O.W. Effects of liraglutide on diastolic function parameters in patients with type 2 diabetes and coronary artery disease: A randomized crossover study. Cardiovasc. Diabetol. 2021, 20, 12. [Google Scholar] [CrossRef]
- Huixing, L.; Di, F.; Daoquan, P. Effect of Glucagon-like Peptide-1 Receptor Agonists on Prognosis of Heart Failure and Cardiac Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Ther. 2023, 45, 17–30. [Google Scholar] [CrossRef]
- A Study of Tirzepatide (LY3298176) in Participants with Heart Failure with Preserved Ejection Fraction and Obesity (SUMMIT). ClinicalTrials.gov Identifier: NCT04847557. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04847557 (accessed on 14 January 2024).
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2022–2031. [Google Scholar] [CrossRef]
- Baviera, M.; Foresta, A.; Colacioppo, P.; Macaluso, G.; Roncaglioni, M.C.; Tettamanti, M.; Fortino, I.; Genovese, S.; Caruso, I.; Giorgino, F. Effectiveness and safety of GLP-1 receptor agonists versus SGLT-2 inhibitors in type 2 diabetes: An Italian cohort study. Cardiovasc. Diabetol. 2022, 21, 162. [Google Scholar] [CrossRef]
- Fu, E.L.; Clase, C.M.; Janse, R.J.; Lindholm, B.; Dekker, F.W.; Jardine, M.J.; Carrero, J.J. Comparative effectiveness of SGLT2i versus GLP1-RA on cardiovascular outcomes in routine clinical practice. Int. J. Cardiol. 2022, 352, 172–179. [Google Scholar] [CrossRef]
- Lugner, M.; Sattar, N.; Miftaraj, M.; Ekelund, J.; Franzén, S.; Svensson, A.M.; Eliasson, B. Cardiorenal and other diabetes related outcomes with SGLT-2 inhibitors compared to GLP-1 receptor agonists in type 2 diabetes: Nationwide observational study. Cardiovasc. Diabetol. 2021, 20, 67. [Google Scholar] [CrossRef]
- Volpe, M.; Gallo, G. Cardiometabolic phenotype of heart failure with preserved ejection fraction as a target of sodium-glucose co-transporter 2 inhibitors and glucagon-like peptide receptor agonists. Cardiovasc. Res. 2021, 117, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Comprehensive Management of Cardiovascular Risk Factors for Adults with Type 2 Diabetes: A Scientific Statement from the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Vellanki, P.; Blonde, L.; Christofides, E.A.; Galindo, R.J.; Hirsch, I.B.; Isaacs, S.D.; Izuora, K.E.; Low Wang, C.C.; Twining, C.L.; et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm—2023 Update. Endocr. Pract. 2023, 29, 305–340. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Shen, L.; Jhund, P.S.; Docherty, K.F.; Vaduganathan, M.; Petrie, M.C.; Desai, A.S.; Køber, L.; Schou, M.; Packer, M.; Solomon, S.D.; et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur. Heart J. 2022, 43, 2573–2587. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Barone, L.; Bellia, A.; Sergi, D.; Lecis, D.; Prandi, F.R.; Milite, M.; Galluccio, C.; Muscoli, S.; Romeo, F.; et al. Treatment of HFpEF beyond the SGLT2-Is: Does the Addition of GLP-1 RA Improve Cardiometabolic Risk and Outcomes in Diabetic Patients? Int. J. Mol. Sci. 2022, 23, 14598. [Google Scholar] [CrossRef]
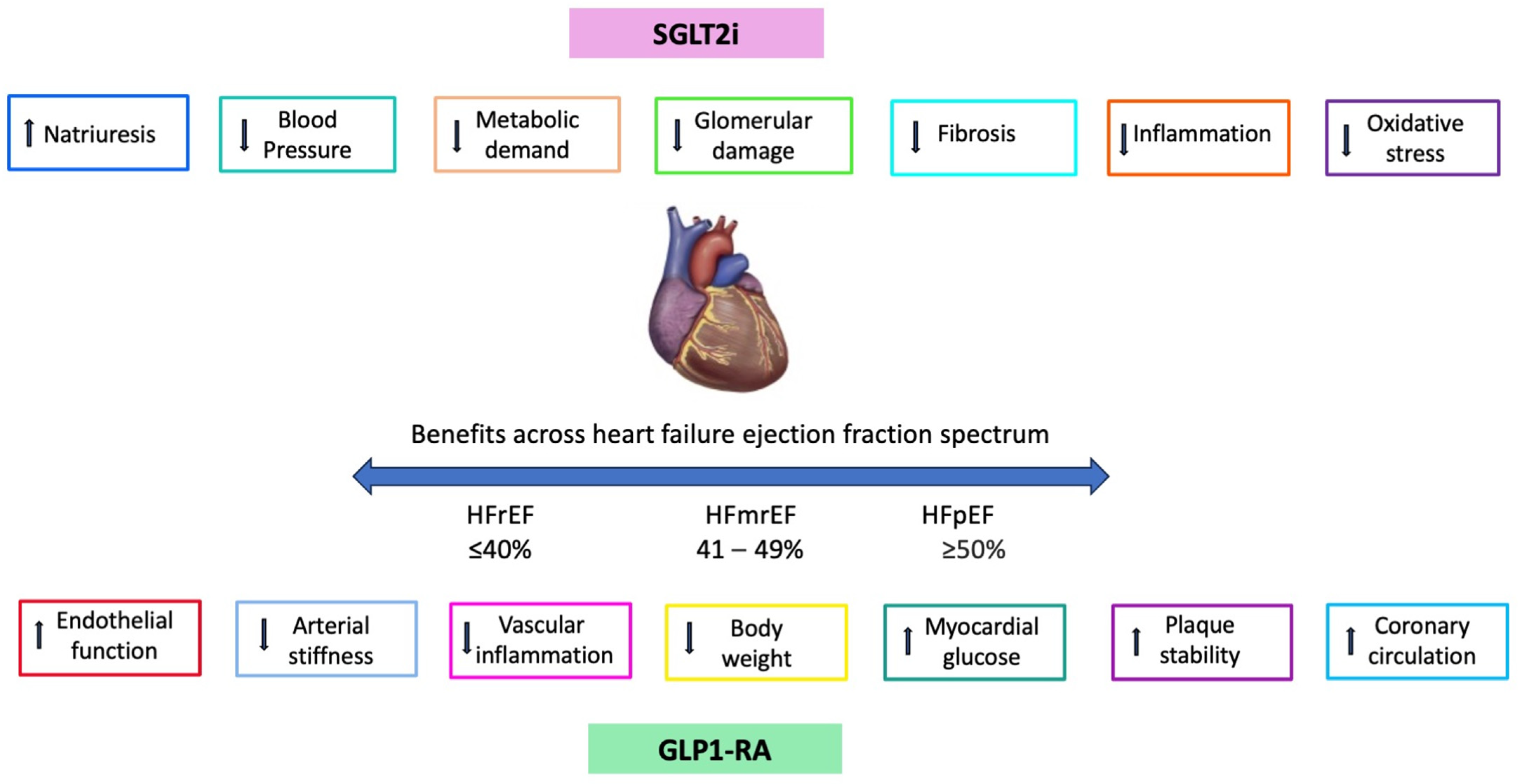

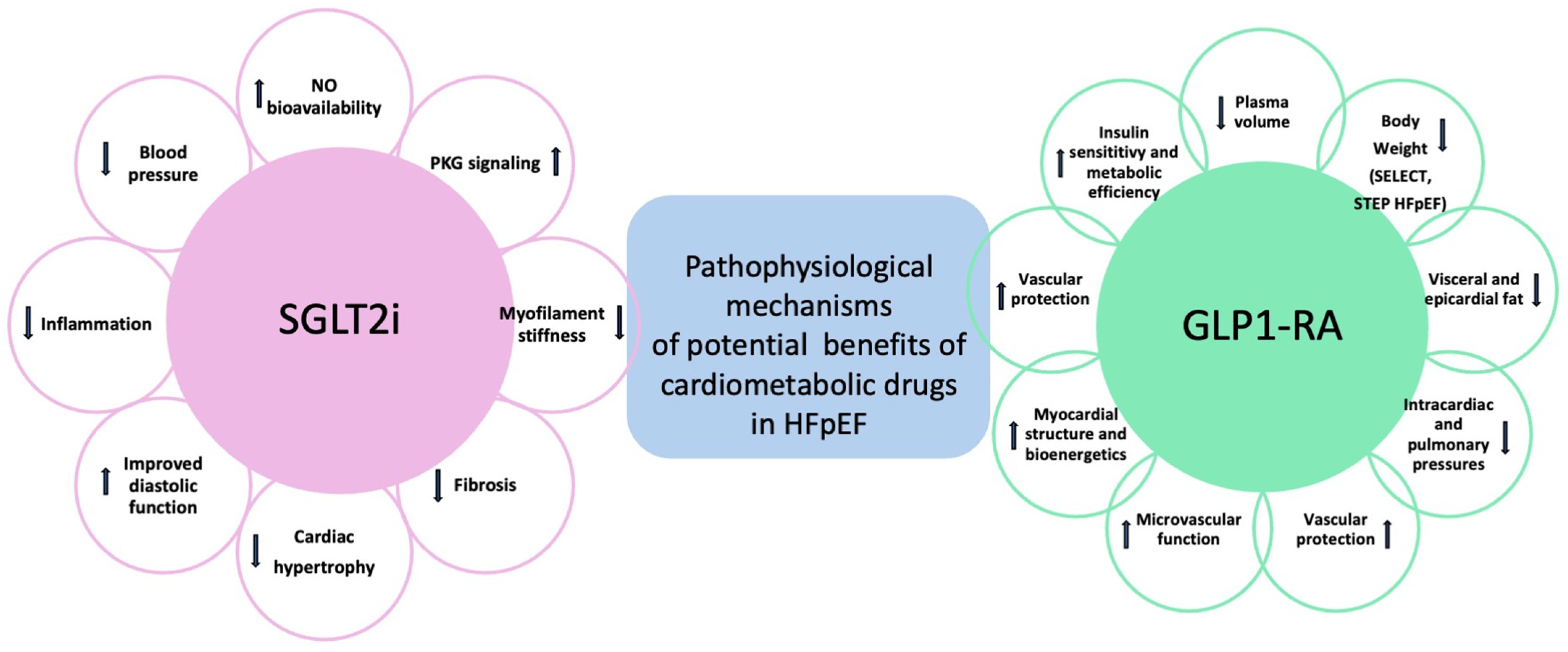
| Study | Study Design | Study Population | Results |
|---|---|---|---|
| EMPA-REG BP [41] | Randomized controlled trial Empagliflozin vs. placebo | 825 patients with both hypertension and type 2 diabetes | Significantly greater reductions in SBP, DBP, and seated office SBP and DBP in the empagliflozin group |
| SACRA [42] | Randomized controlled trial Empagliflozin vs. placebo | 132 patients with type 2 diabetes and uncontrolled nocturnal hypertension receiving stable antihypertensive therapy including ARBs | Significant reduction in office SBP and 24 h SBP in the empagliflozin group |
| Weber et al. [43] | Randomized controlled trial Dapagliflozin vs. placebo | 311 patients with uncontrolled type 2 diabetes (HbA1c 70–105%) and hypertension (SBP 140–165 mmHg and DBP 85–105 mmHg) receiving oral antihyperglycemic drugs, insulin, or both, plus a RAAS blocker and an additional antihypertensive drug | Dapagliflozin (10 mg) significantly reduced office SBP and 24 h SBP with a synergistic BP-lowering effect with calcium channel blockers and beta-blockers |
| Pfeifer et al. [44] | Post hoc analysis canagliflozin vs. placebo | Pooled data from four 26-week, randomized, double-blind, placebo-controlled studies in patients with type 2 diabetes (n = 2313) and a 6-week, randomized, double-blind, placebo-controlled, ABPM study in patients with type 2 diabetes and hypertension (n = 169). | Canagliflozin significantly reduced SBP, 24 h SBP, and DBP |
| CREDENCE post hoc analysis [45] | Post hoc analysis canagliflozin vs. placebo | 4401 patients with type 2 diabetes and CKD | Canagliflozin increased the likelihood of achieving a 30% reduction in UACR with a lower risk of kidney outcomes, MACEs and hospitalization for HF or cardiovascular death |
| EMPEROR-Reduced [50] | Randomized controlled trial empagliflozin vs. placebo | 3730 patients with HFrEF | Empagliflozin reduced the composite outcome of cardiovascular death or hospitalization for worsening HF (−25%) |
| DAPA-HF [51] | Randomized controlled trial dapagliflozin vs. placebo | 4744 patients with HFrEF | Dapagliflozin reduced the composite outcome of cardiovascular death or worsening HF (−26%) |
| EMPEROR-Preserved [52] | Randomized controlled trial empagliflozin vs. placebo | 5988 HF patients with EF > 40% | Empagliflozin reduced the composite outcome of cardiovascular death or worsening HF (−21%) |
| DELIVER [53] | Randomized controlled trial dapagliflozin vs. placebo | 6263 HF patients with EF > 40% | Dapagliflozin reduced the composite outcome of cardiovascular death or worsening HF (−18%) |
| SOLOIST-WHF [54] | Randomized controlled trial sotagliflozin vs. placebo | 1222 HF patients with type 2 diabetes who were recently hospitalized for worsening HF | Sotagliflozin reduced the composite outcome of cardiovascular death or worsening HF (−33%) |
| EMPULSE [55] | Randomized controlled trial empagliflozin vs. placebo | 530 patients with acute de novo or decompensated HF | Empagliflozin reduced the primary hierarchical composite outcome of all-cause death, total HF events, time to first HF event, or a ≥5-point change from KCCQ symptom score |
| EMPAG-HF [56] | Randomized controlled trial empagliflozin vs. placebo | 60 patients hospitalized for acute decompensated HF | Addition of empagliflozin to standard medical treatment resulted in a 25% increase in cumulative urine output over 5 days without affecting markers of renal function or injury |
| Cardoso et al. [57] | Meta-analysis of placebo-controlled, randomized trials of SGLT2i in patients with HF | 20,241 patients with HFrEF and HFpEF | SGLT2i reduced all-cause and cardiovascular mortality (−14%), the composite of cardiovascular mortality, HF hospitalizations, or urgent visits for HF (−25%) |
| LEADER [74] | Randomized controlled trial liraglutide vs. placebo | 9340 patients with type 2 diabetes and high cardiovascular risk | Liraglutide reduced the risk of a composite outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke independently from HF. Liraglutide produced a 13% reduction in HF risk, although not reaching statistical significance |
| SUSTAIN-6 [75] | Randomized controlled trial once-weekly subcutaneous semaglutide vs. placebo | 3297 patients with type 2 diabetes | Semaglutide reduced the composite outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke |
| REWIND [76] | Randomized controlled trial dulaglutide vs. placebo | 9901 patients with type 2 diabetes at high cardiovascular risk with high HbA1c | Dulaglutide reduced the composite outcome of non-fatal myocardial infarction, non-fatal stroke, or death from cardiovascular causes |
| PIONEER-6 [77] | Randomized controlled trial oral semaglutide vs. placebo | 3183 patients with type 2 diabetes at high cardiovascular risk | Semaglutide was not inferior compared to placebo in reducing MACEs (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) |
| EXSCEL [79] | Randomized controlled trial exenatide vs. placebo | 14,752 diabetic patients, 2389 with HF | No significant effects of exenatide in the subgroup of HF patients with regard to all-cause mortality and to the composite of all-cause mortality and HF hospitalization. Significant effects in patients without HF |
| LIVE [80] | Randomized controlled trial liraglutide vs. placebo | 241 patients with HFrEF | No significant effects of liraglutide on LVEF, quality of life, or functional class |
| FIGHT [81] | Randomized controlled trial liraglutide vs. placebo | 300 patients with recently decompensated HFrEF | Liraglutide did not significantly reduce the primary endpoint of time to death, time to rehospitalization for HF, and time-averaged proportional change in NT-proBNP level |
| HARMONY [83] | Randomized controlled trial albiglutide vs. placebo | 309 patients with type 2 diabetes | Albiglutide reduced the exploratory endpoint of HF hospitalizations by 29% |
| SELECT [29] | Randomized controlled trial subcutaneous once-weekly semaglutide vs. placebo | 17,604 patients with pre-existing cardiovascular disease, with overweight or obesity, without diabetes | Semaglutide reduced the composite endpoint of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke |
| STEP-HFpEF [28] | Randomized controlled trial subcutaneous once-weekly semaglutide vs. placebo | 529 patients with HFpEF and obesity | Semaglutide reduced KCCQ clinical summary score and improved 6MWT distance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, G.; Volpe, M. Potential Mechanisms of the Protective Effects of the Cardiometabolic Drugs Type-2 Sodium–Glucose Transporter Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Heart Failure. Int. J. Mol. Sci. 2024, 25, 2484. https://doi.org/10.3390/ijms25052484
Gallo G, Volpe M. Potential Mechanisms of the Protective Effects of the Cardiometabolic Drugs Type-2 Sodium–Glucose Transporter Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Heart Failure. International Journal of Molecular Sciences. 2024; 25(5):2484. https://doi.org/10.3390/ijms25052484
Chicago/Turabian StyleGallo, Giovanna, and Massimo Volpe. 2024. "Potential Mechanisms of the Protective Effects of the Cardiometabolic Drugs Type-2 Sodium–Glucose Transporter Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Heart Failure" International Journal of Molecular Sciences 25, no. 5: 2484. https://doi.org/10.3390/ijms25052484
APA StyleGallo, G., & Volpe, M. (2024). Potential Mechanisms of the Protective Effects of the Cardiometabolic Drugs Type-2 Sodium–Glucose Transporter Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Heart Failure. International Journal of Molecular Sciences, 25(5), 2484. https://doi.org/10.3390/ijms25052484






