Current Strategies for Increasing Knock-In Efficiency in CRISPR/Cas9-Based Approaches
Abstract
1. Introduction
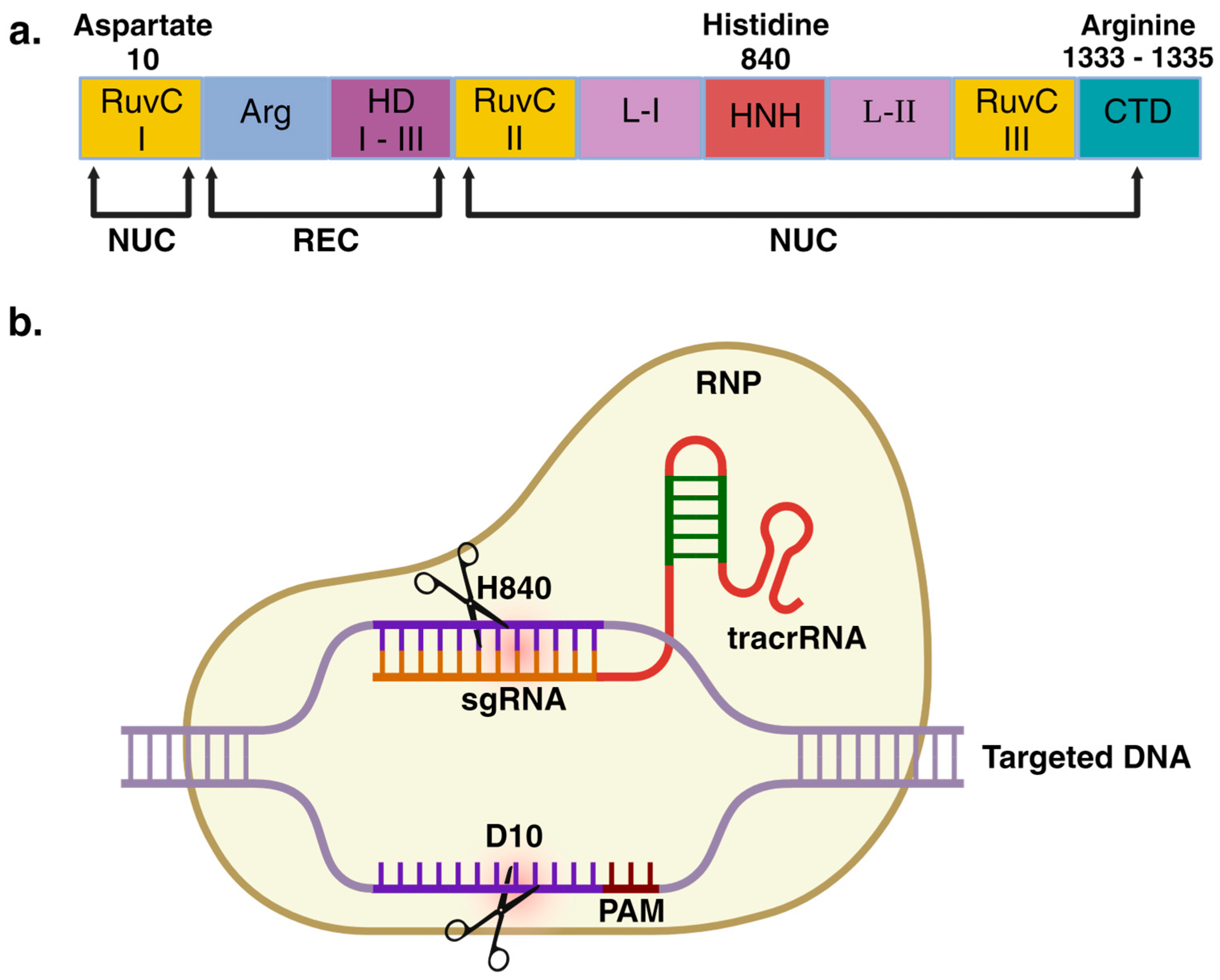
2. The CRISPR/Cas9 System: A Biological Perspective
3. The CRISPR/Cas9 System: A Genome Editing Tool
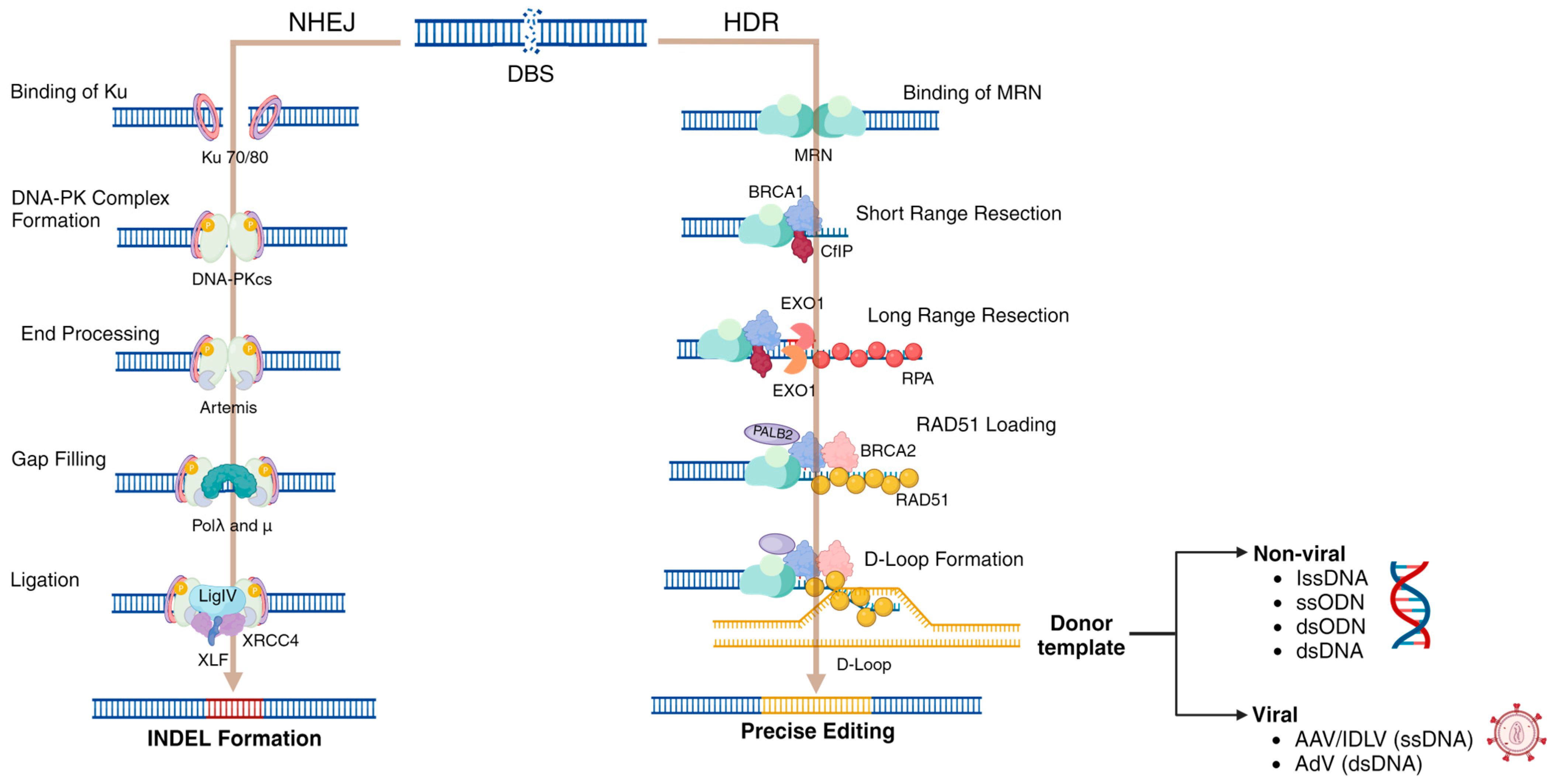
3.1. NHEJ Pathway
3.2. HDR Pathway
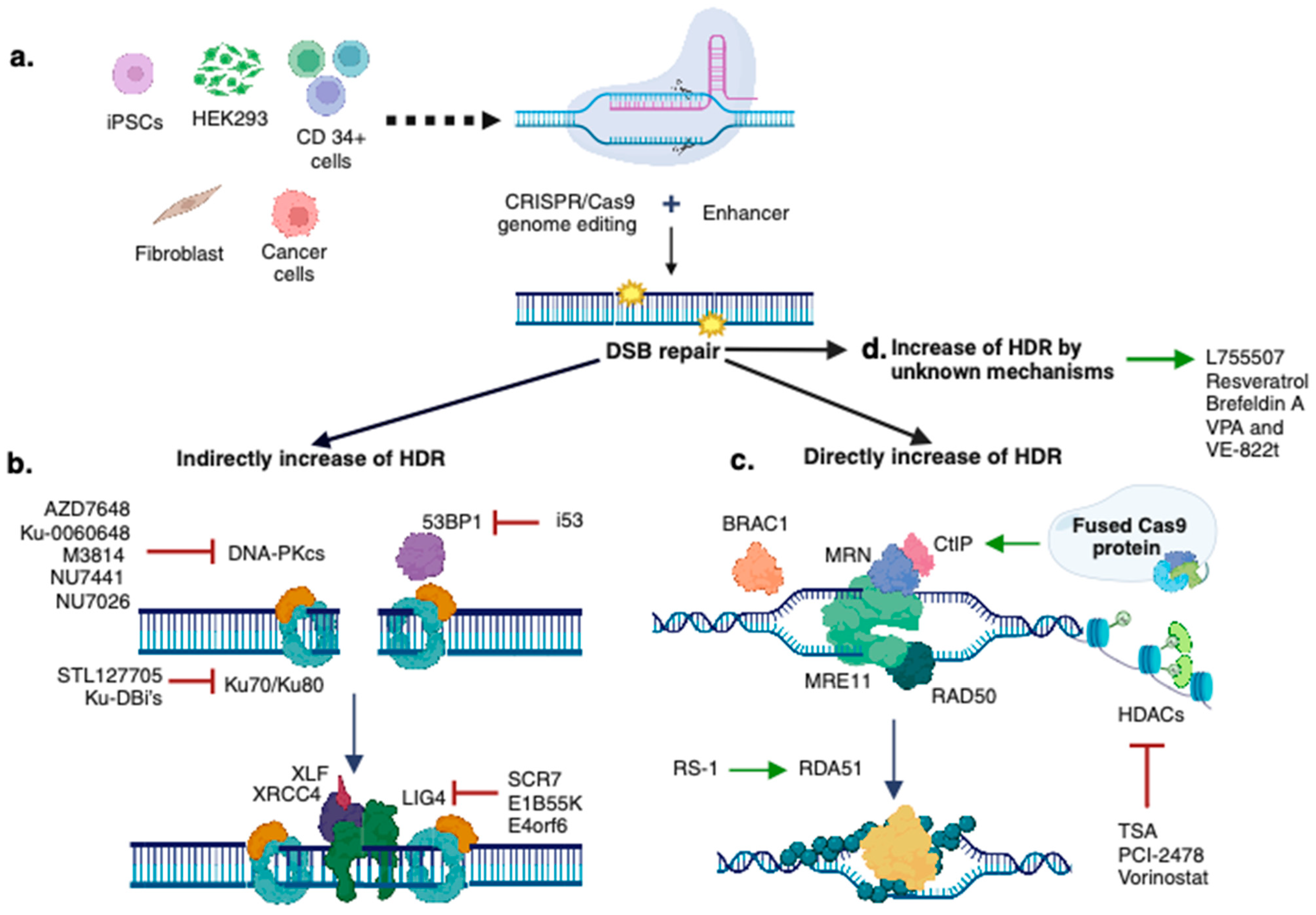
4. Strategies for Increasing CRISPR/Cas9-Mediated HDR Efficiency
4.1. CRISPR/Cas9 Design Considerations
4.1.1. Cas9 Delivery Platforms
4.1.2. Cas9 Fusion Variants
4.1.3. sgRNA
4.1.4. Donor Template
4.2. Blocking the NHEJ Pathway
4.2.1. NHEJ Starting Pathway
4.2.2. Initial DSB Recognition by DNA-PKcs
4.2.3. DNA Ligase IV-Dependent Ligation
4.2.4. Small Interference (si) and Short Harping (sh) RNA NHEJ Protein Downregulation
4.3. Stimulating the HDR Pathway
4.3.1. Small Molecules
4.3.2. Modulating Chromatin Remodeling Factors
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef]
- Leal, A.F.; Fnu, N.; Benincore-Flórez, E.; Herreño-Pachón, A.M.; Echeverri-Peña, O.Y.; Alméciga-Díaz, C.J.; Tomatsu, S. The landscape of CRISPR/Cas9 for inborn errors of metabolism. Mol. Genet. Metab. 2022, 138, 106968. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Malik, F.; Andrabi, K.I. Expansion of the CRISPR/Cas Genome-Sculpting Toolbox: Innovations, Applications and Challenges. Mol. Diagn. Ther. 2021, 25, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Zhou, J.; Xu, B.; Zhang, H. In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges. Acta Pharm. Sin. B 2021, 11, 2150–2171. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Shahryari, A.; Moya, N.; Siehler, J.; Wang, X.; Burtscher, I.; Lickert, H. Increasing Gene Editing Efficiency for CRISPR-Cas9 by Small RNAs in Pluripotent Stem Cells. CRISPR J. 2021, 4, 491–501. [Google Scholar] [CrossRef]
- Sun, Y.; McCorvie, T.J.; Yates, L.A.; Zhang, X. Structural basis of homologous recombination. Cell. Mol. Life Sci. 2020, 77, 3–18. [Google Scholar] [CrossRef]
- Chaplin, A.K.; Hardwick, S.W.; Stavridi, A.K.; Buehl, C.J.; Goff, N.J.; Ropars, V.; Liang, S.; De Oliveira, T.M.; Chirgadze, D.Y.; Meek, K.; et al. Cryo-EM of NHEJ supercomplexes provides insights into DNA repair. Mol. Cell 2021, 81, 3400–3409.e3. [Google Scholar] [CrossRef]
- Liu, M.; Rehman, S.; Tang, X.; Gu, K.; Fan, Q.; Chen, D.; Ma, W. Methodologies for Improving HDR Efficiency. Front. Genet. 2018, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.M.; Quinn, P.M.; da Costa, B.L.; Tsang, S.H. CRISPR/Cas therapeutic strategies for autosomal dominant disorders. J. Clin. Investig. 2022, 132, 1–11. [Google Scholar] [CrossRef]
- FDA. FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease (accessed on 26 December 2023).
- Torres-Ruiz, R.; Rodriguez-Perales, S. CRISPR-Cas9 technology: Applications and human disease modelling. Brief. Funct. Genom. 2017, 16, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Modell, A.E.; Lim, D.; Nguyen, T.M.; Sreekanth, V.; Choudhary, A. CRISPR-based therapeutics: Current challenges and future applications. Trends Pharmacol. Sci. 2022, 43, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Sowbhagya, R.; Muktha, H.; Ramakrishnaiah, T.N.; Surendra, A.S.; Tanvi, Y.; Nivitha, K.; Rajashekara, S. CRISPR/Cas-mediated genome editing in mice for the development of drug delivery mechanism. Mol. Biol. Rep. 2023, 50, 7729–7743. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Banerjee, A.; Vanden Broeck, A.; Klinge, S. Rapid clonal identification of biallelic CRISPR/Cas9 knock-ins using SNEAK PEEC. Sci. Rep. 2023, 13, 1719. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Ikeda, K.; Cromer, M.K.; Uchida, N.; Nishimura, T.; Romano, R.; Tong, A.J.; Lemgart, V.T.; Camarena, J.; Pavel-Dinu, M.; et al. Highly Efficient and Marker-free Genome Editing of Human Pluripotent Stem Cells by CRISPR-Cas9 RNP and AAV6 Donor-Mediated Homologous Recombination. Cell Stem Cell 2019, 24, 821–828.e5. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Choudhary, N.; Tandi, D.; Verma, R.K.; Yadav, V.K.; Dhingra, N.; Ghosh, T.; Choudhary, M.; Gaur, R.K.; Abdellatif, M.H.; Gacem, A.; et al. A comprehensive appraisal of mechanism of anti-CRISPR proteins: An advanced genome editor to amend the CRISPR gene editing. Front. Plant Sci. 2023, 14, 1164461. [Google Scholar] [CrossRef]
- Bharathkumar, N.; Sunil, A.; Meera, P.; Aksah, S.; Kannan, M.; Saravanan, K.M.; Anand, T. CRISPR/Cas-Based Modifications for Therapeutic Applications: A Review. Mol. Biotechnol. 2022, 64, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Beisel, C.L. The tracrRNA in CRISPR Biology and Technologies. Annu. Rev. Genet. 2021, 55, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.M.; Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Investig. 2014, 124, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.W.; le Sage, C.; Larrieu, D.; Demir, M.; Jackson, S.P. CRISPR-Cas9(D10A) nickase-based genotypic and phenotypic screening to enhance genome editing. Sci. Rep. 2016, 6, 24356. [Google Scholar] [CrossRef] [PubMed]
- Vakulskas, C.A.; Dever, D.P.; Rettig, G.R.; Turk, R.; Jacobi, A.M.; Collingwood, M.A.; Bode, N.M.; McNeill, M.S.; Yan, S.; Camarena, J.; et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 2018, 24, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ruan, J.; Song, J.; Wen, L.; Yang, D.; Zhao, J.; Xia, X.; Chen, Y.E.; Zhang, J.; Xu, J. MiCas9 increases large size gene knock-in rates and reduces undesirable on-target and off-target indel edits. Nat. Commun. 2020, 11, 6082. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Gutschner, T.; Haemmerle, M.; Genovese, G.; Draetta, G.F.; Chin, L. Post-translational Regulation of Cas9 during G1 Enhances Homology-Directed Repair. Cell Rep. 2016, 14, 1555–1566. [Google Scholar] [CrossRef]
- Charpentier, M.; Khedher, A.H.Y.; Menoret, S.; Brion, A.; Lamribet, K.; Dardillac, E.; Boix, C.; Perrouault, L.; Tesson, L.; Geny, S.; et al. CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair. Nat. Commun. 2018, 9, 1133. [Google Scholar] [CrossRef]
- Jayavaradhan, R.; Pillis, D.M.; Goodman, M.; Zhang, F.; Zhang, Y.; Andreassen, P.R.; Malik, P. CRISPR-Cas9 fusion to dominant-negative 53BP1 enhances HDR and inhibits NHEJ specifically at Cas9 target sites. Nat. Commun. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Maggio, I.; Zittersteijn, H.A.; Wang, Q.; Liu, J.; Janssen, J.M.; Ojeda, I.T.; van der Maarel, S.M.; Lankester, A.C.; Hoeben, R.C.; Gonçalves, M.A.F.V. Integrating gene delivery and gene-editing technologies by adenoviral vector transfer of optimized CRISPR-Cas9 components. Gene Ther. 2020, 27, 209–225. [Google Scholar] [CrossRef]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2016, 351, 84–88. [Google Scholar] [CrossRef]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z.; et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.; Kim, Y.; Park, J.; Min, S.; Choi, J.W.; Huang, T.P.; Yoon, S.; Liu, D.R.; Kim, H.H. High-throughput analysis of the activities of xCas9, SpCas9-NG and SpCas9 at matched and mismatched target sequences in human cells. Nat. Biomed. Eng. 2020, 4, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ren, K.; Zhu, Y.; Tang, Z.; Wang, Y.; Zhang, B.; Huang, Z. Structural insights into a high fidelity variant of SpCas9. Cell Res. 2019, 29, 183–192. [Google Scholar] [CrossRef]
- Chen, J.S.; Dagdas, Y.S.; Kleinstiver, B.P.; Welch, M.M.; Sousa, A.A.; Harrington, L.B.; Sternberg, S.H.; Joung, J.K.; Yildiz, A.; Doudna, J.A. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 2017, 550, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Fujii, W.; Sugiura, K.; Naito, K. High-fidelity endonuclease variant HypaCas9 facilitates accurate allele-specific gene modification in mouse zygotes. Commun. Biol. 2019, 2, 371. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Whinn, K.S.; Kaur, G.; Lewis, J.S.; Schauer, G.D.; Mueller, S.H.; Jergic, S.; Maynard, H.; Gan, Z.Y.; Naganbabu, M.; Bruchez, M.P.; et al. Nuclease dead Cas9 is a programmable roadblock for DNA replication. Sci. Rep. 2019, 9, 13292. [Google Scholar] [CrossRef]
- Kanafi, M.M.; Tavallaei, M. Overview of advances in CRISPR/deadCas9 technology and its applications in human diseases. Gene 2022, 830, 146518. [Google Scholar] [CrossRef]
- Senturk, S.; Shirole, N.H.; Nowak, D.G.; Corbo, V.; Pal, D.; Vaughan, A.; Tuveson, D.A.; Trotman, L.C.; Kinney, J.B.; Sordella, R. Rapid and tunable method to temporally control gene editing based on conditional Cas9 stabilization. Nat. Commun. 2017, 8, 14370. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Uchida, T.; Tilson, S.; Hu, Z.; Ma, C.D.; Leek, M.; Eichner, M.; Hong, S.G.; Liang, T.J. A dual conditional CRISPR-Cas9 system to activate gene editing and reduce off-target effects in human stem cells. Mol. Ther. Nucleic Acids 2022, 28, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Tisdale, J.F. Gene therapy for sickle cell disease. Hematol. Am. Soc. Hematol. Educ. Program. 2023, 2023, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. The world’s first CRISPR therapy is approved: Who will receive it? Nat. Biotechnol. 2023, 42, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Jensen, R.B.; Rothenberg, E. Preserving genome integrity in human cells via DNA double-strand break repair. Mol. Biol. Cell 2020, 31, 859–865. [Google Scholar] [CrossRef]
- Hustedt, N.; Durocher, D. The control of DNA repair by the cell cycle. Nat. Cell Biol. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Nakamura, A.J.; Rao, V.A.; Pommier, Y.; Bonner, W.M. The complexity of phosphorylated H2AX foci formation and DNA repair assembly at DNA double-strand breaks. Cell Cycle 2010, 9, 389–397. [Google Scholar] [CrossRef]
- Daley, J.M.; Sung, P. 53BP1, BRCA1, and the choice between recombination and end joining at DNA double-strand breaks. Mol. Cell. Biol. 2014, 34, 1380–1388. [Google Scholar] [CrossRef]
- Shakirova, A.; Karpov, T.; Komarova, Y.; Lepik, K. In search of an ideal template for therapeutic genome editing: A review of current developments for structure optimization. Front. Genome Ed. 2023, 5, 1068637. [Google Scholar] [CrossRef]
- Ghosh, D.; Raghavan, S.C. Nonhomologous end joining: New accessory factors fine tune the machinery. Trends Genet. 2021, 37, 582–599. [Google Scholar] [CrossRef]
- Davis, A.J.; Chen, D.J. DNA double strand break repair via non-homologous end-joining. Transl. Cancer Res. 2013, 2, 130–143. [Google Scholar] [CrossRef]
- Davis, A.J.; Chen, B.P.; Chen, D.J. DNA-PK: A dynamic enzyme in a versatile DSB repair pathway. DNA Repair 2014, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Rothenberg, E.; Ramsden, D.A.; Lieber, M.R. The molecular basis and disease relevance of non-homologous DNA end joining. Nat. Rev. Mol. Cell Biol. 2020, 21, 765–781. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Watanabe, G.; Gerodimos, C.A.; Ochi, T.; Blundell, T.L.; Jackson, S.P.; Lieber, M.R. Different DNA End Configurations Dictate Which NHEJ Components Are Most Important for Joining Efficiency. J. Biol. Chem. 2016, 291, 24377–24389. [Google Scholar] [CrossRef]
- Schulte-Uentrop, L.; El-Awady, R.A.; Schliecker, L.; Willers, H.; Dahm-Daphi, J. Distinct roles of XRCC4 and Ku80 in non-homologous end-joining of endonuclease- and ionizing radiation-induced DNA double-strand breaks. Nucleic Acids Res. 2008, 36, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Nemoz, C.; Ropars, V.; Frit, P.; Gontier, A.; Drevet, P.; Yu, J.; Guerois, R.; Pitois, A.; Comte, A.; Delteil, C.; et al. XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining. Nat. Struct. Mol. Biol. 2018, 25, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Watanabe, G.; Lieber, M.R. Unifying the DNA end-processing roles of the artemis nuclease: Ku-dependent artemis resection at blunt DNA ends. J. Biol. Chem. 2015, 290, 24036–24050. [Google Scholar] [CrossRef]
- Tadi, S.K.; Tellier-Lebègue, C.; Nemoz, C.; Drevet, P.; Audebert, S.; Roy, S.; Meek, K.; Charbonnier, J.B.; Modesti, M. PAXX Is an Accessory c-NHEJ Factor that Associates with Ku70 and Has Overlapping Functions with XLF. Cell Rep. 2016, 17, 541–555. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Pryor, J.M.; Waters, C.A.; Aza, A.; Asagoshi, K.; Strom, C.; Mieczkowski, P.A.; Blanco, L.; Ramsden, D.A. Essential role for polymerase specialization in cellular nonhomologous end joining. Proc. Natl. Acad. Sci. USA 2015, 112, E4537–E4545. [Google Scholar] [CrossRef] [PubMed]
- Conlin, M.P.; Reid, D.A.; Small, G.W.; Chang, H.H.; Watanabe, G.; Lieber, M.R.; Ramsden, D.A.; Rothenberg, E. DNA Ligase IV Guides End-Processing Choice during Nonhomologous End Joining. Cell Rep. 2017, 20, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Sturzenegger, A.; Burdova, K.; Kanagaraj, R.; Levikova, M.; Pinto, C.; Cejka, P.; Janscak, P. DNA2 cooperates with the WRN and BLM RecQ helicases to mediate long-range DNA end resection in human cells. J. Biol. Chem. 2014, 289, 27314–27326. [Google Scholar] [CrossRef]
- Nimonkar, A.V.; Genschel, J.; Kinoshita, E.; Polaczek, P.; Campbell, J.L.; Wyman, C.; Modrich, P.; Kowalczykowski, S.C. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011, 25, 350–362. [Google Scholar] [CrossRef]
- Chen, H.; Lisby, M.; Symington, L.S. RPA coordinates DNA end resection and prevents formation of DNA hairpins. Mol. Cell 2013, 50, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.; Cortez, D. RPA and RAD51: Fork reversal, fork protection, and genome stability. Nat. Struct. Mol. Biol. 2018, 25, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Renkawitz, J.; Lademann, C.A.; Jentsch, S. Mechanisms and principles of homology search during recombination. Nat. Rev. Mol. Cell Biol. 2014, 15, 369–383. [Google Scholar] [CrossRef]
- Ma, C.J.; Gibb, B.; Kwon, Y.; Sung, P.; Greene, E.C. Protein dynamics of human RPA and RAD51 on ssDNA during assembly and disassembly of the RAD51 filament. Nucleic Acids Res. 2017, 45, 749–761. [Google Scholar] [CrossRef]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Kowalczykowski, S.C. An Overview of the Molecular Mechanisms of Recombinational DNA Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a016410. [Google Scholar] [CrossRef]
- Ma, Y.; Han, X.; Quintana Bustamante, O.; Bessa de Castro, R.; Zhang, K.; Zhang, P.; Li, Y.; Liu, Z.; Liu, X.; Ferrari, M.; et al. Highly efficient genome editing of human hematopoietic stem cells via a nano-silicon-blade delivery approach. Integr. Biol. 2017, 9, 548–554. [Google Scholar] [CrossRef]
- Kouranova, E.; Forbes, K.; Zhao, G.; Warren, J.; Bartels, A.; Wu, Y.; Cui, X. CRISPRs for Optimal Targeting: Delivery of CRISPR Components as DNA, RNA, and Protein into Cultured Cells and Single-Cell Embryos. Hum. Gene Ther. 2016, 27, 464–475. [Google Scholar] [CrossRef]
- Leal, A.F.; Alméciga-Díaz, C.J. Efficient CRISPR/Cas9 nickase-mediated genome editing in an in vitro model of mucopolysaccharidosis IVA. Gene Ther. 2022, 30, 107–114. [Google Scholar] [CrossRef]
- Leal, A.; Celik, B.; FNU, N.; Khan, S.; Tomatsu, S.; Almáciga-Díaz, C. Iron oxide-coupled CRISPR/nCas9-based genome editing assessment in mucopolysaccharidosis IVA mice. Mol. Ther. Methods Clin. Dev. 2023, 31, 101153. [Google Scholar] [CrossRef] [PubMed]
- Finney, M.; Romanowski, J.; Adelman, Z.N. Strategies to improve homology-based repair outcomes following CRISPR-based gene editing in mosquitoes: Lessons in how to keep any repair disruptions local. Virol. J. 2022, 19, 128. [Google Scholar] [CrossRef]
- Matsumoto, D.; Kishi, K.; Matsugi, E.; Inoue, Y.; Nigorikawa, K.; Nomura, W. Cas9-Geminin and Cdt1-fused anti-CRISPR protein synergistically increase editing accuracy. FEBS Lett. 2023, 597, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Nguyen, V.; Park, S. Recent advances in the engineering and application of streptavidin-like molecules. Appl. Microbiol. Biotechnol. 2019, 103, 7355–7365. [Google Scholar] [CrossRef]
- Paulsen, B.S.; Mandal, P.K.; Frock, R.L.; Boyraz, B.; Yadav, R.; Upadhyayula, S.; Gutierrez-Martinez, P.; Ebina, W.; Fasth, A.; Kirchhausen, T.; et al. Ectopic expression of RAD52 and dn53BP1 improves homology-directed repair during CRISPR-Cas9 genome editing. Nat. Biomed. Eng. 2017, 1, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Pupo, A.; Fernández, A.; Low, S.H.; François, A.; Suárez-Amarán, L.; Samulski, R.J. AAV vectors: The Rubik’s cube of human gene therapy. Mol. Ther. 2022, 30, 3515–3541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2022, 7, e10258. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Staahl, B.T.; Alla, R.K.; Doudna, J.A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 2014, 3, e04766. [Google Scholar] [CrossRef]
- Marino, N.D.; Pinilla-Redondo, R.; Csörgő, B.; Bondy-Denomy, J. Anti-CRISPR protein applications: Natural brakes for CRISPR-Cas technologies. Nat. Methods 2020, 17, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, D.; Tamamura, H.; Nomura, W. A cell cycle-dependent CRISPR-Cas9 activation system based on an anti-CRISPR protein shows improved genome editing accuracy. Commun. Biol. 2020, 3, 601. [Google Scholar] [CrossRef]
- Richardson, R.R.; Steyert, M.; Khim, S.N.; Crutcher, G.W.; Brandenburg, C.; Robertson, C.D.; Romanowski, A.J.; Inen, J.; Altas, B.; Poulopoulos, A. Enhancing Precision and Efficiency of Cas9-Mediated Knockin Through Combinatorial Fusions of DNA Repair Proteins. CRISPR J. 2023, 6, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Bashir, S.; Li, X.; Rossius, J.; Chu, V.T.; Rajewsky, K.; Kühn, R. Enhancement of Precise Gene Editing by the Association of Cas9 With Homologous Recombination Factors. Front. Genet. 2019, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Duan, X.; Cai, P.; Gao, L.; Wu, X.; Yao, L.; Zhou, Y.J. Fusing an exonuclease with Cas9 enhances homologous recombination in Pichia pastoris. Microb. Cell Factories 2022, 21, 182. [Google Scholar] [CrossRef]
- Di Primio, C.; Galli, A.; Cervelli, T.; Zoppè, M.; Rainaldi, G. Potentiation of gene targeting in human cells by expression of Saccharomyces cerevisiae Rad52. Nucleic Acids Res. 2005, 33, 4639–4648. [Google Scholar] [CrossRef]
- Shao, S.; Ren, C.; Liu, Z.; Bai, Y.; Chen, Z.; Wei, Z.; Wang, X.; Zhang, Z.; Xu, K. Enhancing CRISPR/Cas9-mediated homology-directed repair in mammalian cells by expressing Saccharomyces cerevisiae Rad52. Int. J. Biochem. Cell Biol. 2017, 92, 43–52. [Google Scholar] [CrossRef]
- Carneiro, P.; de Freitas, M.V.; Matte, U. In silico analysis of potential off-target sites to gene editing for Mucopolysaccharidosis type I using the CRISPR/Cas9 system: Implications for population-specific treatments. PLoS ONE 2022, 17, e0262299. [Google Scholar] [CrossRef]
- Bao, X.R.; Pan, Y.; Lee, C.M.; Davis, T.H.; Bao, G. Tools for experimental and computational analyses of off-target editing by programmable nucleases. Nat. Protoc. 2021, 16, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, S.; Kim, Y.; Kweon, J.; Kim, H.S.; Bae, S.; Kim, J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014, 24, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S.; Thommandru, B.; Woodley, J.; Turk, R.; Yan, S.; Kurgan, G.; McNeill, M.S.; Rettig, G.R. Optimized design parameters for CRISPR Cas9 and Cas12a homology-directed repair. Sci. Rep. 2021, 11, 19482. [Google Scholar] [CrossRef]
- Gomez-Ospina, N.; Scharenberg, S.G.; Mostrel, N.; Bak, R.O.; Mantri, S.; Quadros, R.M.; Gurumurthy, C.B.; Lee, C.; Bao, G.; Suarez, C.J.; et al. Human genome-edited hematopoietic stem cells phenotypically correct Mucopolysaccharidosis type I. Nat. Commun. 2019, 10, 4045. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.I.; Sutrisnoh, N.B.; Srinivasan, H.; Zhang, J.; Li, J.; Zhang, F.; Lalith, C.R.J.; Xing, H.; Shanmugam, R.; et al. Systematic evaluation of CRISPR-Cas systems reveals design principles for genome editing in human cells. Genome Biol. 2018, 19, 62. [Google Scholar] [CrossRef]
- Richardson, C.D.; Ray, G.J.; DeWitt, M.A.; Curie, G.L.; Corn, J.E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 2016, 34, 339–344. [Google Scholar] [CrossRef]
- Lee, A.B.C.; Tan, M.H.; Chai, C.L.L. Small-molecule enhancers of CRISPR-induced homology-directed repair in gene therapy: A medicinal chemist’s perspective. Drug Discov. Today 2022, 27, 2510–2525. [Google Scholar] [CrossRef]
- Bischoff, N.; Wimberger, S.; Maresca, M.; Brakebusch, C. Improving Precise CRISPR Genome Editing by Small Molecules: Is there a Magic Potion? Cells 2020, 9, 1318. [Google Scholar] [CrossRef]
- Shams, F.; Bayat, H.; Mohammadian, O.; Mahboudi, S.; Vahidnezhad, H.; Soosanabadi, M.; Rahimpour, A. Advance trends in targeting homology-directed repair for accurate gene editing: An inclusive review of small molecules and modified CRISPR-Cas9 systems. Bioimpacts 2022, 12, 371–391. [Google Scholar] [CrossRef]
- Canny, M.D.; Moatti, N.; Wan, L.C.K.; Fradet-Turcotte, A.; Krasner, D.; Mateos-Gomez, P.A.; Zimmermann, M.; Orthwein, A.; Juang, Y.C.; Zhang, W.; et al. Inhibition of 53BP1 favors homology-dependent DNA repair and increases CRISPR-Cas9 genome-editing efficiency. Nat. Biotechnol. 2018, 36, 95–102. [Google Scholar] [CrossRef] [PubMed]
- De Ravin, S.S.; Brault, J.; Meis, R.J.; Liu, S.; Li, L.; Pavel-Dinu, M.; Lazzarotto, C.R.; Liu, T.; Koontz, S.M.; Choi, U.; et al. Enhanced homology-directed repair for highly efficient gene editing in hematopoietic stem/progenitor cells. Blood 2021, 137, 2598–2608. [Google Scholar] [CrossRef]
- Riesenberg, S.; Maricic, T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat. Commun. 2018, 9, 2164. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, M.; Wang, X.; Ying, W.; Hu, X.; Dai, P.; Meng, F.; Shi, L.; Sun, Y.; Yao, N.; et al. Tild-CRISPR Allows for Efficient and Precise Gene Knockin in Mouse and Human Cells. Dev. Cell 2018, 45, 526–536.e5. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, X.; Hu, X.; Liu, Z.; Liu, J.; Zhou, H.; Shen, X.; Wei, Y.; Huang, Z.; Ying, W.; et al. Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res. 2017, 27, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.W.; Dai, X.Y.; Wang, W.T.; Yang, Z.X.; Zhao, J.J.; Zhang, J.P.; Wen, W.; Zhang, F.; Oberg, K.C.; Zhang, L.; et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res. 2021, 49, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, S.; Chintalapati, M.; Macak, D.; Kanis, P.; Maricic, T.; Pääbo, S. Simultaneous precise editing of multiple genes in human cells. Nucleic Acids Res. 2019, 47, e116. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, S.; Kanis, P.; Macak, D.; Wollny, D.; Düsterhöft, D.; Kowalewski, J.; Helmbrecht, N.; Maricic, T.; Pääbo, S. Efficient high-precision homology-directed repair-dependent genome editing by HDRobust. Nat. Methods 2023, 20, 1388–1399. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, Z.; Guo, X.; Jiang, B.; Wang, G.; Luo, D.; Chen, Y.; Zhu, Y.S. Ligase IV inhibitor SCR7 enhances gene editing directed by CRISPR-Cas9 and ssODN in human cancer cells. Cell Biosci. 2018, 8, 12. [Google Scholar] [CrossRef]
- Selvaraj, S.; Feist, W.N.; Viel, S.; Vaidyanathan, S.; Dudek, A.M.; Gastou, M.; Rockwood, S.J.; Ekman, F.K.; Oseghale, A.R.; Xu, L.; et al. High-efficiency transgene integration by homology-directed repair in human primary cells using DNA-PKcs inhibition. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Anuchina, A.A.; Zaynitdinova, M.I.; Demchenko, A.G.; Evtushenko, N.A.; Lavrov, A.V.; Smirnikhina, S.A. Bridging Gaps in HDR Improvement: The Role of MAD2L2, SCAI, and SCR7. Int. J. Mol. Sci. 2023, 24, 6704. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Dougan, S.K.; Truttmann, M.C.; Bilate, A.M.; Ingram, J.R.; Ploegh, H.L. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 2015, 33, 538–542. [Google Scholar] [CrossRef]
- Tanihara, F.; Hirata, M.; Namula, Z.; Do, L.T.K.; Yoshimura, N.; Lin, Q.; Takebayashi, K.; Sakuma, T.; Yamamoto, T.; Otoi, T. Pigs with an INS point mutation derived from zygotes electroporated with CRISPR/Cas9 and ssODN. Front. Cell Dev. Biol. 2023, 11, 884340. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.T.; Weber, T.; Wefers, B.; Wurst, W.; Sander, S.; Rajewsky, K.; Kühn, R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 2015, 33, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, F.; Schreiner, S.; Blair, G.E.; Dobner, T.; Branton, P.E.; Blanchette, P. The Human Adenovirus Type 5 E4orf6/E1B55K E3 Ubiquitin Ligase Complex Enhances E1A Functional Activity. mSphere 2016, 1, e00015-15. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Zhang, X.; Quan, R.; Zhong, C.; Mo, J.; Huang, Y.; Wang, H.; Ruan, X.; Xu, Z.; et al. Suppressing Ku70/Ku80 expression elevates homology-directed repair efficiency in primary fibroblasts. Int. J. Biochem. Cell Biol. 2018, 99, 154–160. [Google Scholar] [CrossRef]
- Li, K.; Wang, G.; Andersen, T.; Zhou, P.; Pu, W.T. Optimization of genome engineering approaches with the CRISPR/Cas9 system. PLoS ONE 2014, 9, e105779. [Google Scholar] [CrossRef]
- Chan, J.K.; Blansit, K.; Kiet, T.; Sherman, A.; Wong, G.; Earle, C.; Bourguignon, L.Y. The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol. Oncol. 2014, 132, 739–744. [Google Scholar] [CrossRef]
- Jenike, A.E.; Halushka, M.K. miR-21: A non-specific biomarker of all maladies. Biomark. Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Jayathilaka, K.; Sheridan, S.D.; Bold, T.D.; Bochenska, K.; Logan, H.L.; Weichselbaum, R.R.; Bishop, D.K.; Connell, P.P. A chemical compound that stimulates the human homologous recombination protein RAD51. Proc. Natl. Acad. Sci. USA 2008, 105, 15848–15853. [Google Scholar] [CrossRef]
- Song, J.; Yang, D.; Xu, J.; Zhu, T.; Chen, Y.E.; Zhang, J. RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efficiency. Nat. Commun. 2016, 7, 10548. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, X.; Wang, H.; Liu, D.; Li, Z.; Wu, Z.; Yang, H. Increasing CRISPR/Cas9-mediated homology-directed DNA repair by histone deacetylase inhibitors. Int. J. Biochem. Cell Biol. 2020, 125, 105790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Yang, Z.X.; Zhang, F.; Fu, Y.W.; Dai, X.Y.; Wen, W.; Zhang, B.; Choi, H.; Chen, W.; Brown, M.; et al. HDAC inhibitors improve CRISPR-mediated HDR editing efficiency in iPSCs. Sci. China Life Sci. 2021, 64, 1449–1462. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, L.; Lin, G.; Hu, Y.; Jiao, Y.; Wang, Y.; Liu, J.; Yang, S.; Yao, S. HDAC inhibitors improve CRISPR-Cas9 mediated prime editing and base editing. Mol. Ther. Nucleic Acids 2022, 29, 36–46. [Google Scholar] [CrossRef]
- Molugu, K.; Khajanchi, N.; Lazzarotto, C.R.; Tsai, S.Q.; Saha, K. Trichostatin A for Efficient CRISPR-Cas9 Gene Editing of Human Pluripotent Stem Cells. CRISPR J. 2023, 6, 473–485. [Google Scholar] [CrossRef]
- Liang, Z.; Kumar, V.; Le Bouteiller, M.; Zurita, J.; Kenrick, J.; Lin, S.G.; Lou, J.; Hu, J.; Ye, A.Y.; Boboila, C.; et al. Ku70 suppresses alternative end joining in G1-arrested progenitor B cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2103630118. [Google Scholar] [CrossRef]
- Kotnis, A.; Du, L.; Liu, C.; Popov, S.W.; Pan-Hammarström, Q. Non-homologous end joining in class switch recombination: The beginning of the end. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 653–665. [Google Scholar] [CrossRef][Green Version]
- Sishc, B.J.; Davis, A.J. The Role of the Core Non-Homologous End Joining Factors in Carcinogenesis and Cancer. Cancers 2017, 9, 81. [Google Scholar] [CrossRef]
| Variant | Type of DNA Cutting | Characteristics | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| wtCas9 | DSB | Induces DSB | High DBS efficiency Simplest sgRNA design | High off-target | [7] |
| HF-Cas9 | DBS | High-fidelity | Low off-target | * Reduced on-target cut | [26,27,28] |
| HDR-Cas9 | DBS | Fusion of several motifs to interact with HDR protein | Increases HDR | ** Large in-size proteins upon fusion | [27,29,30,31] |
| eCas9 | DSB | Presence of 4X NLS | High-fidelity High nuclear localization | Reduced on-target cut | [32,33] |
| xCas9 | DSB | Recognizes several PAMs, NGG, NG, NNG, GAT, and CAA | Broader PAM recognition Low off-target | Altered interactions between Cas9 and sgRNA/DNA duplex | [34,35,36] |
| HA-Cas9 | DSB | High fidelity | Higher DSB cutting as compared to wtCas9 | Moderate off-target | [37,38] |
| nCas9 | SSB | HNH or RuvC mutated Requires paired sgRNA for DSB cutting | High DSB efficiency | Lowest off-target Require accurate sgRNA design | [39] |
| dCas9 | NA | HNH and RuvC mutated No DNA cutting | Allows transcriptional studies without permanent modification in the DNA | Large-in-size proteins Moderate off-target | [40,41] |
| CD-Cas9 | NA | Conditional Cas9 expression in the presence of FKBP12 | Temporal control of Cas9 Marginal off-target effect | Large in-size protein Background mRNA expression | [42,43] |
| Strategy | Locus | * Cas9 Variant | Cells | ** % HDR Efficiency (Fold-Change) | Ref. |
|---|---|---|---|---|---|
| Cas9-Brex27 (miCas9) | AAVS1 | wtCas9 | Fibroblast | 2.9% (2.5-fold) | [27] |
| AAVS1 | wtCas9 | AECs | 1.5% (2.1-fold) | ||
| AAVS1 | wtCas9 | iPSCs | 1.2% (2.1-fold) | ||
| CtIP fusion to Cas9 | AAVS1 | wtCas9 | Fibroblast | 2.3-fold | [30] |
| AAVS1 | wtCas9 | HEK293 | 2.4-fold | ||
| ATF4 | wtCas9 | HEK293 | 2.5-fold | ||
| GABP | wtCas9 | HEK293 | 1-fold | ||
| TGIF2 | wtCas9 | HEK293 | 2.1-fold | ||
| RAD21 | wtCas9 | HEK293 | 2.5-fold | ||
| CREB | wtCas9 | HEK293 | 1.4-fold | ||
| AAVS1 | wtCas9 | iPSCs | 1.5-fold | ||
| Cas9-RC | Actβ | wtCas9 | Neuron P7 | 3.7-fold | [86] |
| DN1S fusion to Cas9 | AAVS1 | wtCas9 | HEK293 | 33.3% (1.6-fold) | [31] |
| LMO2 | wtCas9 | HEK293 | 54.6%(2-fold) | ||
| CD45 | wtCas9 | K562 | 17% (1.3-fold) | ||
| CCR5 | wtCas9 | Jurkat | 26% (1.1-fold) | ||
| Geminin fusion to Cas9 | MALAT1 | wtCas9 | HEK293 | 14% (1.4-fold) | [29] |
| AcrIIA4-hCdt1 co-expressed with Cas9 | AAVS1 | wtCas9 | HEK293 | 1.7-fold | [85] |
| EMX1 | wtCas9 | HEK293 | 4-fold | ||
| VEGFA | wtCas9 | HEK293 | 4.5-fold | ||
| yRAD52-Cas9 fusion protein | VEGF | wtCas9 | HEK293 | 3.3-fold | [90] |
| CCR5 | wtCas9 | HEK293 | 3.8-fold | ||
| IGF2 | wtCas9 | PK15 | 2.2-fold | ||
| i53 | LMNA | wtCas9 | U2OS | 8.6% (1.8-fold) | [102] |
| HIST1H2BK | wtCas9 | HEK293 | 2.1% (1.3-fold) | ||
| HIST1H2BK | wtCas9 | K562 | 12% (1.8-fold) | ||
| CYBB | wtCas9 | CD34+ | 2.3-fold | [103] | |
| NU7026 | CALD1 | wtCas9 | iPSC | 25.5% (1.5-fold) | [104] |
| CALD1 | nCas9 | iPSC | 11.7% (1.5-fold) | ||
| KATNA1 | wtCas9 | iPSC | 5.2% (1.6-fold) | ||
| KATNA1 | nCas9 | iPSC | 18.7% (2.6-fold) | ||
| SLITRK1 | wtCas9 | iPSC | 6.2% (1.2-fold) | ||
| SLITRK1 | nCas9 | iPSC | 11.9% (2.5-fold) | ||
| Trichostatin A | EEF1A1 | wtCas9 | iPSCs | 30% (2.2-fold) | [107] |
| EEF2 | wtCas9 | T cells | 50% (1.3-fold) | ||
| Trichostatin A | CALD1 | wtCas9 | iPSCs | 12.3% (0.8-fold) | [104] |
| CALD1 | nCas9 | iPSCs | 10.1% (1.5-fold) | ||
| KATNA1 | wtCas9 | iPSCs | 3.4% (1-fold) | ||
| KATNA1 | nCas9 | iPSCs | 17.6% (2.2-fold) | ||
| SLITRK1 | wtCas9 | iPSCs | 5% (1-fold | ||
| SLITRK1 | nCas9 | iPSCs | 8.7% (1.8-fold) | ||
| RS-1 | CALD1 | wtCas9 | iPSCs | 22.2% (1-fold) | [104] |
| CALD1 | nCas9 | iPSCs | 8.5% (1.1-fold) | ||
| KATNA1 | wtCas9 | iPSCs | 2.5% (0.8-fold) | ||
| KATNA1 | nCas9 | iPSCs | 8% (1.2-fold) | ||
| SLITRK1 | wtCas9 | iPSCs | 4.4% (0.9-fold) | ||
| SLITRK1 | nCas9 | iPSCs | 5.3% (1-fold) | ||
| Resveratrol | CALD1 | wtCas9 | iPSCs | 13.3% (1.1-fold) | [104] |
| CALD1 | nCas9 | iPSCs | 7.1% (1-fold) | ||
| KATNA1 | wtCas9 | iPSCs | 2.7% (0.8-fold) | ||
| KATNA1 | nCas9 | iPSCs | 8% (1.1-fold) | ||
| SLITRK1 | wtCas9 | iPSCs | 4.3% (0.9-fold) | ||
| SLITRK1 | nCas9 | iPSCs | 6.2% (0.9-fold) | ||
| SCR7 | TSG101 | wtCas9 | MelJuSo | 19-fold | [113] |
| Tap1 | wtCas9 | DC2.4 | 58.3% (13-fold) | [113] | |
| AAVS1 | wtCas9 | MCF-7 | 4% (3-fold) | [110] | |
| AAVS1 | wtCas9 | HCT-116 | 3.1% (2.4-fold) | ||
| CALD1 | wtCas9 | iPSCs | 12.5% (0.9-fold) | [104] | |
| CALD1 | nCas9 | iPSCs | 7.1% (1.1-fold) | ||
| KATNA1 | wtCas9 | iPSCs | 2.8% (0.8-fold) | ||
| KATNA1 | nCas9 | iPSCs | 6.3% (1-fold) | ||
| SLITRK1 | wtCas9 | iPSCs | 4.7% (0.9-fold) | ||
| SLITRK1 | nCas9 | iPSCs | 5.9% (1.1-fold) | ||
| L755507 | CALD1 | wtCas9 | iPSCs | 11.6% (0.9-fold) | [104] |
| CALD1 | nCas9 | iPSCs | 5.5% (1-fold) | ||
| KATNA1 | wtCas9 | iPSCs | 3.4% (0.9-fold) | ||
| KATNA1 | nCas9 | iPSCs | 5.6% (1-fold) | ||
| SLITRK1 | wtCas9 | iPSCs | 3.9% (0.7-fold) | ||
| SLITRK1 | nCas9 | iPSCs | 4.6% (0.9-fold) | ||
| STL127685 | CALD1 | wtCas9 | iPSCs | 12.8% (1-fold) | [104] |
| CALD1 | nCas9 | iPSCs | 7.4% (0.9-fold) | ||
| KATNA1 | wtCas9 | iPSCs | 2.7% (0.8-fold) | ||
| KATNA1 | nCas9 | iPSCs | 6.4% (0.9-fold) | ||
| SLITRK1 | wtCas9 | iPSCs | 4.9% (1-fold) | ||
| SLITRK1 | nCas9 | iPSCs | 4.9% (0.9-fold) | ||
| M3814 | EEF1A1 | wtCas9 | iPSCs | 40% (2.9-fold) | [107] |
| EEF2 | wtCas9 | T cells | 70% (2-fold) | ||
| LAG3 | wtCas9 | T cells | 2.8-fold | [109] | |
| AZD7648 | CCR5 | wtCas9 | CD34+ | 3-fold | [111] |
| CCR5 | wtCas9 | iPSCs | 8.5-fold | ||
| CCR5 | wtCas9 | T cells | 7-fold | ||
| HBB | wtCas9 | CD34+ | 2.6-fold | ||
| STING1 | wtCas9 | CD34+ | 2.3-fold | ||
| STING1 | wtCas9 | iPSCs | 50-fold | ||
| STING1 | wtCas9 | CD34+ | 6-fod | ||
| E1B55K and E4orf6 | Rosa26 | wtCas9 | NIH3T3 | 8-fold | [115] |
| siRNA targeting Ku70/Ku80 | B-actin | wtCas9 | PFF | 5.6-fold | [117] |
| H11 | wtCas9 | PFF | 1.9-fold | ||
| shRNA targeting Ku70 | Rosa26 | wtCas9 | NIH3T3 | 2.1-fold | [115] |
| shRNA targeting LIG4 | Rosa26 | wtCas9 | NIH3T3 | 2.8-fold | |
| shRNA targeting Ku70/Ku80 | Rosa26 | wtCas9 | NIH3T3 | 3.0-fold | |
| shRNA targeting Ku80/LIG4 | Rosa26 | wtCas9 | NIH3T3 | 3.8-fold | |
| shRNA targeting Ku70/LIG4 | Rosa26 | wtCas9 | NIH3T3 | 5-fold | |
| miRNA-21 | SOX2 | wtCas9 | iPSCs | 3-fold | [8] |
| RS-1 | RLL | wtCas9 | R-Em | 26.1% (6-fold) | [122] |
| CFTR | wtCas9 | R-Em | 30% (2.4-fold) | ||
| Trichostatin A | HIST1H2BJ | wtCas9 | iPSCs | 4-fold | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, A.F.; Herreno-Pachón, A.M.; Benincore-Flórez, E.; Karunathilaka, A.; Tomatsu, S. Current Strategies for Increasing Knock-In Efficiency in CRISPR/Cas9-Based Approaches. Int. J. Mol. Sci. 2024, 25, 2456. https://doi.org/10.3390/ijms25052456
Leal AF, Herreno-Pachón AM, Benincore-Flórez E, Karunathilaka A, Tomatsu S. Current Strategies for Increasing Knock-In Efficiency in CRISPR/Cas9-Based Approaches. International Journal of Molecular Sciences. 2024; 25(5):2456. https://doi.org/10.3390/ijms25052456
Chicago/Turabian StyleLeal, Andrés Felipe, Angelica María Herreno-Pachón, Eliana Benincore-Flórez, Amali Karunathilaka, and Shunji Tomatsu. 2024. "Current Strategies for Increasing Knock-In Efficiency in CRISPR/Cas9-Based Approaches" International Journal of Molecular Sciences 25, no. 5: 2456. https://doi.org/10.3390/ijms25052456
APA StyleLeal, A. F., Herreno-Pachón, A. M., Benincore-Flórez, E., Karunathilaka, A., & Tomatsu, S. (2024). Current Strategies for Increasing Knock-In Efficiency in CRISPR/Cas9-Based Approaches. International Journal of Molecular Sciences, 25(5), 2456. https://doi.org/10.3390/ijms25052456








