The Proteome of Extracellular Vesicles Released from Pulmonary Microvascular Endothelium Reveals Impact of Oxygen Conditions on Biotrauma
Abstract
1. Introduction
2. Results
2.1. Proteomic Analysis of Extracellular Vesicles
2.2. Functional Enrichment Analysis
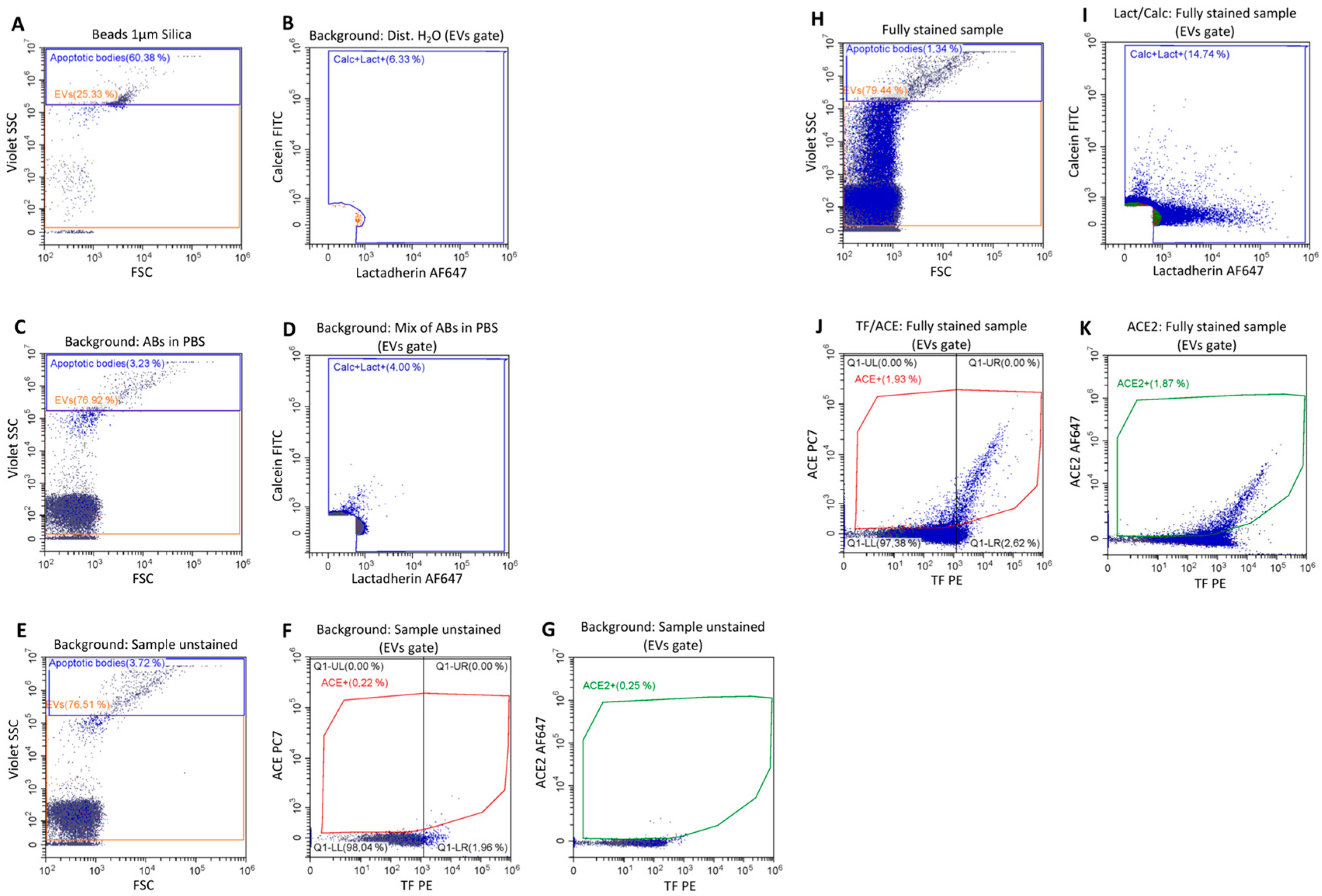
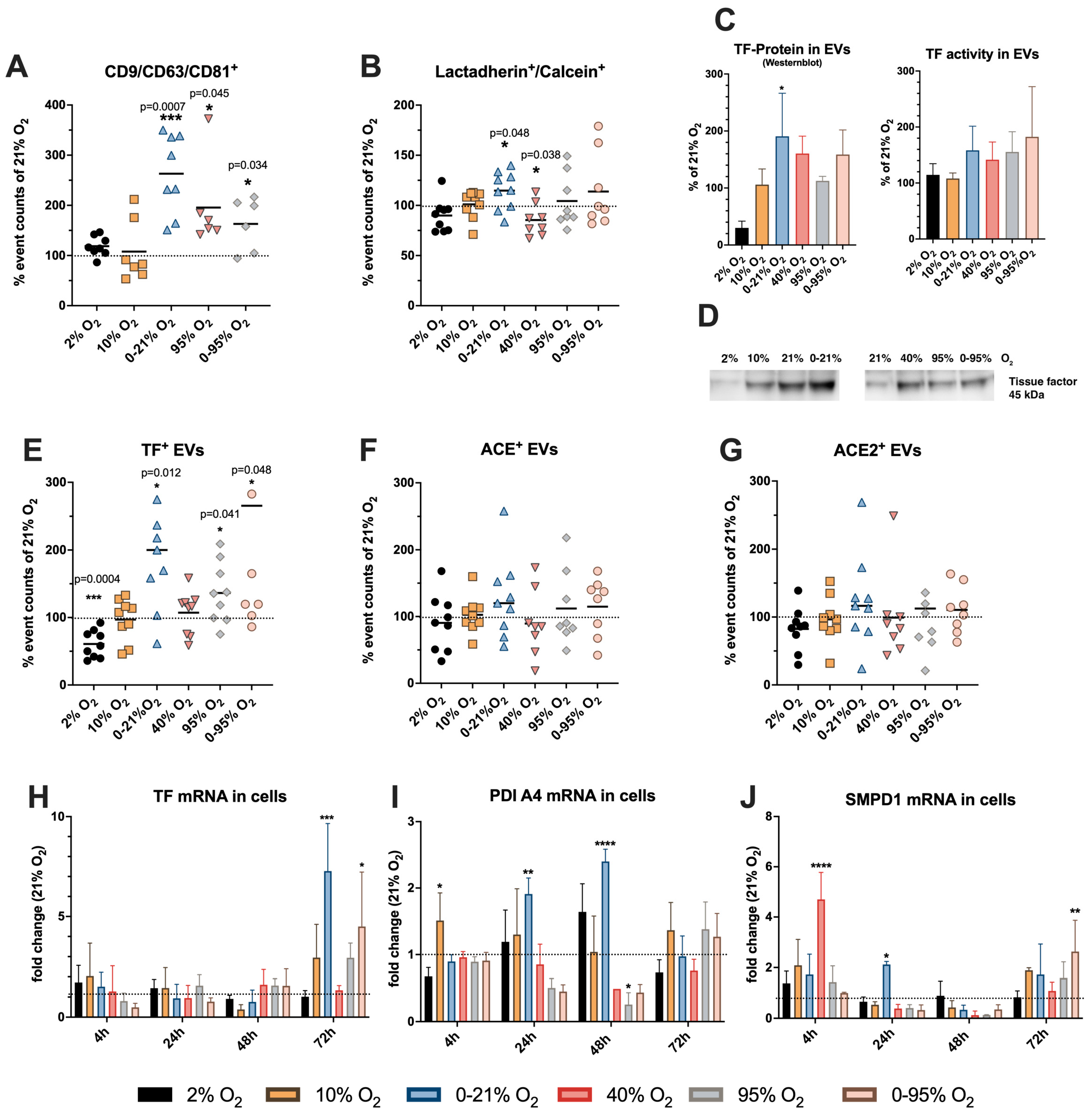
2.3. Release of Different Types of Vesicles as Detected by Flow Cytometry
2.4. Cellular Expression of mRNA of Tissue Factor and Its Activity Regulators SMPD1 and PDI
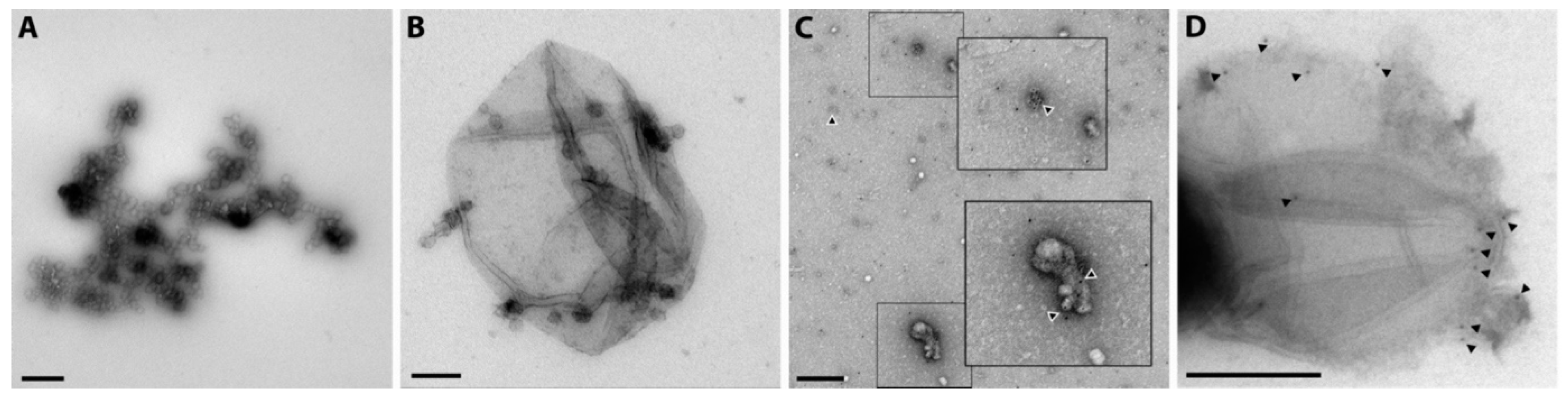
2.5. Release of Different Types of Vesicles as Detected by Transmission Electron Microscopy Presence of Tissue Factor
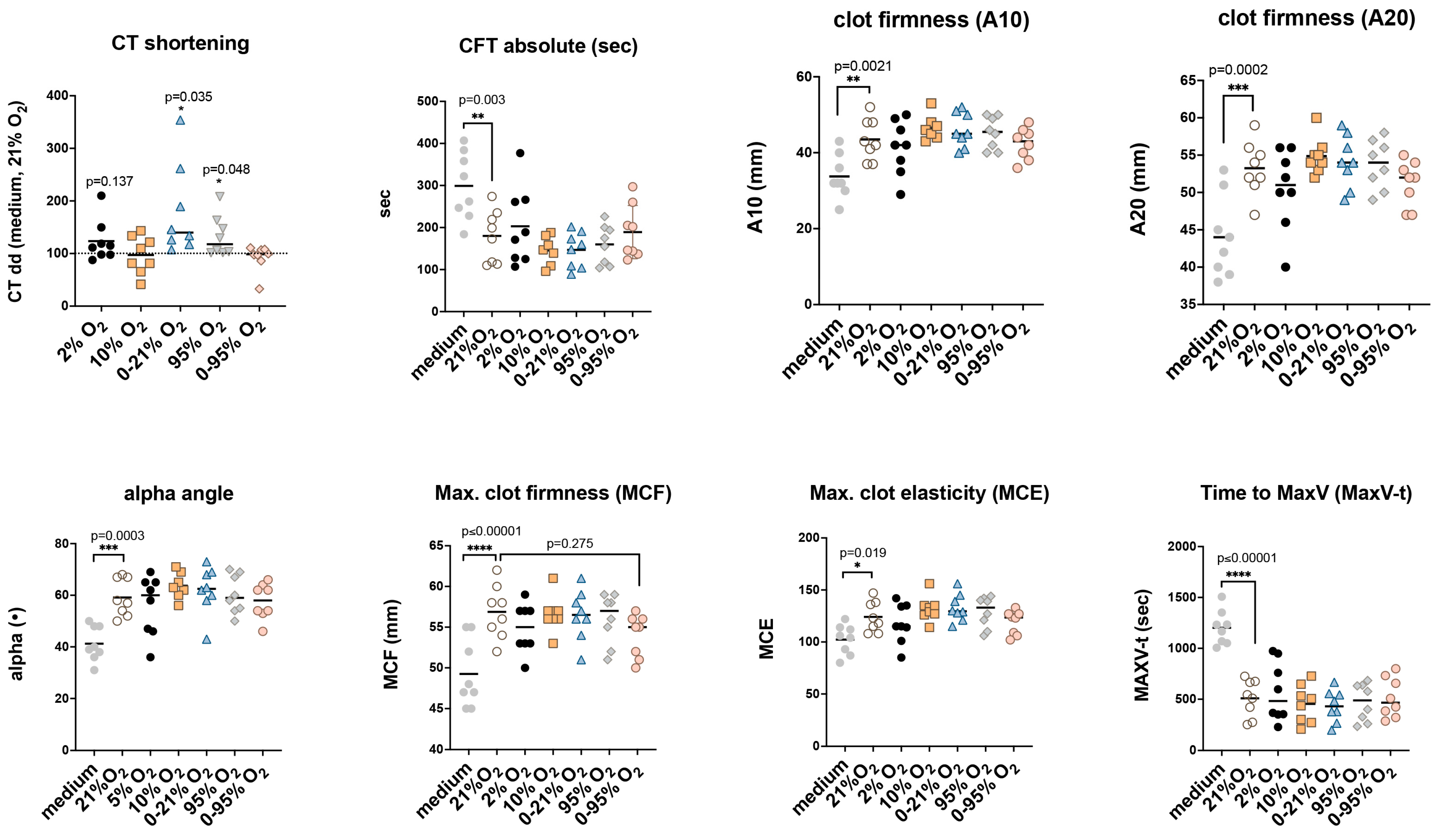
2.6. Functional Implications of Tissue Factor in Extracellular Vesicles: Non-Activated Thromboelastometry
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Gas Exposure
4.2. Isolation of Extracellular Vesicles
4.3. Proteomic Analysis
4.3.1. Sample Preparation and LC-MSMS Analysis
4.3.2. Mass Spectrometry Analysis
4.3.3. Data Analysis, Statistics
4.4. Pathway Enrichment and Protein Network Analysis
4.5. Flow Cytometry Analysis of EVs
EV Staining Protocol for Flow Cytometry
4.6. Tissue Factor Activity Assay
4.7. Nanoparticle Tracking Analysis (NTA)
4.8. Non-Activated Thromboelastometry Test (NATEM)
4.9. Transmission Electron Microscopy
4.10. Real-Time Quantitative Polymerase Chain Reaction
- Actb forward: (AGACGCAGGATGGCATGGG); Actb reverse (GAGACCTTCAACACCCCAGCC)
- F3(TF) forward (GGCGCTTCAGGCACTACAA); F3(TF) reverse (TTGATTGACGGGTTTGGGTTC)
- PDIA4 forward (GGCAGGCTGTAGACTACGAG); PDIA4 reverse (TTGGTCAACACAAGCGTGACT)
- SMPD1 forward (CTGTCTGACTCTCGGGTTCTC); SMPD1 reverse (CTATGCGATGTAACCTGGCAG)
4.11. Western Blot Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest 2016, 150, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.U.; Boehme, S.; Hartmann, E.K.; Szczyrba, M.; Heylen, L.; Liu, T.; David, M.; Werner, C.; Markstaller, K.; Engelhard, K. Transmission of arterial oxygen partial pressure oscillations to the cerebral microcirculation in a porcine model of acute lung injury caused by cyclic recruitment and derecruitment. Br. J. Anaesth. 2013, 110, 266–273. [Google Scholar] [CrossRef][Green Version]
- Thomas, R.; Mollmann, C.; Ziebart, A.; Liu, T.; David, M.; Hartmann, E.K. Cyclic PaO2 oscillations assessed in the renal microcirculation: Correlation with tidal volume in a porcine model of lung lavage. BMC Anesthesiol. 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.; Hartmann, E.K.; Tripp, T.; Thal, S.C.; David, M.; Abraham, D.; Baumgardner, J.E.; Markstaller, K.; Klein, K.U. PO2 oscillations induce lung injury and inflammation. Crit. Care 2019, 23, 102. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, J.; Nanduri, R.P. Cellular mechanisms associated with intermittent hypoxia. Essays Biochem. 2007, 43, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef]
- Morimoto, M.; Maishi, N.; Hida, K. Acquisition of drug resistance in endothelial cells by tumor-derived extracellular vesicles and cancer progression. Cancer Drug Resist. 2024, 7, 1. [Google Scholar] [CrossRef]
- Wang, S.E. Extracellular Vesicles and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, 102. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lischnig, A.; Bergqvist, M.; Ochiya, T.; Lasser, C. Quantitative Proteomics Identifies Proteins Enriched in Large and Small Extracellular Vesicles. Mol. Cell. Proteom. 2022, 21, 100273. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Biol. 2019, 2, 325. [Google Scholar] [CrossRef] [PubMed]
- Mallia, A.; Gianazza, E.; Zoanni, B.; Brioschi, M.; Barbieri, S.S.; Banfi, C. Proteomics of Extracellular Vesicles: Update on Their Composition, Biological Roles and Potential Use as Diagnostic Tools in Atherosclerotic Cardiovascular Diseases. Diagnostics 2020, 10, 843. [Google Scholar] [CrossRef]
- Tesovnik, T.; Jenko Bizjan, B.; Sket, R.; Debeljak, M.; Battelino, T.; Kovac, J. Technological Approaches in the Analysis of Extracellular Vesicle Nucleotide Sequences. Front. Bioeng. Biotechnol. 2021, 9, 787551. [Google Scholar] [CrossRef]
- Tirlapur, N. The Role of Microvesicles in Systemic Inflammatory Signalling in Acute Distress Syndrome. Ph.D. Thesis, Imperial College London, London, UK, 2019. [Google Scholar]
- Kornilov, R.; Puhka, M.; Mannerstrom, B.; Hiidenmaa, H.; Peltoniemi, H.; Siljander, P.; Seppanen-Kaijansinkko, R.; Kaur, S. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2018, 7, 1422674. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef]
- Holnthoner, W.; Bonstingl, C.; Hromada, C.; Muehleder, S.; Zipperle, J.; Stojkovic, S.; Redl, H.; Wojta, J.; Schochl, H.; Grillari, J.; et al. Endothelial Cell-derived Extracellular Vesicles Size-dependently Exert Procoagulant Activity Detected by Thromboelastometry. Sci. Rep. 2017, 7, 3707. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, D.; Laskin, D.L.; Jin, Y. Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation. J. Immunol. 2018, 201, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Osterud, B.; Latysheva, N.; Schoergenhofer, C.; Jilma, B.; Hansen, J.B.; Snir, O. A rapid, sensitive, and specific assay to measure TF activity based on chromogenic determination of thrombin generation. J. Thromb. Haemost. 2022, 20, 866–876. [Google Scholar] [CrossRef]
- El-Shennawy, L.; Hoffmann, A.D.; Dashzeveg, N.K.; McAndrews, K.M.; Mehl, P.J.; Cornish, D.; Yu, Z.; Tokars, V.L.; Nicolaescu, V.; Tomatsidou, A.; et al. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat. Commun. 2022, 13, 405. [Google Scholar] [CrossRef]
- Takei, Y.; Yamada, M.; Saito, K.; Kameyama, Y.; Sugiura, H.; Makiguchi, T.; Fujino, N.; Koarai, A.; Toyama, H.; Saito, K.; et al. Increase in circulating ACE-positive endothelial microparticles during acute lung injury. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Simioni, P.; Campello, E.; Spiezia, L.; Gavasso, S.; Bizzaro, D.; Cardin, R.; D’Amico, F.; Gringeri, E.; Cillo, U.; et al. Endothelial Damage of the Portal Vein is Associated with Heparin-Like Effect in Advanced Stages of Cirrhosis. Thromb. Haemost. 2020, 120, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, P.; Sokou, R.; Tsantes, A.G.; Parastatidou, S.; Konstantinidi, A.; Houhoula, D.; Kokoris, S.; Iacovidou, N.; Tsantes, A.E. The Non-Activated Thromboelastometry (NATEM) Assay’s Application among Adults and Neonatal/Pediatric Population: A Systematic Review. Diagnostics 2022, 12, 658. [Google Scholar] [CrossRef]
- Millar, F.R.; Summers, C.; Griffiths, M.J.; Toshner, M.R.; Proudfoot, A.G. The pulmonary endothelium in acute respiratory distress syndrome: Insights and therapeutic opportunities. Thorax 2016, 71, 462–473. [Google Scholar] [CrossRef]
- Hisada, Y.; Sachetto, A.T.A.; Mackman, N. Circulating tissue factor-positive extracellular vesicles and their association with thrombosis in different diseases. Immunol. Rev. 2022, 312, 61–75. [Google Scholar] [CrossRef]
- McVey, M.; Tabuchi, A.; Kuebler, W.M. Microparticles and acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L364–L381. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Du, Y.; Peng, L.; Qin, Y.; Liu, H.; Ma, X.; Wei, Y. Extracellular vesicle microRNA cargoes from intermittent hypoxia-exposed cardiomyocytes and their effect on endothelium. Biochem. Biophys. Res. Commun. 2021, 548, 182–188. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, M.; Chen, J.; Li, S.; Deng, Y.; Yang, S.; Ou, Q.; Li, J.; Gao, P.; Luo, Z.; et al. Extracellular vesicles derived from lung cancer cells exposed to intermittent hypoxia upregulate programmed death ligand 1 expression in macrophages. Sleep. Breath. 2022, 26, 893–906. [Google Scholar] [CrossRef]
- Klein, K.U.; Hartmann, E.K.; Boehme, S.; Szczyrba, M.; Heylen, L.; Liu, T.; David, M.; Werner, C.; Markstaller, K.; Engelhard, K. PaO2 oscillations caused by cyclic alveolar recruitment can be monitored in pig buccal mucosa microcirculation. Acta Anaesthesiol. Scand. 2013, 57, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Ruf, W.; Riewald, M. Regulation of Tissue Factor Expression. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Koizume, S.; Miyagi, Y. Tissue factor in cancer-associated thromboembolism: Possible mechanisms and clinical applications. Br. J. Cancer 2022, 127, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Pendurthi, U.R.; Rao, L.V.M. Role of Cell Surface Lipids and Thiol-Disulphide Exchange Pathways in Regulating the Encryption and Decryption of Tissue Factor. Thromb. Haemost. 2019, 119, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Abid Hussein, M.N.; Boing, A.N.; Biro, E.; Hoek, F.J.; Vogel, G.M.; Meuleman, D.G.; Sturk, A.; Nieuwland, R. Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb. Res. 2008, 121, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Hisada, Y.; Grover, S.P.; Schug, W.J.; Paul, D.S.; Bergmeier, W.; Mackman, N. Decreased Platelet Reactivity and Function in a Mouse Model of Human Pancreatic Cancer. Thromb. Haemost. 2023, 123, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Frantzeskaki, F.; Armaganidis, A.; Orfanos, S.E. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration 2017, 93, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Caporali, E.; Gauthier, L.G.; Pianezzi, E.; Balbi, C.; Rigamonti, E.; Bolis, S.; Lazzarini, E.; Biemmi, V.; Burrello, A.; et al. Risk stratification of patients with SARS-CoV-2 by tissue factor expression in circulating extracellular vesicles. Vasc. Pharmacol. 2022, 145, 106999. [Google Scholar] [CrossRef]
- Guervilly, C.; Bonifay, A.; Burtey, S.; Sabatier, F.; Cauchois, R.; Abdili, E.; Arnaud, L.; Lano, G.; Pietri, L.; Robert, T.; et al. Dissemination of extreme levels of extracellular vesicles: Tissue factor activity in patients with severe COVID-19. Blood Adv. 2021, 5, 628–634. [Google Scholar] [CrossRef]
- Wang, J.; Pendurthi, U.R.; Yi, G.; Rao, L.V.M. SARS-CoV-2 infection induces the activation of tissue factor-mediated coagulation via activation of acid sphingomyelinase. Blood 2021, 138, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 6522. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Khatun, M.; Steele, R.; Isbell, T.S.; Ray, R.; Ray, R.B. Exosomes from COVID-19 Patients Carry Tenascin-C and Fibrinogen-beta in Triggering Inflammatory Signals in Cells of Distant Organ. Int. J. Mol. Sci. 2021, 22, 3184. [Google Scholar] [CrossRef]
- Moraes, E.; Martins-Goncalves, R.; da Silva, L.R.; Mandacaru, S.C.; Melo, R.M.; Azevedo-Quintanilha, I.; Perales, J.; Bozza, F.A.; Souza, T.M.L.; Castro-Faria-Neto, H.C.; et al. Proteomic Profile of Procoagulant Extracellular Vesicles Reflects Complement System Activation and Platelet Hyperreactivity of Patients with Severe COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 926352. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, J.; Wang, N.; Yuan, G.; Khan, S.A.; Souvannakitti, D.; Peng, Y.J.; Kumar, G.K.; Garcia, J.A.; Prabhakar, N.R. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc. Natl. Acad. Sci. USA 2009, 106, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Ngo, J.M.; Lehman, I.M.; Schekman, R. Annexin A6 mediates calcium-dependent exosome secretion during plasma membrane repair. eLife 2023, 12, 86556. [Google Scholar] [CrossRef]
- Castillo-Galan, S.; Arenas, G.A.; Reyes, R.V.; Krause, B.J.; Iturriaga, R. Stim-activated TRPC-ORAI channels in pulmonary hypertension induced by chronic intermittent hypoxia. Pulm. Circ. 2020, 10, 13–22. [Google Scholar] [CrossRef]
- Sanz-Rubio, D.; Khalyfa, A.; Qiao, Z.; Ullate, J.; Marin, J.M.; Kheirandish-Gozal, L.; Gozal, D. Cell-Selective Altered Cargo Properties of Extracellular Vesicles Following In Vitro Exposures to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 5604. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Jose, P.A.; Yang, J.; Zeng, C. Importance of extracellular vesicles in hypertension. Exp. Biol. Med. 2021, 246, 342–353. [Google Scholar] [CrossRef]
- Tey, S.K.; Lam, H.; Wong, S.W.K.; Zhao, H.; To, K.K.; Yam, J.W.P. ACE2-enriched extracellular vesicles enhance infectivity of live SARS-CoV-2 virus. J. Extracell. Vesicles 2022, 11, e12231. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Fernandes, L.; Rocha, V.B.; Carregari, V.C.; Urbani, A.; Palmisano, G. A Perspective on Extracellular Vesicles Proteomics. Front. Chem. 2017, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Tiboldi, A.; Fuhrer, J.; Schaubmayr, W.; Hunyadi-Gulyas, E.; Zach, M.L.; Hochreiter, B.; Spittler, A.; Ullrich, R.; Markstaller, K.; Altmann, F.; et al. Oxygen-Dependent Changes in the N-Glycome of Murine Pulmonary Endothelial Cells. Antioxidants 2021, 10, 1947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—a Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wisgrill, L.; Lamm, C.; Hartmann, J.; Preissing, F.; Dragosits, K.; Bee, A.; Hell, L.; Thaler, J.; Ay, C.; Pabinger, I.; et al. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytom. A 2016, 89, 663–672. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Neville, D.M., Jr.; Glossmann, H. Plasma membrane protein subunit composition. A comparative study by discontinuous electrophoresis in sodium dodecyl sulfate. J. Biol. Chem. 1971, 246, 6335–6338. [Google Scholar] [CrossRef]
- Glossmann, H.; Neville, D.M., Jr. Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J. Biol. Chem. 1971, 246, 6339–6346. [Google Scholar] [CrossRef]


| 2% O2 | 10% O2 | 0–21% O2 | 40% O2 | 95% O2 | 0–95% O2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDIA4 | SMPD1 | PDIA4 | SMPD1 | PDIA4 | SMPD1 | PDIA4 | SMPD1 | PDIA4 | SMPD1 | PDIA4 | SMPD1 | |
| 4 h | 0.7 ± 0.01 | 1.4 ± 0.48 | 1.5 ± 0.42 | 2.1 ± 1.03 | 0.9 ± 0.10 | 1.7 ± 0.80 | 0.9 ± 0.08 | 4.7 ± 1.10 | 0.9 ± 0.07 | 1.4 ± 0.64 | 0.9 ± 0.12 | 1.0 ± 0.03 |
| 24 h | 1.2 ± 0.40 | 0.7 ± 0.18 | 1.3 ± 0.69 | 0.5 ± 0.12 | 1.9 ± 0.2 | 2.1 ± 0.12 * | 0.8 ± 0.3 | 0.4 ± 0.16 | 0.5 ± 0.14 * | 0.4 ± 0.13 | 0.5 ± 0.10 * | 0.3 ± 0.20 |
| 48 h | 1.6 ± 0.42 | 0.9 ± 0.56 | 1.0 ± 0.54 | 0.4 ± 0.27 | 2.4 ± 0.18 * | 0.3 ± 0.18 * | 0.5 ± 0.01 | 0.1 ± 0.16 | 0.3 ± 0.16 | 0.1 ± 0.01 ** | 0.4 ± 0.12 | 0.4 ± 0.18 |
| 72 h | 0.7 ± 0.19 | 0.8 ± 0.25 | 1.4 ± 0.42 | 1.9 ± 0.09 * | 0.9 ± 0.30 | 1.7 ± 1.20 | 0.8 ± 0.17 | 1.1 ± 0.34 | 1.4 ± 0.41 | 1.6 ± 0.64 | 1.3 ± 0.35 | 2.6 ± 1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaubmayr, W.; Hochreiter, B.; Hunyadi-Gulyas, E.; Riegler, L.; Schmidt, K.; Tiboldi, A.; Moser, B.; Klein, K.U.; Krenn, K.; Scharbert, G.; et al. The Proteome of Extracellular Vesicles Released from Pulmonary Microvascular Endothelium Reveals Impact of Oxygen Conditions on Biotrauma. Int. J. Mol. Sci. 2024, 25, 2415. https://doi.org/10.3390/ijms25042415
Schaubmayr W, Hochreiter B, Hunyadi-Gulyas E, Riegler L, Schmidt K, Tiboldi A, Moser B, Klein KU, Krenn K, Scharbert G, et al. The Proteome of Extracellular Vesicles Released from Pulmonary Microvascular Endothelium Reveals Impact of Oxygen Conditions on Biotrauma. International Journal of Molecular Sciences. 2024; 25(4):2415. https://doi.org/10.3390/ijms25042415
Chicago/Turabian StyleSchaubmayr, Wolfgang, Beatrix Hochreiter, Eva Hunyadi-Gulyas, Louise Riegler, Katy Schmidt, Akos Tiboldi, Bernhard Moser, Klaus U. Klein, Katharina Krenn, Gisela Scharbert, and et al. 2024. "The Proteome of Extracellular Vesicles Released from Pulmonary Microvascular Endothelium Reveals Impact of Oxygen Conditions on Biotrauma" International Journal of Molecular Sciences 25, no. 4: 2415. https://doi.org/10.3390/ijms25042415
APA StyleSchaubmayr, W., Hochreiter, B., Hunyadi-Gulyas, E., Riegler, L., Schmidt, K., Tiboldi, A., Moser, B., Klein, K. U., Krenn, K., Scharbert, G., Mohr, T., Schmid, J. A., Spittler, A., & Tretter, V. (2024). The Proteome of Extracellular Vesicles Released from Pulmonary Microvascular Endothelium Reveals Impact of Oxygen Conditions on Biotrauma. International Journal of Molecular Sciences, 25(4), 2415. https://doi.org/10.3390/ijms25042415









