Future-Proofing Agriculture: De Novo Domestication for Sustainable and Resilient Crops
Abstract
1. Introduction
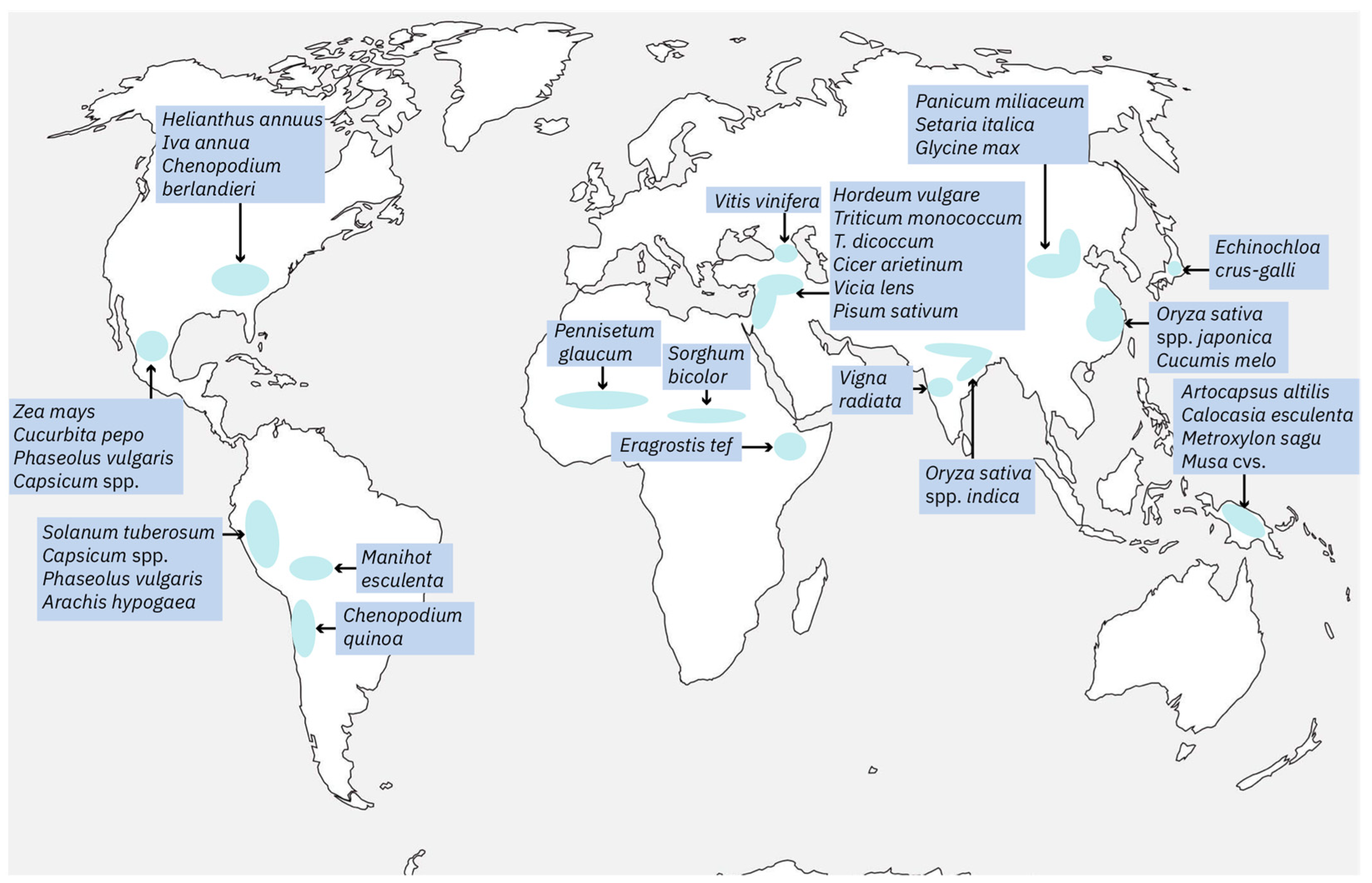
2. The Origin of Gene Domestication
| Crop | Gene | Sequence Variation | Phenotype | References |
|---|---|---|---|---|
| Wheat | APETALA 2-LIKE (WAP2, Q) | Single amino acid variation | Free-threshing | [58] |
| REDUCED HEIGHT 1 (Rht1) | In-frame insertion | Semidwarfism | [69] | |
| TENACIOUS GLUME (Tg1) | - | Free-threshing | [70,71] | |
| VERNALIZATION1 (Vrn1) | Mutation in the regulatory region | Vernalization response | [72] | |
| Rice | QTL of seed shattering in chromosome 1 (qSH1) | SNP in the 5′-regulatory region | Grain shattering | [60] |
| SHATTERING 4 (sh4) | Amino acid substitution | Grain shattering | [38,61] | |
| SHATTERING ABORTION 1 (shat1) | Frameshift mutation | Grain shattering | [73] | |
| PROSTRATE GROWTH 1 (PROG1) | SNPs in the coding region | Plant architecture | [74,75] | |
| GRAIN INCOMPLETE FILLING 1 (GIF1) | Mutation in the regulatory region | Grain-filling | [76] | |
| Maize | TEOSINTE BRANCHED 1 (Tb1) | Retrotransposon insertion in the regulatory region | Plant architecture and photosynthesis | [47,62] |
| ZmSWEET4c | SNPs in the promoter region | Grain-filling | [77] | |
| TEOSINTE GLUME ARCHITECTURE 1(Tga1) | SNP | Grain development | [78,79] | |
| ramosa1 (ra1) | Mutation in the regulatory region | Plant architecture | [80] | |
| LIGULELESS1 (Lg1) | Transposon insertion | Plant architecture | [81] | |
| Tomato | MULTIFLORA (S) | Missense mutation | Inflorescence development | [63] |
| SUN | Gene duplication mediated by retrotransposon | Fruit shape | [82] | |
| fasciated (fas) | Large insertion (6–8 kb) in the first intron | Locule number | [83] | |
| locule number (lc) | Two SNPs 1200 bp downstream a stop codon | Locule number | [84] | |
| SELF-PRUNING (SP) | Amino acid substitution | Growth habit | [85] | |
| OVATE | Premature stop codon | Fruit shape | [86] | |
| FRUIT WEIGHT 2.2 (Fw2.2) | Mutations in coding and upstream region | Fruit size | [87] | |
| Soybean | Dt1 (ortholog of GmTERMINAL FLOWER 1) | SNP: substitution from Arg in the Dt1 allele to Trp in the dt1 allele at residue 166 | Growth habit | [88] |
| Time of Flowering 11 (Tof11) and Tof12 | - | Control flowering time at maturity | [64] | |
| Protein, Oil, Weight, Regulator 1 (POWR1) | Transposon insertion | Grain-filling | [65] | |
| Grapevine | SWEET1 | - | Berry sugar content | [66] |
| Anthocyanin biosynthesis regulator in Vitis labrusca (VlmybA1) | Gret1 retrotransposon insertion | Fruit colour | [67] | |
| Vvi AINTEGUMENTA-like (VviANT1) | - | Fruit size | [89] | |
| Ethylene overproducer-1 (ETO1) | - | Putative candidate gene for the control of sexual traits in grapevine | [68] |
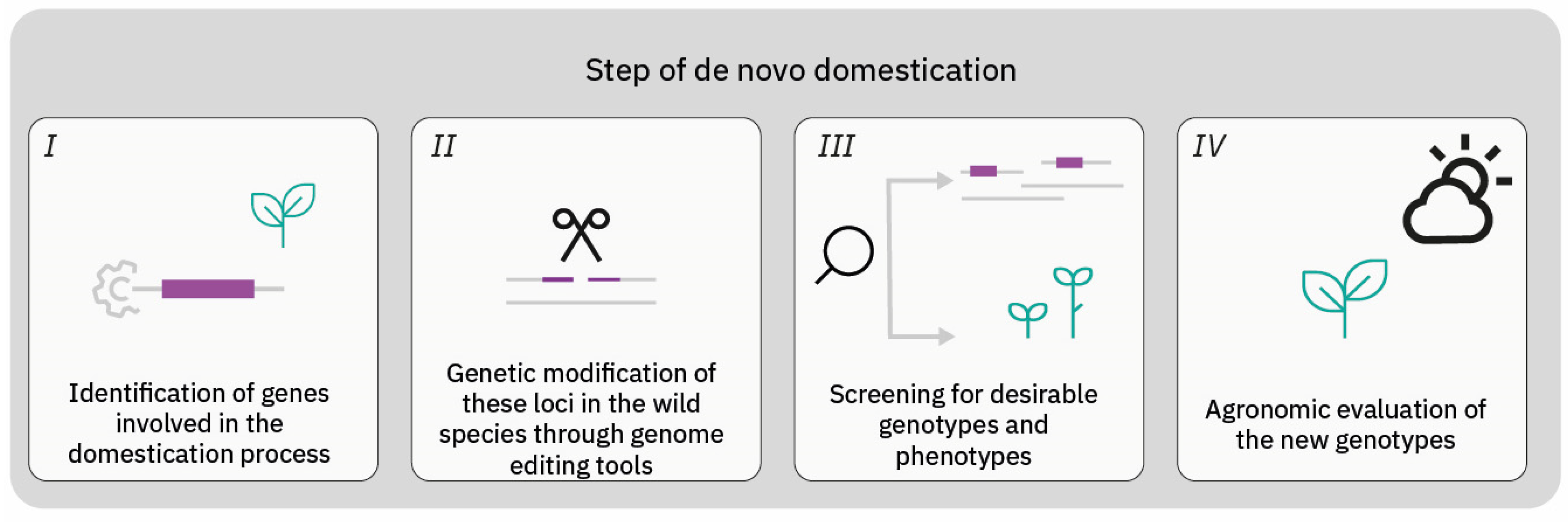
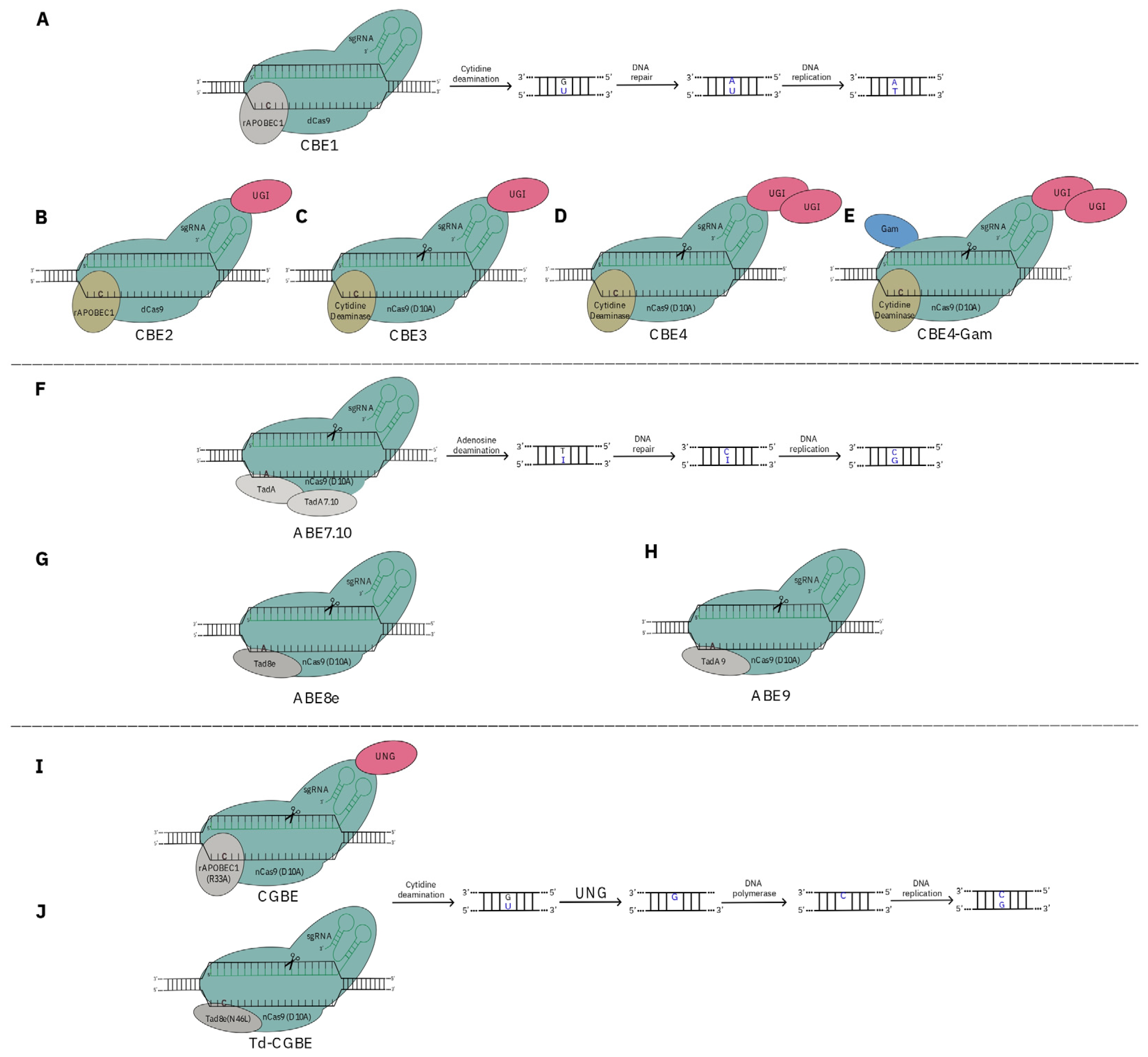
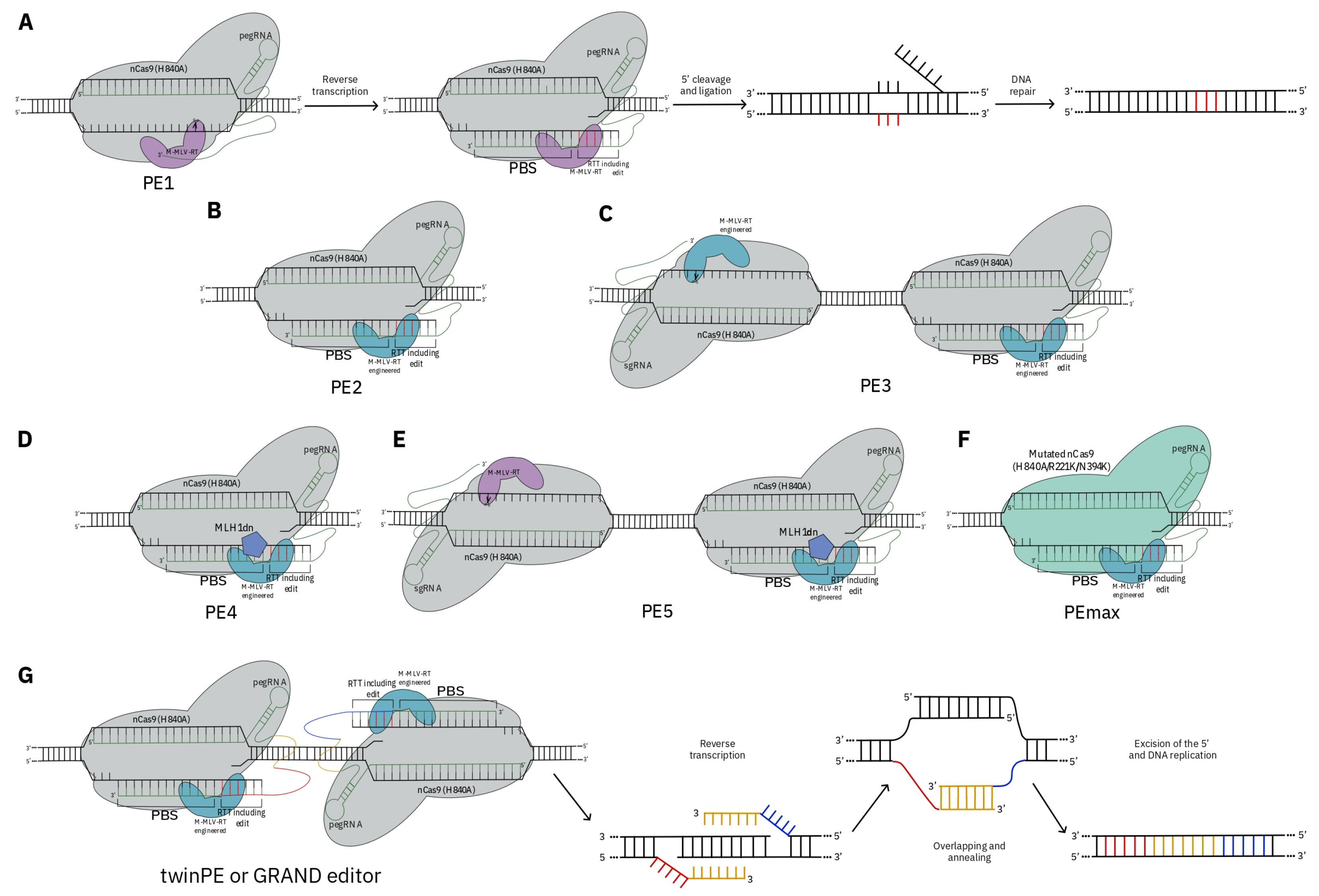
3. CRISPR as a Tool for De Novo Domestication
3.1. Trailblazing the Frontier: Pioneer Studies in De Novo Domestication of Plants through Genome Editing
3.2. New Delivery Techniques and Nanotechnology Advancements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABEs | adenine base editors |
| AP lyase | apurinic or apyrimidinic site lyase |
| BE | base editing |
| BER | base excision repair |
| Cas9 | CRISPR-associated protein 9 |
| CBEs | cytosine base editors |
| CGBEs | C-to g base editors |
| CNTs | carbon nano tubes |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| DSBs | double-strand breaks |
| GE | genome editing |
| HDR | Homologous-Directed Repair |
| HR | homologous recombination |
| MMEJ | microhomology-mediated end-joining |
| M-MLV-RT | Moloney murine leukaemia virus reverse transcriptase |
| NBTs | new breeding techniques |
| NGS | next-generation sequencing |
| NHEJ | Non-Homologous End Joining |
| NMD | nonsense-mediated mRNA decay |
| PBS | primer binding site |
| PE | prime editing |
| pegRNAs | prime editing guide RNAs |
| rAPOBEC1 | cytidine deaminase |
| RNPs | ribonucleoprotein particles |
| RTT | reverse transcription template |
| sgRNA | single-guide RNA |
| SSTR | single-strand template repair |
| TadA | wild-type adenine deaminase |
| TE | transposable element |
| twinPE | Twin prime editing |
| UGI | uracil DNA glycosylase inhibitor |
| UNG | uracil N-glycosylase |
| VIGE | virus-induced genome editing |
References
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Genetics of domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef]
- Dempewolf, H.; Hodgins, K.A.; Rummell, S.E.; Ellstrand, N.C.; Rieseberg, L.H. Reproductive isolation during domestication. Plant Cell 2012, 24, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J. Isozymic evidence and the evolution of crop plants. In Isozymes in Plant Biology; Soltis, D.E., Soltis, P.S., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 165–191. [Google Scholar]
- Kumar, K.; Mandal, S.N.; Pradhan, B.; Kaur, P.; Kaur, K.; Neelam, K. From evolution to revolution: Accelerating crop domestication through genome editing. Plant Cell Physiol. 2022, 63, 1607–1623. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front. Plant Sci. 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.R.; Devanna, B.N.; Kiran, K.; Singh, P.K.; Arora, K.; Jain, P.; Tiwari, I.M.; Dubey, H.; Saklani, B.; Kumari, M.; et al. Status and prospects of next generation sequencing technologies in crop plants. Curr. Issues Mol. Biol. 2018, 27, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, S.; Han, B.; Li, J. The integrated genomics of crop domestication and breeding. Cell 2022, 185, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple wheat genomes reveal global variation in modern breeding. Nature 2020, 588, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mauleon, R.; Hu, Z.; Chebotarov, D.; Tai, S.; Wu, Z.; Li, M.; Zheng, T.; Fuentes, R.R.; Zhang, F.; et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 2018, 557, 43–49. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, Q.; Lu, H.; Li, Y.; Wang, A.; Tian, Q.; Zhan, Q.; Lu, Y.; Zhang, L.; Huang, T.; et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018, 50, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2021, 39, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gonda, I.; Sun, H.; Ma, Q.; Bao, K.; Tieman, D.M.; Burzynski-Chang, E.A.; Fish, T.L.; Stromberg, K.A.; Sacks, G.L.; et al. The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 2019, 51, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Hübner, S.; Bercovich, N.; Todesco, M.; Mandel, J.R.; Odenheimer, J.; Ziegler, E.; Lee, J.S.; Baute, G.J.; Owens, G.L.; Grassa, C.J.; et al. Sunflower pan-genome analysis shows that hybridization altered gene content and disease resistance. Nat. Plants 2018, 5, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Yan, J. De novo domestication: An alternative route toward new crops for the future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Najafi, J.; Correia, P.M.P.; Trinh, M.D.L.; Chapman, E.A.; Østerberg, J.T.; Thomsen, H.C.; Pedas, P.R.; Larson, S.; Gao, C.; et al. Accelerated domestication of new crops: Yield is key. Plant Cell Physiol. 2022, 63, 1624–1640. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, K.; dos Reis Moreira, J.; Peres, L.E.P.; Zsögön, A. De novo domestication of wild species to create crops with increased resilience and nutritional value. Curr. Opin. Plant Biol. 2021, 60, 102006. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Li, J. De novo domestication: Retrace the history of agriculture to design future crops. Curr. Opin. Biotechnol. 2023, 81, 102946. [Google Scholar] [CrossRef]
- Rogo, U.; Fambrini, M.; Pugliesi, C. Embryo rescue in plant breeding. Plants 2023, 12, 3106. [Google Scholar] [CrossRef]
- Viviani, A.; Spada, M.; Giordani, T.; Fambrini, M.; Pugliesi, C. Origin of the genome editing systems: Application for crop improvement. Biologia (Bratisl) 2022, 77, 3353–3383. [Google Scholar] [CrossRef]
- Liu, M.; Rehman, S.; Tang, X.; Gu, K.; Fan, Q.; Chen, D.; Ma, W. Methodologies for improving HDR efficiency. Front. Genet. 2019, 9, 691. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef]
- Hilton, I.B.; D’Ippolito, A.M.; Vockley, C.M.; Thakore, P.I.; Crawford, G.E.; Reddy, T.E.; Gersbach, C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015, 33, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Vavilov, N.I. Origin and Geography of Cultivated Plants; Cambridge University Press: Cambridge, UK, 1926. [Google Scholar]
- Kantar, M.B.; Nashoba, A.R.; Anderson, J.E.; Blackman, B.K.; Rieseberg, L.H. The genetics and genomics of plant domestication. Bioscience 2017, 67, 971–982. [Google Scholar] [CrossRef]
- Schaal, B. Plants and people: Our shared history and future. PLANTS PEOPLE PLANET 2019, 1, 14–19. [Google Scholar] [CrossRef]
- Feldman, M.; Lupton, F.G.H.; and Miller, T.E. Wheats. In Evolution of Crop Plants, 2nd ed.; Smartt, J., Simmonds, N.W., Eds.; Longman Scientific: London, UK, 1995; pp. 184–192. [Google Scholar]
- Lev-Yadun, S.; Gopher, A.; Abbo, S. The cradle of agriculture. Science 2000, 288, 1602–1603. [Google Scholar] [CrossRef] [PubMed]
- Morrell, P.L.; Clegg, M.T. Genetic evidence for a second domestication of barley (Hordeum vulgare) east of the Fertile Crescent. Proc. Natl. Acad. Sci. USA 2007, 104, 3289–3294. [Google Scholar] [CrossRef]
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef]
- Abbo, S.; Pinhasi van-Oss, R.; Gopher, A.; Saranga, Y.; Ofner, I.; Peleg, Z. Plant domestication versus crop evolution: A conceptual framework for cereals and grain legumes. Trends Plant Sci. 2014, 19, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, M.; Monneveux, P. Cultivated einkorn wheat (Triticum monococcum L. subsp. monococcum): The long life of a founder crop of agriculture. Genet. Resour. Crop Evol. 2014, 61, 677–706. [Google Scholar] [CrossRef]
- Mascher, M.; Schuenemann, V.J.; Davidovich, U.; Marom, N.; Himmelbach, A.; Hübner, S.; Korol, A.; David, M.; Reiter, E.; Riehl, S.; et al. Genomic analysis of 6,000-year-old cultivated grain illuminates the domestication history of barley. Nat. Genet. 2016, 48, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Kislev, M.E.; Hartmann, A.; Bar-Yosef, O. Early domesticated fig in the Jordan Valley. Science 2006, 312, 1372–1374. [Google Scholar] [CrossRef]
- Li, C.; Zhou, A.; Sang, T. Rice domestication by reducing shattering. Science 2006, 311, 1936–1939. [Google Scholar] [CrossRef]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.-X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Haberer, G.; Marri, P.R.; Fan, C.; Goicoechea, J.L.; Zuccolo, A.; Song, X.; Kudrna, D.; Ammiraju, J.S.S.; et al. The genome sequence of African rice (Oryza glaberrima) and evidence for independent domestication. Nat. Genet. 2014, 46, 982–988. [Google Scholar] [CrossRef]
- Johnson, E. Archeological evidence for utilization of wild rice. Science 1969, 163, 276–277. [Google Scholar] [CrossRef]
- Winchell, F.; Stevens, C.J.; Murphy, C.; Champion, L.; Fuller, D.Q. Evidence for Sorghum domestication in fourth millennium BC Eastern Sudan: Spikelet morphology from ceramic impressions of the Butana Group. Curr. Anthropol. 2017, 58, 673–683. [Google Scholar] [CrossRef]
- Scarcelli, N.; Cubry, P.; Akakpo, R.; Thuillet, A.-C.; Obidiegwu, J.; Baco, M.N.; Otoo, E.; Sonké, B.; Dansi, A.; Djedatin, G.; et al. Yam genomics supports West Africa as a major cradle of crop domestication. Sci. Adv. 2019, 5, eaaw1947. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.Q.; Barron, A.; Champion, L.; Dupuy, C.; Commelin, D.; Raimbault, M.; Denham, T. Transition from wild to domesticated pearl illet (Pennisetum glaucum) revealed in ceramic temper at three Middle Holocene sites in Northern Mali. Afr. Archaeol. Rev. 2021, 38, 211–230. [Google Scholar] [CrossRef]
- Lira, R.; Eguiarte, L.; Montes, S.; Zizumbo-Villarreal, D.; Marín, P.C.-G.; Quesada, M. Homo sapiens–Cucurbita interaction in Mesoamerica: Domestication, dissemination, and diversification. In Ethnobotany of Mexico. Ethnobiology; Lira, R., Casas, A., Blancas, J., Eds.; Springer: New York, NY, USA, 2016; pp. 389–401. [Google Scholar]
- Doebley, J.; Stec, A.; Gustus, C. Teosinte branched1 and the origin of maize: Evidence for epistasis and the evolution of dominance. Genetics 1995, 141, 333–346. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Tang, S.; Knapp, S.J.; Rieseberg, L.H. Genetic analysis of sunflower domestication. Genetics 2002, 161, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Harter, A.V.; Gardner, K.A.; Falush, D.; Lentz, D.L.; Bye, R.A.; Rieseberg, L.H. Origin of extant domesticated sunflowers in eastern North America. Nature 2004, 430, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Demirel, U.; Yousaf, M.F.; Caliskan, S.; Caliskan, M.E. Overview on domestication, breeding, genetic gain and improvement of tuber quality traits of potato using fast forwarding technique (GWAS): A review. Plant Breed. 2021, 140, 519–542. [Google Scholar] [CrossRef]
- Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 2002, 418, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.Q.; Denham, T.; Arroyo-Kalin, M.; Lucas, L.; Stevens, C.J.; Qin, L.; Allaby, R.G.; Purugganan, M.D. Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proc. Natl. Acad. Sci. USA 2014, 111, 6147–6152. [Google Scholar] [CrossRef] [PubMed]
- Purugganan, M.D. What is domestication? Trends Ecol. Evol. 2022, 37, 663–671. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Zhang, D.; Liang, J. Endomembrane-biased dimerization of ABCG16 and ABCG25 transporters determines their substrate selectivity in ABA-regulated plant growth and stress responses. Mol. Plant 2024. [Google Scholar] [CrossRef]
- Chen, Q.; Li, W.; Tan, L.; Tian, F. Harnessing knowledge from maize and rice domestication for new crop breeding. Mol. Plant 2021, 14, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Lyzenga, W.J.; Pozniak, C.J.; Kagale, S. Advanced domestication: Harnessing the precision of gene editing in crop breeding. Plant Biotechnol. J. 2021, 19, 660–670. [Google Scholar] [CrossRef]
- Yu, H.; Li, J. Breeding future crops to feed the world through de novo domestication. Nat. Commun. 2022, 13, 1171. [Google Scholar] [CrossRef]
- Simons, K.J.; Fellers, J.P.; Trick, H.N.; Zhang, Z.; Tai, Y.-S.; Gill, B.S.; Faris, J.D. Molecular characterization of the major wheat domestication gene Q. Genetics 2006, 172, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Belcram, H.; Gornicki, P.; Charles, M.; Just, J.; Huneau, C.; Magdelenat, G.; Couloux, A.; Samain, S.; Gill, B.S.; et al. Duplication and partitioning in evolution and function of homoeologous Q loci governing domestication characters in polyploid wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 18737–18742. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Izawa, T.; Lin, S.Y.; Ebana, K.; Fukuta, Y.; Sasaki, T.; Yano, M. An SNP caused loss of seed shattering during rice domestication. Science 2006, 312, 1392–1396. [Google Scholar] [CrossRef]
- Lin, Z.; Griffith, M.E.; Li, X.; Zhu, Z.; Tan, L.; Fu, Y.; Zhang, W.; Wang, X.; Xie, D.; Sun, C. Origin of seed shattering in rice (Oryza sativa L.). Planta 2007, 226, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.; Zhao, Q.; Ross-Ibarra, J.; Doebley, J. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat. Genet. 2011, 43, 1160–1163. [Google Scholar] [CrossRef]
- Lippman, Z.B.; Cohen, O.; Alvarez, J.P.; Abu-Abied, M.; Pekker, I.; Paran, I.; Eshed, Y.; Zamir, D. The making of a compound inflorescence in tomato and related nightshades. PLoS Biol. 2008, 6, e288. [Google Scholar] [CrossRef]
- Lu, S.; Dong, L.; Fang, C.; Liu, S.; Kong, L.; Cheng, Q.; Chen, L.; Su, T.; Nan, H.; Zhang, D.; et al. Stepwise selection on homeologous PRR genes controlling flowering and maturity during soybean domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef]
- Goettel, W.; Zhang, H.; Li, Y.; Qiao, Z.; Jiang, H.; Hou, D.; Song, Q.; Pantalone, V.R.; Song, B.-H.; Yu, D.; et al. POWR1 is a domestication gene pleiotropically regulating seed quality and yield in soybean. Nat. Commun. 2022, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Massonnet, M.; Sanjak, J.S.; Cantu, D.; Gaut, B.S. Evolutionary genomics of grape (Vitis vinifera ssp. vinifera) domestication. Proc. Natl. Acad. Sci. USA 2017, 114, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon-induced mutations in grape skin color. Science 2004, 304, 982. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, D.; Bellido, A.; Vargas, A.M.; Picq, S.; Bacilieri, R.; This, P.; Arroyo-Garcia, R. Comparative analysis of the expression of sex candidate genes in flower of dioecious and hermaphrodite grapevine (Vitis vinifera L. ssp.). Sci. Hortic. 2020, 274, 109639. [Google Scholar] [CrossRef]
- Pearce, S.; Saville, R.; Vaughan, S.P.; Chandler, P.M.; Wilhelm, E.P.; Sparks, C.A.; Al-Kaff, N.; Korolev, A.; Boulton, M.I.; Phillips, A.L.; et al. Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat. Plant Physiol. 2011, 157, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Nalam, V.J.; Vales, M.I.; Watson, C.J.W.; Johnson, E.B.; Riera-Lizarazu, O. Map-based analysis of genetic loci on chromosome 2D that affect glume tenacity and threshability, components of the free-threshing habit in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 116, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Kuraparthy, V.; Bai, G.; Gill, B.S. The major threshability genes soft glume (sog) and tenacious glume (Tg), of diploid and polyploid wheat, trace their origin to independent mutations at non-orthologous loci. Teor. Appl. Genet. 2009, 119, 341–351. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, D.; Li, C.; Luo, J.; Zhu, B.-F.; Zhu, J.; Shangguan, Y.; Wang, Z.; Sang, T.; Zhou, B.; et al. Genetic control of seed shattering in rice by the APETALA2 transcription factor SHATTERING ABORTION1. Plant Cell 2012, 24, 1034–1048. [Google Scholar] [CrossRef]
- Jin, J.; Huang, W.; Gao, J.-P.; Yang, J.; Shi, M.; Zhu, M.-Z.; Luo, D.; Lin, H.-X. Genetic control of rice plant architecture under domestication. Nat. Genet. 2008, 40, 1365–1369. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.; Liu, F.; Sun, X.; Li, C.; Zhu, Z.; Fu, Y.; Cai, H.; Wang, X.; Xie, D.; et al. Control of a key transition from prostrate to erect growth in rice domestication. Nat. Genet. 2008, 40, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, J.; Zhu, X.; Hao, W.; Wang, L.; Li, Q.; Zhang, L.; He, W.; Lu, B.; Lin, H.; et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008, 40, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Sosso, D.; Luo, D.; Li, Q.-B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nussbaum-Wagler, T.; Li, B.; Zhao, Q.; Vigouroux, Y.; Faller, M.; Bomblies, K.; Lukens, L.; Doebley, J.F. The origin of the naked grains of maize. Nature 2005, 436, 714–719. [Google Scholar] [CrossRef]
- Wang, H.; Studer, A.J.; Zhao, Q.; Meeley, R.; Doebley, J.F. Evidence that the origin of naked kernels during maize domestication was caused by a single amino acid substitution in tga1. Genetics 2015, 200, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Sigmon, B.; Vollbrecht, E. Evidence of selection at the ramosa1 locus during maize domestication. Mol. Ecol. 2010, 19, 1296–1311. [Google Scholar] [CrossRef]
- Moreno, M.A.; Harper, L.C.; Krueger, R.W.; Dellaporta, S.L.; Freeling, M. Liguleless1 encodes a nuclear-localized protein required for induction of ligules and auricles during maize leaf organogenesis. Genes Dev. 1997, 11, 616–628. [Google Scholar] [CrossRef]
- Xiao, H.; Jiang, N.; Schaffner, E.; Stockinger, E.J.; van der Knaap, E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 2008, 319, 1527–1530. [Google Scholar] [CrossRef]
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef]
- Muños, S.; Ranc, N.; Botton, E.; Bérard, A.; Rolland, S.; Duffé, P.; Carretero, Y.; Le Paslier, M.-C.; Delalande, C.; Bouzayen, M.; et al. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiol. 2011, 156, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Pnueli, L.; Carmel-Goren, L.; Hareven, D.; Gutfinger, T.; Alvarez, J.; Ganal, M.; Zamir, D.; Lifschitz, E. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 1998, 125, 1979–1989. [Google Scholar] [CrossRef]
- Liu, J.; Van Eck, J.; Cong, B.; Tanksley, S.D. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc. Natl. Acad. Sci. USA 2002, 99, 13302–13306. [Google Scholar] [CrossRef]
- Frary, A.; Nesbitt, T.C.; Grandillo, S.; Knaap, E.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; Tanksley, S.D. Fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, X.; Lee, R.; Li, Y.; Specht, J.E.; Nelson, R.L.; McClean, P.E.; Qiu, L.; Ma, J. Artificial selection for determinate growth habit in soybean. Proc. Natl. Acad. Sci. USA 2010, 107, 8563–8568. [Google Scholar] [CrossRef]
- Chialva, C.; Eichler, E.; Grissi, C.; Muñoz, C.; Gomez-Talquenca, S.; Martínez-Zapater, J.M.; Lijavetzky, D. Expression of grapevine AINTEGUMENTA-like genes is associated with variation in ovary and berry size. Plant Mol. Biol. 2016, 91, 67–80. [Google Scholar] [CrossRef]
- Cardi, T.; Murovec, J.; Bakhsh, A.; Boniecka, J.; Bruegmann, T.; Bull, S.E.; Eeckhaut, T.; Fladung, M.; Galovic, V.; Linkiewicz, A.; et al. CRISPR/Cas-mediated plant genome editing: Outstanding challenges a decade after implementation. Trends Plant Sci. 2023, 28, 1144–1165. [Google Scholar] [CrossRef] [PubMed]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.E.; Dupuis, M.-È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Donohoue, P.D.; Barrangou, R.; May, A.P. Advances in industrial biotechnology using CRISPR-Cas systems. Trends Biotechnol. 2018, 36, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Al-Shayeb, B.; Skopintsev, P.; Soczek, K.M.; Stahl, E.C.; Li, Z.; Groover, E.; Smock, D.; Eggers, A.R.; Pausch, P.; Cress, B.F.; et al. Diverse virus-encoded CRISPR-Cas systems include streamlined genome editors. Cell 2022, 185, 4574–4586.e16. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Jiang, F.; Taylor, D.W.; Sternberg, S.H.; Kaya, E.; Ma, E.; Anders, C.; Hauer, M.; Zhou, K.; Lin, S.; et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 2014, 343, 1247997. [Google Scholar] [CrossRef]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- van der Oost, J.; Patinios, C. The genome editing revolution. Trends Biotechnol. 2023, 41, 396–409. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Bowater, R.; Doherty, A.J. Making ends meet: Repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2006, 2, e8. [Google Scholar] [CrossRef]
- Maquat, L.E. When cells stop making sense: Effects of nonsense codons on RNA metabolism in vertebrate cells. RNA 1995, 1, 453–465. [Google Scholar]
- Lalonde, S.; Stone, O.A.; Lessard, S.; Lavertu, A.; Desjardins, J.; Beaudoin, M.; Rivas, M.; Stainier, D.Y.R.; Lettre, G. Frameshift indels introduced by genome editing can lead to in-frame exon skipping. PLoS ONE 2017, 12, e0178700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; He, Y.; Li, S.; Yan, L.; Li, Y.; Zhu, Z.; Xia, L. Plant base editing and prime editing: The current status and future perspectives. J. Integr. Plant Biol. 2023, 65, 444–467. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774. [Google Scholar] [CrossRef] [PubMed]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef]
- Yan, D.; Ren, B.; Liu, L.; Yan, F.; Li, S.; Wang, G.; Sun, W.; Zhou, X.; Zhou, H. High-efficiency and multiplex adenine base editing in plants using new TadA variants. Mol. Plant 2021, 14, 722–731. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, B.; Ru, G.; Meng, H.; Yan, Y.; Hong, M.; Zhang, D.; Luan, C.; Zhang, S.; Wu, H.; et al. Re-engineering the adenine deaminase TadA-8e for efficient and specific CRISPR-based cytosine base editing. Nat. Biotechnol. 2023, 41, 663–672. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Du, J.; Zhao, Y.; Xia, L. Generation of targeted point mutations in rice by a modified CRISPR/Cas9 system. Mol. Plant 2017, 10, 526–529. [Google Scholar] [CrossRef]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Gosavi, G.; Ren, B.; Cao, Y.; Kuang, Y.; Zhou, C.; Spetz, C.; Yan, F.; Zhou, X.; et al. Targeted base editing in rice with CRISPR/ScCas9 system. Plant Biotechnol. J. 2020, 18, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Song, Q.; Li, C.; Jin, S.; Zhang, D.; Wang, Y.; Qiu, J.-L.; Gao, C. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 2018, 36, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Liu, T.; Tan, J.; Zhang, Y.; Zheng, Z.; Wang, B.; Zhou, D.; Xie, X.; Guo, M.; Liu, Y.-G.; et al. PhieCBEs: Plant high-efficiency cytidine base editors with expanded target range. Mol. Plant 2020, 13, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46. [Google Scholar] [CrossRef]
- Koblan, L.W.; Arbab, M.; Shen, M.W.; Hussmann, J.A.; Anzalone, A.V.; Doman, J.L.; Newby, G.A.; Yang, D.; Mok, B.; Replogle, J.M.; et al. Efficient C•G-to-G•C base editors developed using CRISPRi screens, target-library analysis, and machine learning. Nat. Biotechnol. 2021, 39, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shen, R.; Li, Z.; Yao, Q.; Zhang, X.; Zhong, D.; Tan, X.; Song, M.; Han, H.; Zhu, J.; et al. Efficient C-to-G editing in rice using an optimized base editor. Plant Biotechnol. J. 2022, 20, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zeng, D.; Zhao, Y.; Wang, Y.; Liu, T.; Li, S.; Xue, Y.; Luo, Y.; Xie, X.; Chen, L.; et al. PhieABEs: A PAM-less/free high-efficiency adenine base editor toolbox with wide target scope in plants. Plant Biotechnol. J. 2022, 20, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Lin, Q.; Gao, Q.; Gao, C. Optimized prime editing in monocot plants using PlantPegDesigner and engineered plant prime editors (ePPEs). Nat. Protoc. 2023, 18, 831–853. [Google Scholar] [CrossRef]
- Chen, P.J.; Hussmann, J.A.; Yan, J.; Knipping, F.; Ravisankar, P.; Chen, P.-F.; Chen, C.; Nelson, J.W.; Newby, G.A.; Sahin, M.; et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 2021, 184, 5635–5652.e29. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Gao, X.D.; Podracky, C.J.; Nelson, A.T.; Koblan, L.W.; Raguram, A.; Levy, J.M.; Mercer, J.A.M.; Liu, D.R. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat. Biotechnol. 2022, 40, 731–740. [Google Scholar] [CrossRef]
- Lemmon, Z.H.; Reem, N.T.; Dalrymple, J.; Soyk, S.; Swartwood, K.E.; Rodriguez-Leal, D.; Van Eck, J.; Lippman, Z.B. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 2018, 4, 766–770. [Google Scholar] [CrossRef]
- Kwon, C.-T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Lin, T.; Zhu, G.; Zhang, J.; Xu, X.; Yu, Q.; Zheng, Z.; Zhang, Z.; Lun, Y.; Li, S.; Wang, X.; et al. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 2014, 46, 1220–1226. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, T.; Huang, X.; Xu, C. A two-in-one breeding strategy boosts rapid utilization of wild species and elite cultivars. Plant Biotechnol. J. 2022, 20, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G.; Chen, M.; Jing, Y.; Kou, L.; Li, X.; et al. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, P.; Yu, H.; Meng, X.; Li, J. Protocol for genome editing in wild allotetraploid rice Oryza alta. STAR Protoc. 2022, 3, 101789. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.-Y.; Fan, L. Orphan crops and their wild relatives in the genomic era. Mol. Plant 2021, 14, 27–39. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; Tesdell, O.; Schlautman, B.; Rubin, M.J.; DeHaan, L.R.; Crews, T.E.; Streit Krug, A. New food crop domestication in the age of gene editing: Genetic, agronomic and cultural change remain co-evolutionarily entangled. Front. Plant Sci. 2020, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.; Tariq, A.; Bhat, B.A.; Bhat, K.A.; Nehvi, I.B.; Raza, A.; Djalovic, I.; Prasad, P.V.; Mir, R.A. Integrating genomics and genome editing for orphan crop improvement: A bridge between orphan crops and modern agriculture system. GM Crops Food 2023, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lao, S.; Fan, L. De-domestication: An extension of crop evolution. Trends Plant Sci. 2021, 26, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Pisias, M.T.; Bakala, H.S.; McAlvay, A.C.; Mabry, M.E.; Birchler, J.A.; Yang, B.; Pires, J.C. Prospects of feral crop de novo redomestication. Plant Cell Physiol. 2022, 63, 1641–1653. [Google Scholar] [CrossRef]
- Mabry, M.E.; Bagavathiannan, M.V.; Bullock, J.M.; Wang, H.; Caicedo, A.L.; Dabney, C.J.; Drummond, E.B.M.; Frawley, E.; Gressel, J.; Husband, B.C.; et al. Building a feral future: Open questions in crop ferality. PLANTS PEOPLE PLANET 2023, 5, 635–649. [Google Scholar] [CrossRef]
- Cao, X.; Xie, H.; Song, M.; Lu, J.; Ma, P.; Huang, B.; Wang, M.; Tian, Y.; Chen, F.; Peng, J.; et al. Cut–dip–budding delivery system enables genetic modifications in plants without tissue culture. The Innovation 2023, 4, 100345. [Google Scholar] [CrossRef]
- Nadakuduti, S.S.; Enciso-Rodríguez, F. Advances in genome editing with CRISPR systems and transformation technologies for plant DNA manipulation. Front. Plant Sci. 2021, 11, 637159. [Google Scholar] [CrossRef]
- Daròs, J.-A.; Pasin, F.; Merwaiss, F. CRISPR-Cas-based plant genome engineering goes viral. Mol. Plant 2023, 16, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Han, T.; Liu, G.; Chen, T.; Wang, Y.; Yu, A.Y.L.; Liu, Y. A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Sci. Rep. 2015, 5, 14926. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Wu, M.; Carroll, B.J.; Xu, Z.P.; Zhang, R. Enhancing plant biotechnology by nanoparticle delivery of nucleic acids. Trends Genet. 2024. [Google Scholar] [CrossRef]
- Uranga, M.; Vazquez-Vilar, M.; Orzáez, D.; Daròs, J.-A. CRISPR-Cas12a genome editing at the whole-plant level using two compatible RNA virus vectors. CRISPR J. 2021, 4, 761–769. [Google Scholar] [CrossRef]
- Uranga, M.; Daròs, J. Tools and targets: The dual role of plant viruses in CRISPR–Cas genome editing. Plant Genome 2022, 16, e20220. [Google Scholar] [CrossRef]
- Uranga, M.; Aragonés, V.; Daròs, J.-A.; Pasin, F. Heritable CRISPR-Cas9 editing of plant genomes using RNA virus vectors. STAR Protoc. 2023, 4, 102091. [Google Scholar] [CrossRef] [PubMed]
- Uranga, M.; Aragonés, V.; García, A.; Mirabel, S.; Gianoglio, S.; Presa, S.; Granell, A.; Pasin, F.; Daròs, J.-A. RNA virus-mediated gene editing for tomato trait breeding. Hortic. Res. 2024, 11, uhad279. [Google Scholar] [CrossRef]
- Ellison, E.E.; Nagalakshmi, U.; Gamo, M.E.; Huang, P.; Dinesh-Kumar, S.; Voytas, D.F. Author Correction: Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants 2020, 7, 99. [Google Scholar] [CrossRef]
- Yang, L.; Machin, F.; Wang, S.; Saplaoura, E.; Kragler, F. Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat. Biotechnol. 2023, 41, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.J.; Goh, N.S.; Demirer, G.S.; Matos, J.L.; Landry, M.P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018, 36, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Mitter, N. How nanocarriers delivering cargos in plants can change the GMO landscape. Nat. Nanotechnol. 2019, 14, 512–514. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.-J.; et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]
- Doyle, C.; Higginbottom, K.; Swift, T.A.; Winfield, M.; Bellas, C.; Benito-Alifonso, D.; Fletcher, T.; Galan, M.C.; Edwards, K.; Whitney, H.M. A simple method for spray-on gene editing in planta. bioRxiv 2019, 805036. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed]
- World Population Prospects, The 2019 Revision—Volume I: Comprehensive Tables; UN: New York, NY, USA, 2019; ISBN 9789210046428.
- Stetter, M.G.; Gates, D.J.; Mei, W.; Ross-Ibarra, J. How to make a domesticate. Curr. Biol. 2017, 27, R896–R900. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogo, U.; Simoni, S.; Fambrini, M.; Giordani, T.; Pugliesi, C.; Mascagni, F. Future-Proofing Agriculture: De Novo Domestication for Sustainable and Resilient Crops. Int. J. Mol. Sci. 2024, 25, 2374. https://doi.org/10.3390/ijms25042374
Rogo U, Simoni S, Fambrini M, Giordani T, Pugliesi C, Mascagni F. Future-Proofing Agriculture: De Novo Domestication for Sustainable and Resilient Crops. International Journal of Molecular Sciences. 2024; 25(4):2374. https://doi.org/10.3390/ijms25042374
Chicago/Turabian StyleRogo, Ugo, Samuel Simoni, Marco Fambrini, Tommaso Giordani, Claudio Pugliesi, and Flavia Mascagni. 2024. "Future-Proofing Agriculture: De Novo Domestication for Sustainable and Resilient Crops" International Journal of Molecular Sciences 25, no. 4: 2374. https://doi.org/10.3390/ijms25042374
APA StyleRogo, U., Simoni, S., Fambrini, M., Giordani, T., Pugliesi, C., & Mascagni, F. (2024). Future-Proofing Agriculture: De Novo Domestication for Sustainable and Resilient Crops. International Journal of Molecular Sciences, 25(4), 2374. https://doi.org/10.3390/ijms25042374







