Dysregulation of Glucocorticoid Receptor Homeostasis and Glucocorticoid-Associated Genes in Umbilical Cord Endothelial Cells of Diet-Induced Obese Pregnant Sheep
Abstract
1. Introduction
2. Results
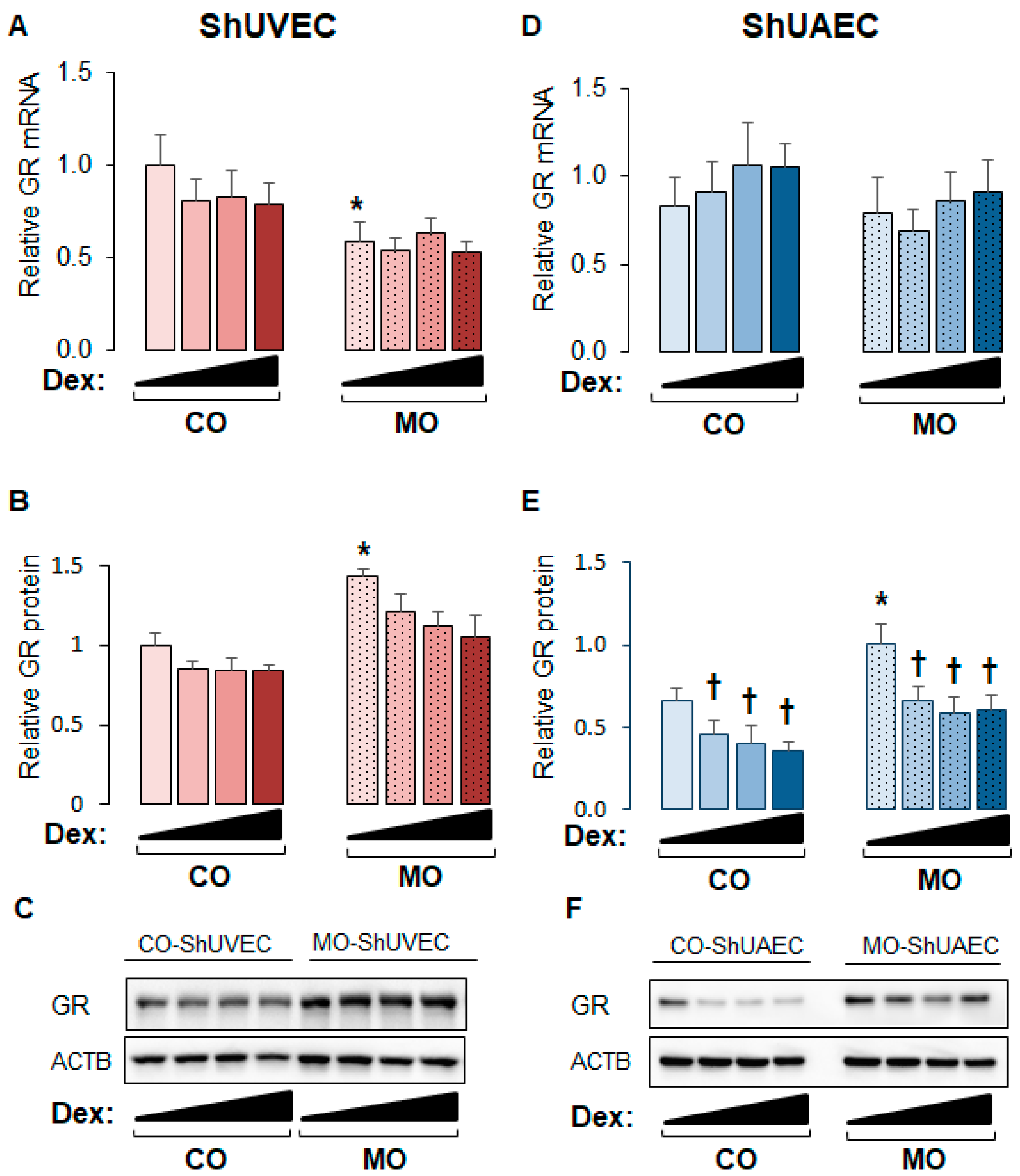
2.1. Maternal Obesity Upregulates GR Expression in ShUVECs and ShUAECs
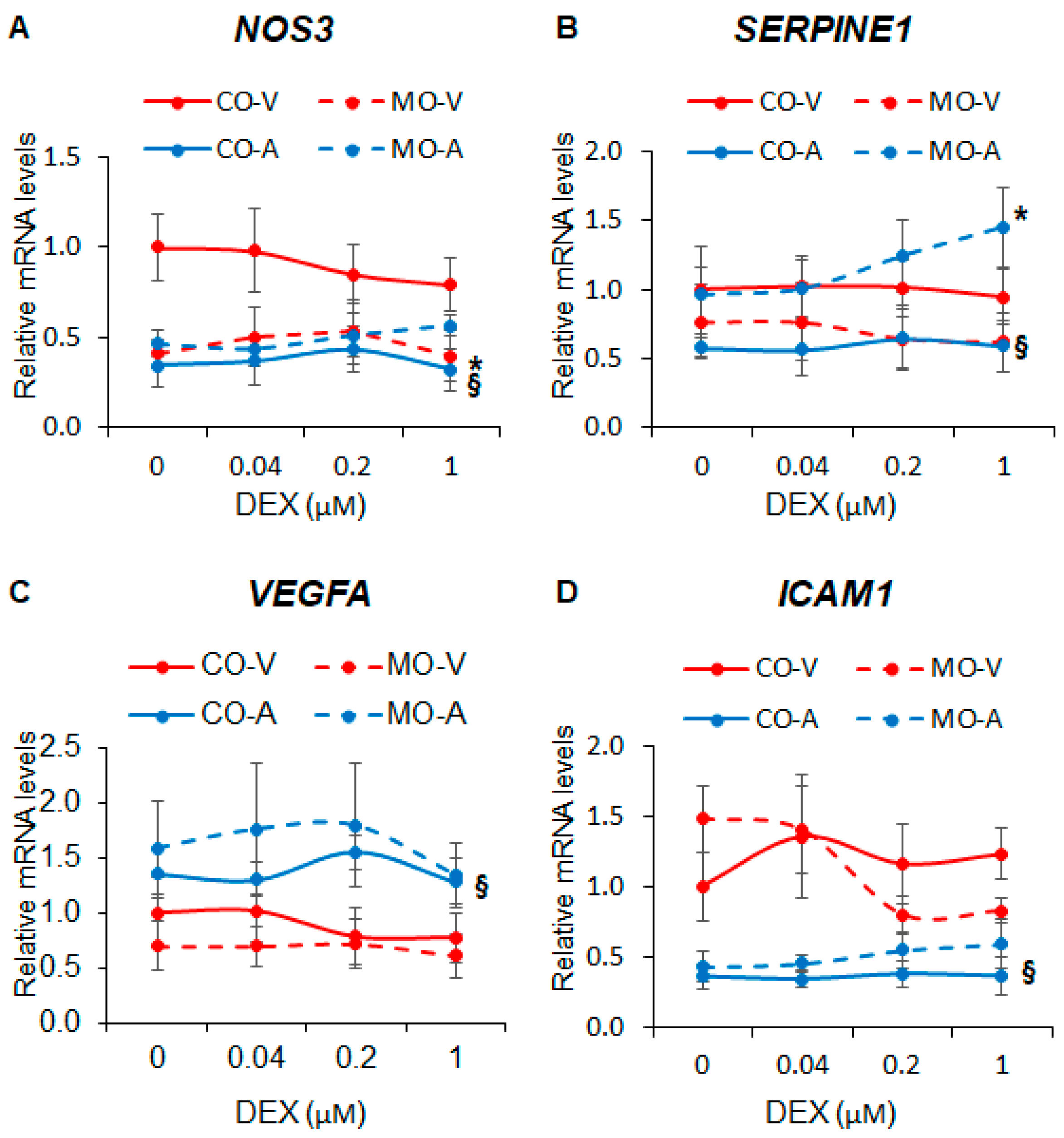
2.2. Maternal Obesity Dysregulates Key Endothelial Gene Expression
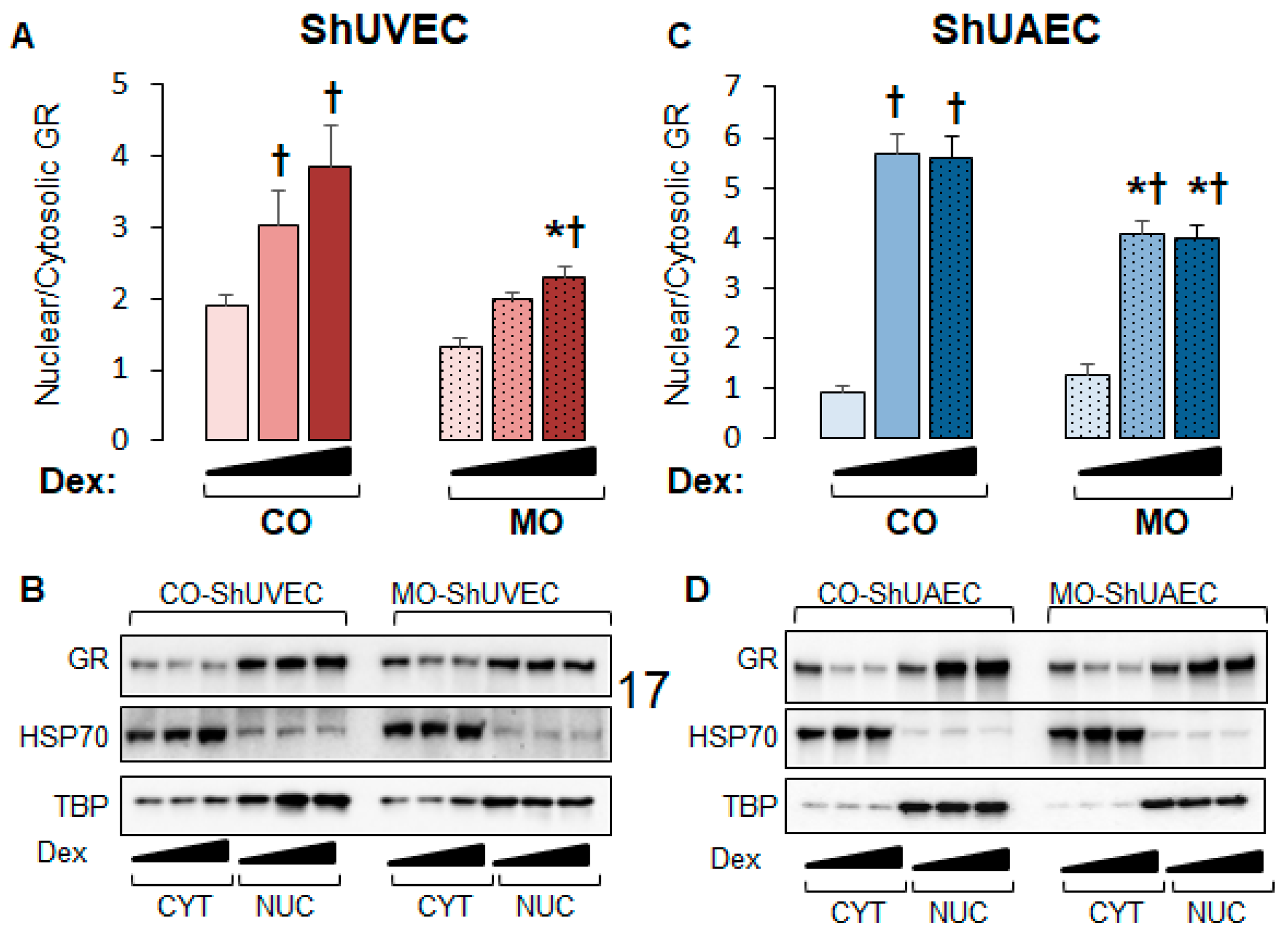
2.3. Maternal Obesity Dysregulates GR-Homeostasis
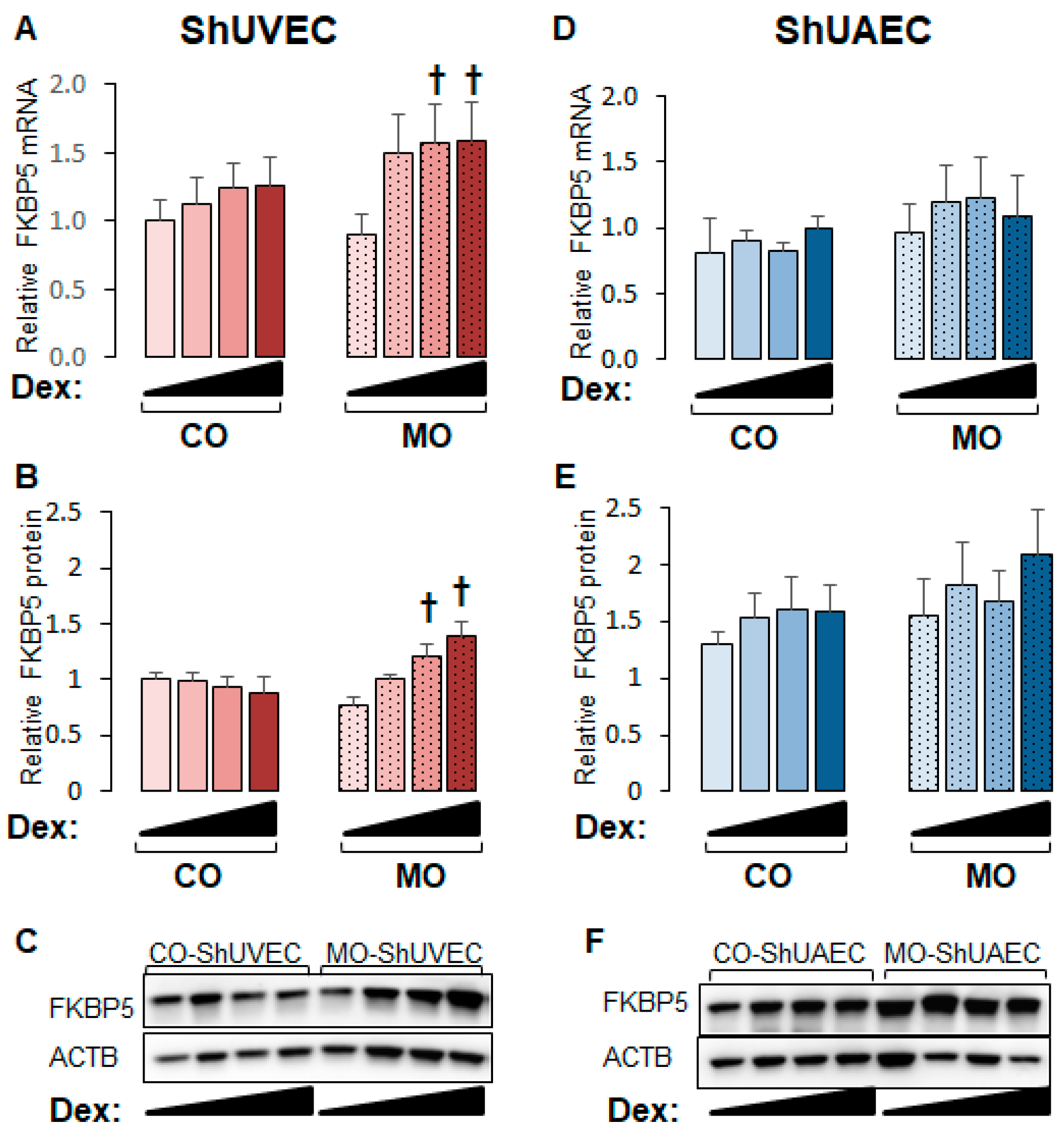
2.4. Maternal Obesity Increases FKBP51 Upregulation as a Negative Feedback Mechanism
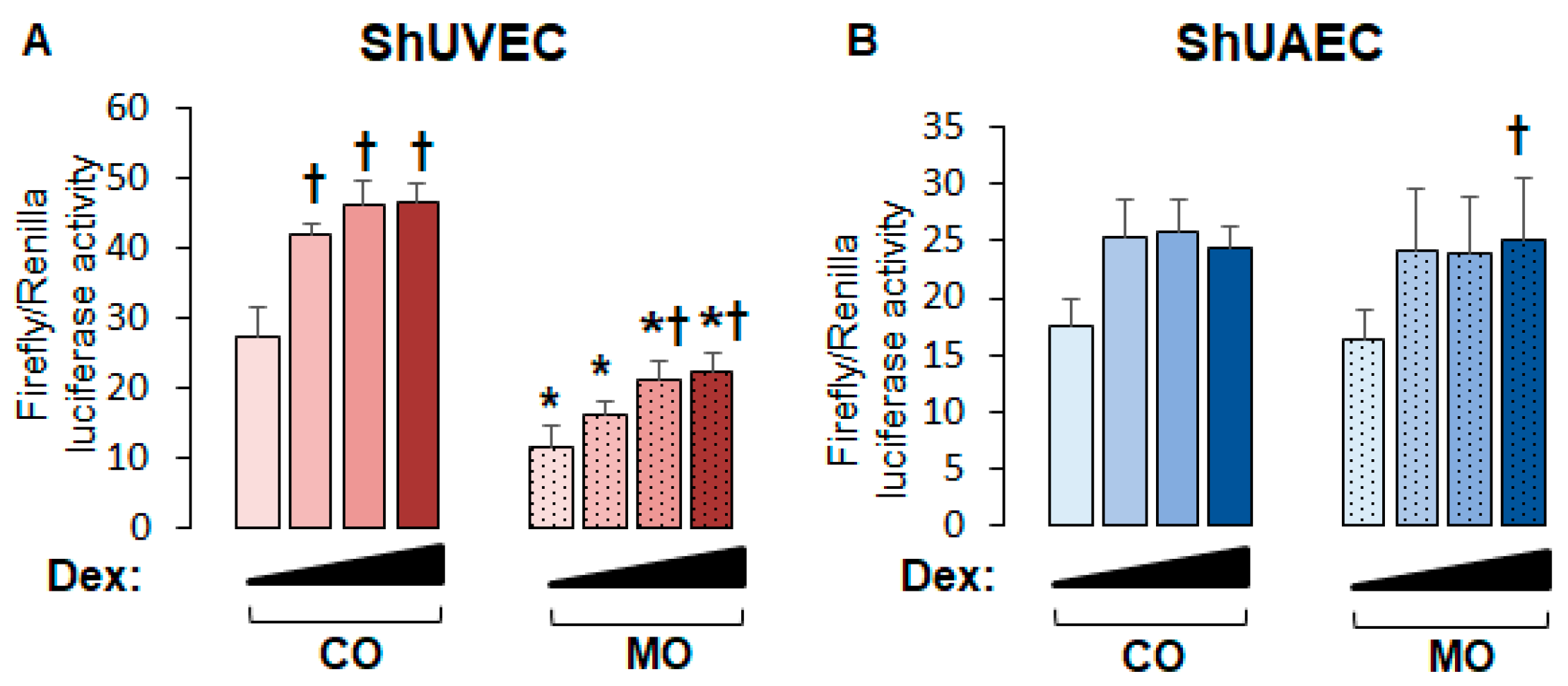
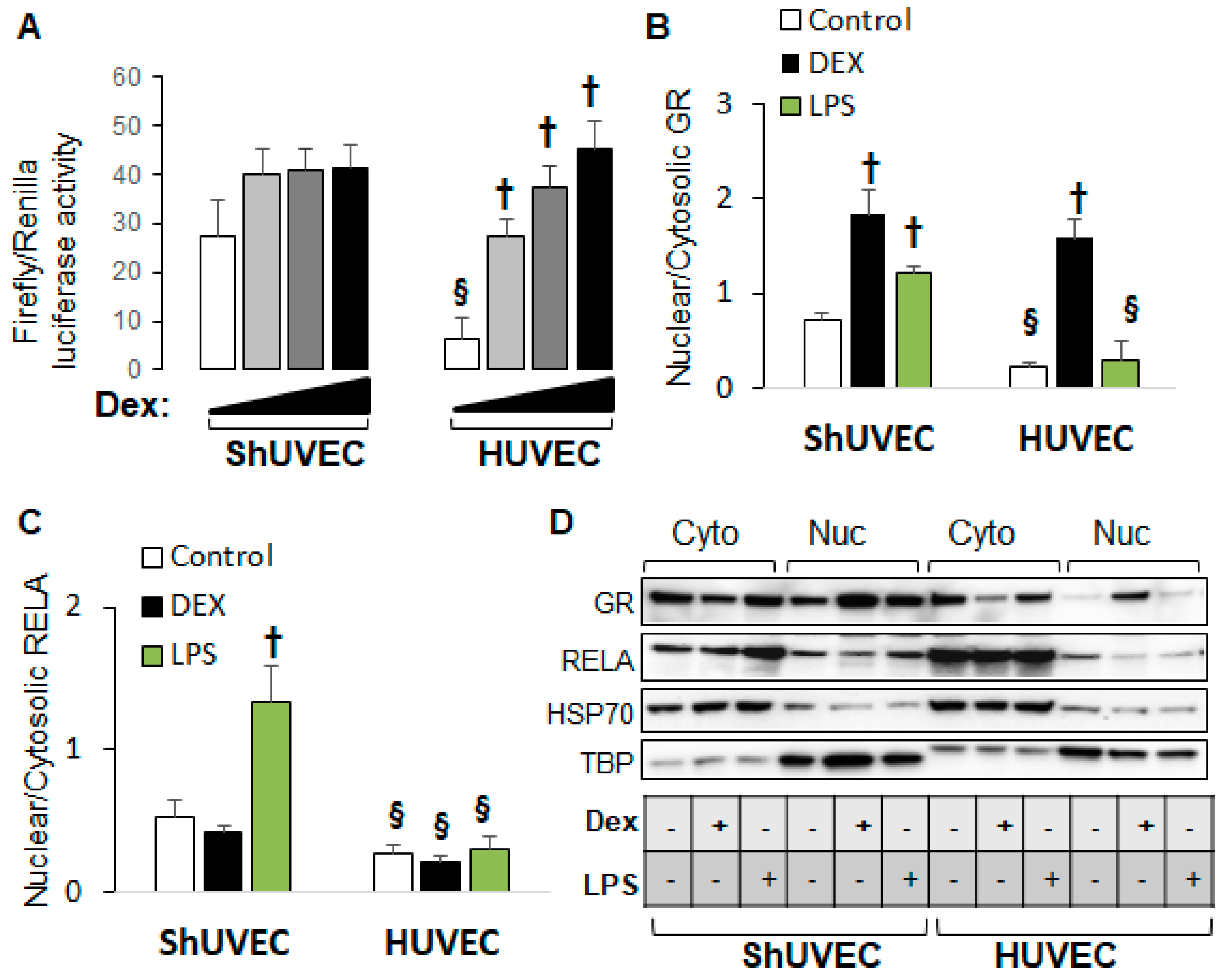
2.5. Decreased Dexamethasone Sensitivity in ShUVECs Compared with HUVECs
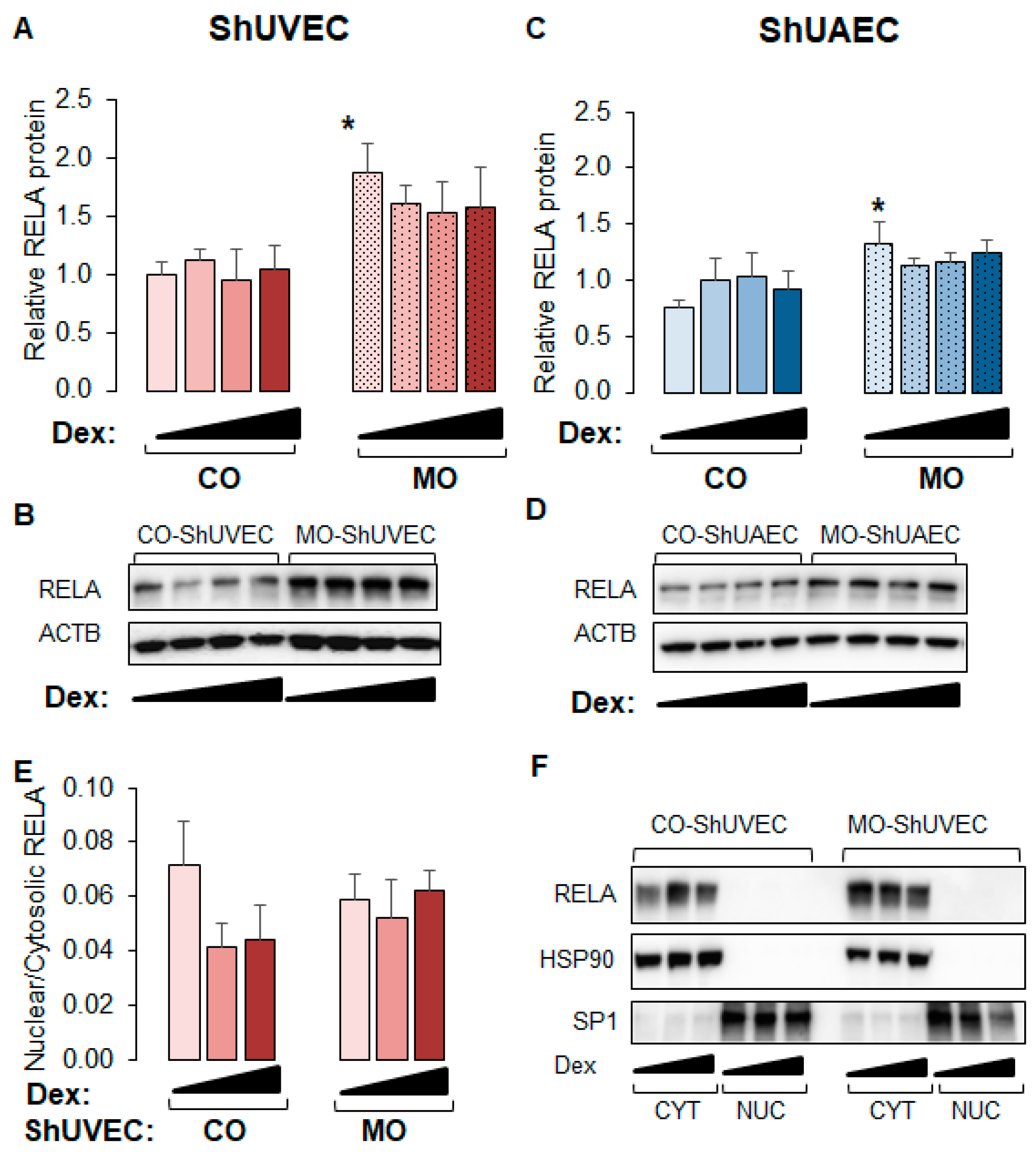
2.6. MO Increases NF-kB Expression in Umbilical Endothelial Cells
3. Discussion
3.1. Maternal Obesity Effects on Fetal Endothelial GR Expression and Function
3.2. Maternal Obesity Effects on Key Endothelial Gene Expression
3.3. Comparative Endothelial GR Physiology: Human versus Sheep
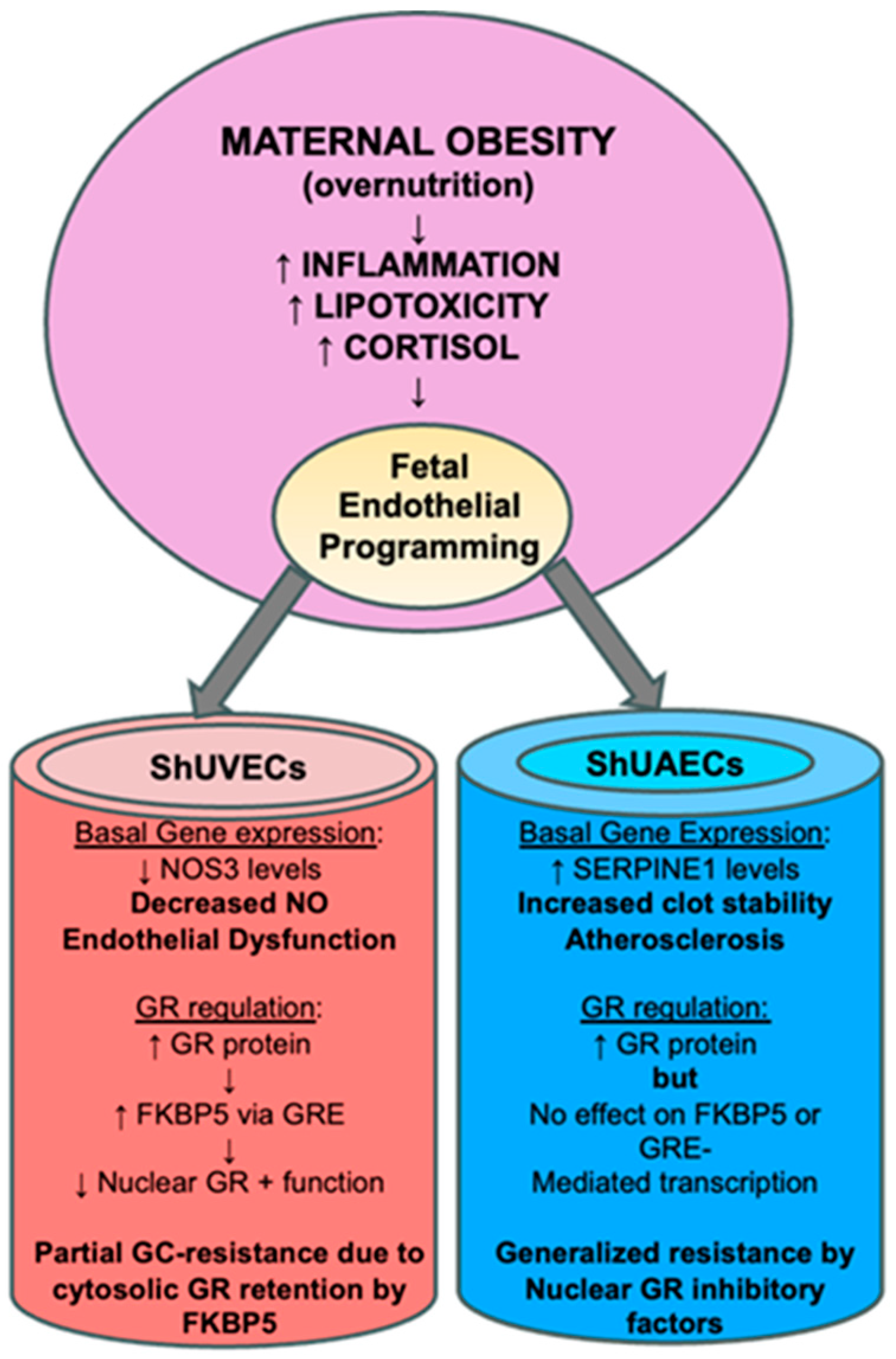
3.4. Conclusions
4. Materials and Methods
4.1. Animal Care and Use
4.2. Umbilical Cord Endothelial Cell Isolation
4.3. Cell Culture, Treatment, and Determination of Glucocorticoid Sensitivity
4.4. Protein Isolation, SDS-PAGE, and Immunoblotting
4.5. RNA Extraction, cDNA Synthesis, and Real-Time PCR
4.6. Cell Transfection and Lucierase Reporter Assays
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poston, L.; Harthoorn, L.F.; Van Der Beek, E.M. Obesity in Pregnancy: Implications for the Mother and Lifelong Health of the Child. A Consensus Statement. Pediatr. Res. 2011, 69, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J. The Impact of Maternal Obesity on Maternal and Fetal Health. Neonatol. Today 2021, 16, 10–12. [Google Scholar] [CrossRef]
- Kislal, S.; Shook, L.L.; Edlow, A.G. Perinatal Exposure to Maternal Obesity: Lasting Cardiometabolic Impact on Offspring. Prenat. Diagn. 2020, 40, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.L.; Barzel, B.; Wood-Bradley, R.J.; Burke, S.L.; Head, G.A.; Armitage, J.A. Developmental Origins of Obesity-related Hypertension. Clin. Exp. Pharmacol. Physiol. 2012, 39, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, P.; Holmes, M.; Schmidt, M.V.; Cirulli, F.; Guzzardi, M.A.; Berry, A.; Balsevich, G.; Andreassi, M.G.; Wesselink, J.-J.; Liistro, T.; et al. Developmental ORIgins of Healthy and Unhealthy AgeiNg: The Role of Maternal Obesity-Introduction to DORIAN. Obes. Facts 2014, 7, 130–151. [Google Scholar] [CrossRef]
- Li, M.; Sloboda, D.M.; Vickers, M.H. Maternal Obesity and Developmental Programming of Metabolic Disorders in Offspring: Evidence from Animal Models. Exp. Diabetes Res. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Ojha, S.; Fainberg, H.P.; Sebert, S.; Budge, H.; Symonds, M.E. Maternal Health and Eating Habits: Metabolic Consequences and Impact on Child Health. Trends Mol. Med. 2015, 21, 126–133. [Google Scholar] [CrossRef]
- Hsu, M.-H.; Chen, Y.-C.; Sheen, J.-M.; Huang, L.-T. Maternal Obesity Programs Offspring Development and Resveratrol Potentially Reprograms the Effects of Maternal Obesity. Int. J. Environ. Res. Public. Health 2020, 17, 1610. [Google Scholar] [CrossRef]
- Wankhade, U.D. Persistent Influence of Maternal Obesity on Offspring Health: Mechanisms from Animal Models and Clinical Studies. Mol. Cell. Endocrinol. 2016, 435, 7–19. [Google Scholar] [CrossRef]
- Zambrano, E.; Nathanielsz, P.W. Mechanisms by Which Maternal Obesity Programs Offspring for Obesity: Evidence from Animal Studies. Nutr. Rev. 2013, 71, S42–S54. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Fukuoka, H. Developmental Origins of Health and Disease Theory in Cardiology. J. Cardiol. 2020, 76, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental Origins of Health and Disease: Current Knowledge and Potential Mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.W.; Bennett, F.I.; Wilks, R.; Thame, M.; Boyne, M.; Osmond, C.; Forrester, T.E. Maternal Body Composition, Offspring Blood Pressure and the Hypothalamic-Pituitary-Adrenal Axis. Paediatr. Perinat. Epidemiol. 2005, 19, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Forbes, S.; Denison, F.C.; Räikkönen, K.; Norman, J.E.; Reynolds, R.M. Maternal Lipids in Pregnancy Are Associated with Increased Offspring Cortisol Reactivity in Childhood. Psychoneuroendocrinology 2017, 83, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Pankey, C.L.; Odhiambo, J.F.; Smith, A.M.; Ford, S.P. Effects of Maternal Obesity in an Ovine Model on Metabolic Outcomes in F2 Adults and F3 Neonates. Domest. Anim. Endocrinol. 2021, 76, 106628. [Google Scholar] [CrossRef] [PubMed]
- Long, N.M.; Nathanielsz, P.W.; Ford, S.P. The Impact of Maternal Overnutrition and Obesity on Hypothalamic-Pituitary-Adrenal Axis Response of Offspring to Stress. Domest. Anim. Endocrinol. 2012, 42, 195–202. [Google Scholar] [CrossRef][Green Version]
- Ghnenis, A.B.; Odhiambo, J.F.; McCormick, R.J.; Nathanielsz, P.W.; Ford, S.P. Maternal Obesity in the Ewe Increases Cardiac Ventricular Expression of Glucocorticoid Receptors, Proinflammatory Cytokines and Fibrosis in Adult Male Offspring. PLoS ONE 2017, 12, e0189977. [Google Scholar] [CrossRef]
- Kandadi, M.R.; Hua, Y.; Zhu, M.; Turdi, S.; Nathanielsz, P.W.; Ford, S.P.; Nair, S.; Ren, J. Influence of Gestational Overfeeding on Myocardial Proinflammatory Mediators in Fetal Sheep Heart. J. Nutr. Biochem. 2013, 24, 1982–1990. [Google Scholar] [CrossRef]
- De Leo, M.; Pivonello, R.; Auriemma, R.S.; Cozzolino, A.; Vitale, P.; Simeoli, C.; De Martino, M.C.; Lombardi, G.; Colao, A. Cardiovascular Disease in Cushing’s Syndrome: Heart versus Vasculature. Neuroendocrinology 2010, 92, 50–54. [Google Scholar] [CrossRef]
- Abraham, S.B.; Abel, B.S.; Rubino, D.; Nansel, T.; Ramsey, S.; Nieman, L.K. A Direct Comparison of Quality of Life in Obese and Cushing’s Syndrome Patients. Eur. J. Endocrinol. 2013, 168, 787–793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Der Sluis, R.J.; Hoekstra, M. Glucocorticoids Are Active Players and Therapeutic Targets in Atherosclerotic Cardiovascular Disease. Mol. Cell. Endocrinol. 2020, 504, 110728. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.; Hadoke, P.W.F.; Nixon, M. Glucocorticoids: Fuelling the Fire of Atherosclerosis or Therapeutic Extinguishers? Int. J. Mol. Sci. 2021, 22, 7622. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, K.A.; Van Moortel, L.; Opdenakker, G.; De Bosscher, K.; Van Den Steen, P.E. Endothelial Response to Glucocorticoids in Inflammatory Diseases. Front. Immunol. 2016, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, M.D.; Wolf, I.M.; Sanchez, E.R.; Witchel, S.F.; DeFranco, D.B. Glucocorticoid Receptor Physiology. Rev. Endocr. Metab. Disord. 2007, 8, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, S.; Nalbantoglu, D.; Gebhardt, J.C.M.; Tuckermann, J. A Guide to Changing Paradigms of Glucocorticoid Receptor Function—A Model System for Genome Regulation and Physiology. FEBS J. 2022, 289, 5718–5743. [Google Scholar] [CrossRef] [PubMed]
- Fadel, L.; Dacic, M.; Fonda, V.; Sokolsky, B.A.; Quagliarini, F.; Rogatsky, I.; Uhlenhaut, N.H. Modulating Glucocorticoid Receptor Actions in Physiology and Pathology: Insights from Coregulators. Pharmacol. Ther. 2023, 251, 108531. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Bali, U.; Phillips, T.; Hunt, H.; Unitt, J. FKBP5 mRNA Expression Is a Biomarker for GR Antagonism. J. Clin. Endocrinol. Metab. 2016, 101, 4305–4312. [Google Scholar] [CrossRef]
- Stechschulte, L.A.; Sanchez, E.R. FKBP51—A Selective Modulator of Glucocorticoid and Androgen Sensitivity. Curr. Opin. Pharmacol. 2011, 11, 332–337. [Google Scholar] [CrossRef]
- Fries, G.; Gassen, N.; Rein, T. The FKBP51 Glucocorticoid Receptor Co-Chaperone: Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2017, 18, 2614. [Google Scholar] [CrossRef]
- Beck, I.M.E.; Vanden Berghe, W.; Vermeulen, L.; Yamamoto, K.R.; Haegeman, G.; De Bosscher, K. Crosstalk in Inflammation: The Interplay of Glucocorticoid Receptor-Based Mechanisms and Kinases and Phosphatases. Endocr. Rev. 2009, 30, 830–882. [Google Scholar] [CrossRef] [PubMed]
- Kassel, O.; Herrlich, P. Crosstalk between the Glucocorticoid Receptor and Other Transcription Factors: Molecular Aspects. Mol. Cell. Endocrinol. 2007, 275, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Pankey, C.L.; Wang, Q.; King, J.; Ford, S.P. Cardiovascular Consequences of Maternal Obesity throughout the Lifespan in First Generation Sheep. PLoS ONE 2022, 17, e0274214. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.D.; Cidlowski, J.A. Proteasome-Mediated Glucocorticoid Receptor Degradation Restricts Transcriptional Signaling by Glucocorticoids. J. Biol. Chem. 2001, 276, 42714–42721. [Google Scholar] [CrossRef]
- Mata-Greenwood, E.; Stewart, J.M.; Steinhorn, R.H.; Pearce, W.J. Role of BCL2-Associated Athanogene 1 in Differential Sensitivity of Human Endothelial Cells to Glucocorticoids. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1046–1055. [Google Scholar] [CrossRef]
- Mata-Greenwood, E.; Jackson, P.N.; Pearce, W.J.; Zhang, L. Endothelial Glucocorticoid Receptor Promoter Methylation According to Dexamethasone Sensitivity. J. Mol. Endocrinol. 2015, 55, 133–146. [Google Scholar] [CrossRef]
- Dovio, A.; Masera, R.G.; Sartori, M.L.; Racca, S.; Angeli, A. Autocrine Up-Regulation of Glucocorticoid Receptors by Interleukin-6 in Human Osteoblast-Like Cells. Calcif. Tissue Int. 2001, 69, 293–298. [Google Scholar] [CrossRef]
- Molina, M.L.; Guerrero, J.; Cidlowski, J.A.; Gatica, H.; Goecke, A. LPS Regulates the Expression of Glucocorticoid Receptor α and β Isoforms and Induces a Selective Glucocorticoid Resistance in Vitro. J. Inflamm. 2017, 14, 22. [Google Scholar] [CrossRef]
- Wang, H.; Gou, X.; Jiang, T.; Ouyang, J. The Effects of microRNAs on Glucocorticoid Responsiveness. J. Cancer Res. Clin. Oncol. 2017, 143, 1005–1011. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Head, G.A.; Kaye, D.M. Say NO to Obesity-Related Hypertension: Role of the l-Arginine–Nitric Oxide Pathway. Hypertension 2016, 67, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Labra, R. Pre-Pregnancy Maternal Obesity Associates with Endoplasmic Reticulum Stress in Human Umbilical Vein Endothelium. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 3195–3210. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Endothelial Dysfunction in Obesity. In Obesity and Lipotoxicity; Advances in Experimental Medicine and Biology; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 960, pp. 345–379. ISBN 978-3-319-48380-1. [Google Scholar]
- Walford, G.; Loscalzo, J. Nitric Oxide in Vascular Biology. J. Thromb. Haemost. 2003, 1, 2112–2118. [Google Scholar] [CrossRef]
- Freedman, J.E.; Loscalzo, J. Nitric Oxide and Its Relationship to Thrombotic Disorders. J. Thromb. Haemost. 2003, 1, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Gozal, D. PAI-1: A Major Player in the Vascular Dysfunction in Obstructive Sleep Apnea? Int. J. Mol. Sci. 2022, 23, 5516. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.M.; Freeman, D.J.; Ramsay, J.E.; Greer, I.A.; Caslake, M.; Ferrell, W.R. Longitudinal Assessment of Maternal Endothelial Function and Markers of Inflammation and Placental Function throughout Pregnancy in Lean and Obese Mothers. J. Clin. Endocrinol. Metab. 2007, 92, 969–975. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Alessi, M.C. PAI-1, Obesity, Insulin Resistance and Risk of Cardiovascular Events. Thromb. Haemost. 1997, 78, 656–660. [Google Scholar] [CrossRef]
- Hildebrand, F.; Pape, H.-C.; Harwood, P.; Müller, K.; Hoevel, P.; Pütz, C.; Siemann, A.; Krettek, C.; Van Griensven, M. Role of Adhesion Molecule ICAM in the Pathogenesis of Polymicrobial Sepsis. Exp. Toxicol. Pathol. 2005, 56, 281–290. [Google Scholar] [CrossRef]
- Kalani, C.; Venigalla, T.; Bailey, J.; Udeani, G.; Surani, S. Sepsis Patients in Critical Care Units with Obesity: Is Obesity Protective? Cureus 2020, 12, e6929. [Google Scholar] [CrossRef]
- Bai, L.; Huang, J.; Wang, D.; Zhu, D.; Zhao, Q.; Li, T.; Zhou, X.; Xu, Y. Association of Body Mass Index with Mortality of Sepsis or Septic Shock: An Updated Meta-Analysis. J. Intensive Care 2023, 11, 27. [Google Scholar] [CrossRef]
- Makó, V.; Czúcz, J.; Weiszhár, Z.; Herczenik, E.; Matkó, J.; Prohászka, Z.; Cervenak, L. Proinflammatory Activation Pattern of Human Umbilical Vein Endothelial Cells Induced by IL-1β, TNF-α, and LPS. Cytometry A 2010, 77A, 962–970. [Google Scholar] [CrossRef]
- Grooby, W.L.; Krishnan, R.; Russ, G.R. Characterization of Ovine Umbilical Vein Endothelial Cells and Their Expression of Cell Adhesion Molecules: Comparative Study with Human Endothelial Cells. Immunol. Cell Biol. 1997, 75, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chriguer, R.S.; Elias, L.L.K.; Da Silva, I.M.; Vieira, J.G.H.; Moreira, A.C.; De Castro, M. Glucocorticoid Sensitivity in Young Healthy Individuals: In Vitro and In Vivo Studies. J. Clin. Endocrinol. Metab. 2005, 90, 5978–5984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quax, R.A.; Manenschijn, L.; Koper, J.W.; Hazes, J.M.; Lamberts, S.W.J.; Van Rossum, E.F.C.; Feelders, R.A. Glucocorticoid Sensitivity in Health and Disease. Nat. Rev. Endocrinol. 2013, 9, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Billings, T.L.; Mansour, T.; Martin, C.; Baylink, D.J.; Longo, L.D.; Pearce, W.J.; Mata-Greenwood, E. Vitamin D Status and Metabolism in an Ovine Pregnancy Model: Effect of Long-Term, High-Altitude Hypoxia. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1062–E1071. [Google Scholar] [CrossRef]
| Characteristics a | Control Diet (n = 6) | Obesogenic DIET (n = 6) | p-Value |
|---|---|---|---|
| Maternal | |||
| Age (yr) | 4.8 ± 0.5 | 4.2 ± 0.6 | 0.405 |
| Pre-pregnancy weight (kg) Necropsy weight (kg) Weight gain (kg) | 65.8 ± 3.9 69.3 ± 3.7 3.6 ± 1.9 | 69.3 ± 3.7 109.6 ± 5.2 38.2 ± 3.6 | 0.294 <0.001 <0.001 |
| Fetal | |||
| Gestational age (days) | 138.5 ± 0.5 | 139.5 ± 0.6 | 0.214 |
| Sex (male/female) | 3/3 | 3/3 | 1.00 |
| Birthweight (kg) | 5.53 ± 0.27 | 6.06 ± 0.18 | 0.128 |
| Name | Vendor | Catalog | Dilution | Observed MW (kDa) |
|---|---|---|---|---|
| Glucocorticoid receptor (GR/NR3C1) | Cell Signaling Technology | 12041 | 1:1000 | 95/90 |
| FKBP prolyl isomerase 5 (FKBP5) | Cell Signaling Technology | 12210 | 1:1000 | 55 |
| NFKB p65 subunit (RELA) | Cell Signaling Technology | 8242 | 1:1000 | 65 |
| TATA binding protein (TBP) | Proteintech | 22006-1-AP | 1:1000 | 38 |
| Specificity protein 1 (SP1) | Proteintech | 21962-1-AP | 1:1000 | 95 |
| Heat shock protein 70 (HSP70) | Cell Signaling Technology | 4872 | 1:1000 | 70 |
| Heat shock protein 90 (HSP90) | Proteintech | 13171-1-AP | 1:2000 | 90 |
| Beta-actin (ACTB) | Sigma Millipore | A5441 | 1:2000 | 41 |
| Name | Sequence | Reference |
|---|---|---|
| Ovine glucocorticoid receptor (NR3C1) | Forward: 5’-CAGTGAAATGGGCAAAGGCAA-3’ Reverse: 5’-TGCGCTTGACTGTCTGTATGA-3’ | NM_001114186.1 |
| Ovine endothelial nitric oxide synthase (NOS3) | Forward: 5’-TCTTCCACCAGGAGATGGTC-3’ Reverse: 5’-AGAGGCGTACAGGATGGTTG-3’ | NM_001129901.1 |
| Ovine plasminogen activator inhibitor 1 (SERPINE1) | Forward: 5’-GACCGCAACGTGGTTTTCTC-3’ Reverse: 5’-CGAGCTCCTTGTACAGTCGG-3’ | NM_001174114.2 |
| Ovine intercellular adhesion molecule 1 (ICAM1) | Forward: 5’-GGTCTGGAGGTGCCGAAATA-3’ Reverse: 5’-CGGCTCACTTCCTCCTTGTT-3’ | NM_001009731.1 |
| Ovine vascular endothelial growth factor (VEGFA) | Forward: 5’-AAGAAAATCCCTGTGGGCCT-3’ Reverse: 5-GGAACATTTACACGTCTGCGG-3’ | AF071015.1 |
| Ovine FKBP prolyl isomerase 5 (FKBP5) | Forward: 5’-GAGCAGGACGCAAAGGAAGA-3’ Reverse: 5’-TGGTGTCATACGTGCCCTTC-3’ | XM_042237053.2 |
| Ovine beta-actin (ACTB) | Forward: 5’-GCAGATGTGGATCAGCAAGC-3’ Reverse: 5’-GGGTGTAACGCAGCTAACAG-3’ | NM_001009784.3 |
| Ovine 18S ribosomal RNA (18S rRNA) | Forward, 5’-AAACGGCTACCACATCCAAG -3’ Reverse, 5’-TCCTGTATTGTTATTTTTCGTCAC-3’ | AY753190.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata-Greenwood, E.; Chow, W.L.; Anti, N.A.O.; Sands, L.D.; Adeoye, O.; Ford, S.P.; Nathanielsz, P.W. Dysregulation of Glucocorticoid Receptor Homeostasis and Glucocorticoid-Associated Genes in Umbilical Cord Endothelial Cells of Diet-Induced Obese Pregnant Sheep. Int. J. Mol. Sci. 2024, 25, 2311. https://doi.org/10.3390/ijms25042311
Mata-Greenwood E, Chow WL, Anti NAO, Sands LD, Adeoye O, Ford SP, Nathanielsz PW. Dysregulation of Glucocorticoid Receptor Homeostasis and Glucocorticoid-Associated Genes in Umbilical Cord Endothelial Cells of Diet-Induced Obese Pregnant Sheep. International Journal of Molecular Sciences. 2024; 25(4):2311. https://doi.org/10.3390/ijms25042311
Chicago/Turabian StyleMata-Greenwood, Eugenia, Wendy L. Chow, Nana A. O. Anti, LeeAnna D. Sands, Olayemi Adeoye, Stephen P. Ford, and Peter W. Nathanielsz. 2024. "Dysregulation of Glucocorticoid Receptor Homeostasis and Glucocorticoid-Associated Genes in Umbilical Cord Endothelial Cells of Diet-Induced Obese Pregnant Sheep" International Journal of Molecular Sciences 25, no. 4: 2311. https://doi.org/10.3390/ijms25042311
APA StyleMata-Greenwood, E., Chow, W. L., Anti, N. A. O., Sands, L. D., Adeoye, O., Ford, S. P., & Nathanielsz, P. W. (2024). Dysregulation of Glucocorticoid Receptor Homeostasis and Glucocorticoid-Associated Genes in Umbilical Cord Endothelial Cells of Diet-Induced Obese Pregnant Sheep. International Journal of Molecular Sciences, 25(4), 2311. https://doi.org/10.3390/ijms25042311










