Generation of Endotoxin-Specific Monoclonal Antibodies by Phage and Yeast Display for Capturing Endotoxin
Abstract
1. Introduction
2. Results and Discussion
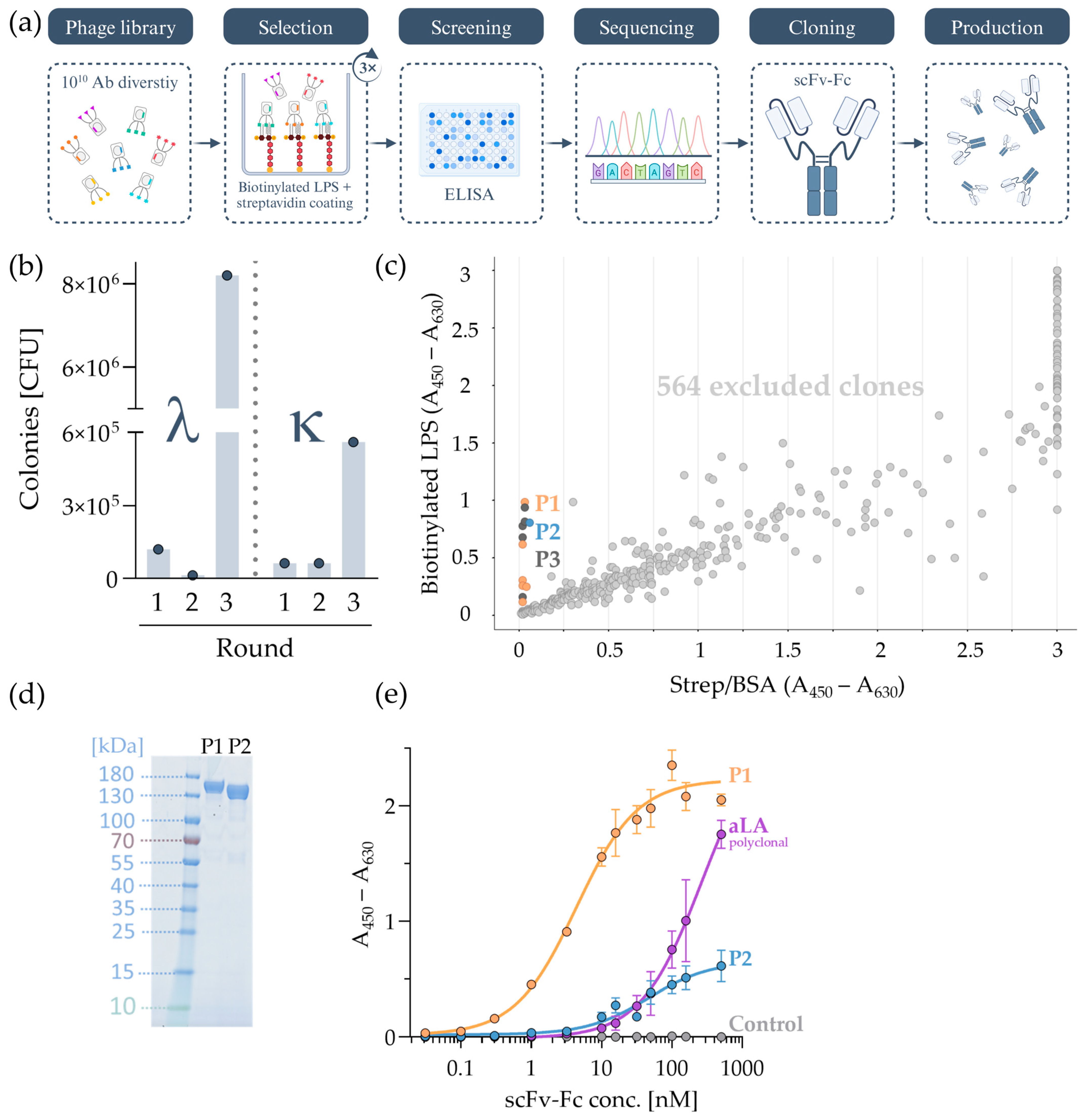
2.1. Screening and Evaluation of LPS Monoclonal Antibodies via Phage Display
2.2. Screening and Evaluation of LPS Monoclonal Antibodies via Yeast Display
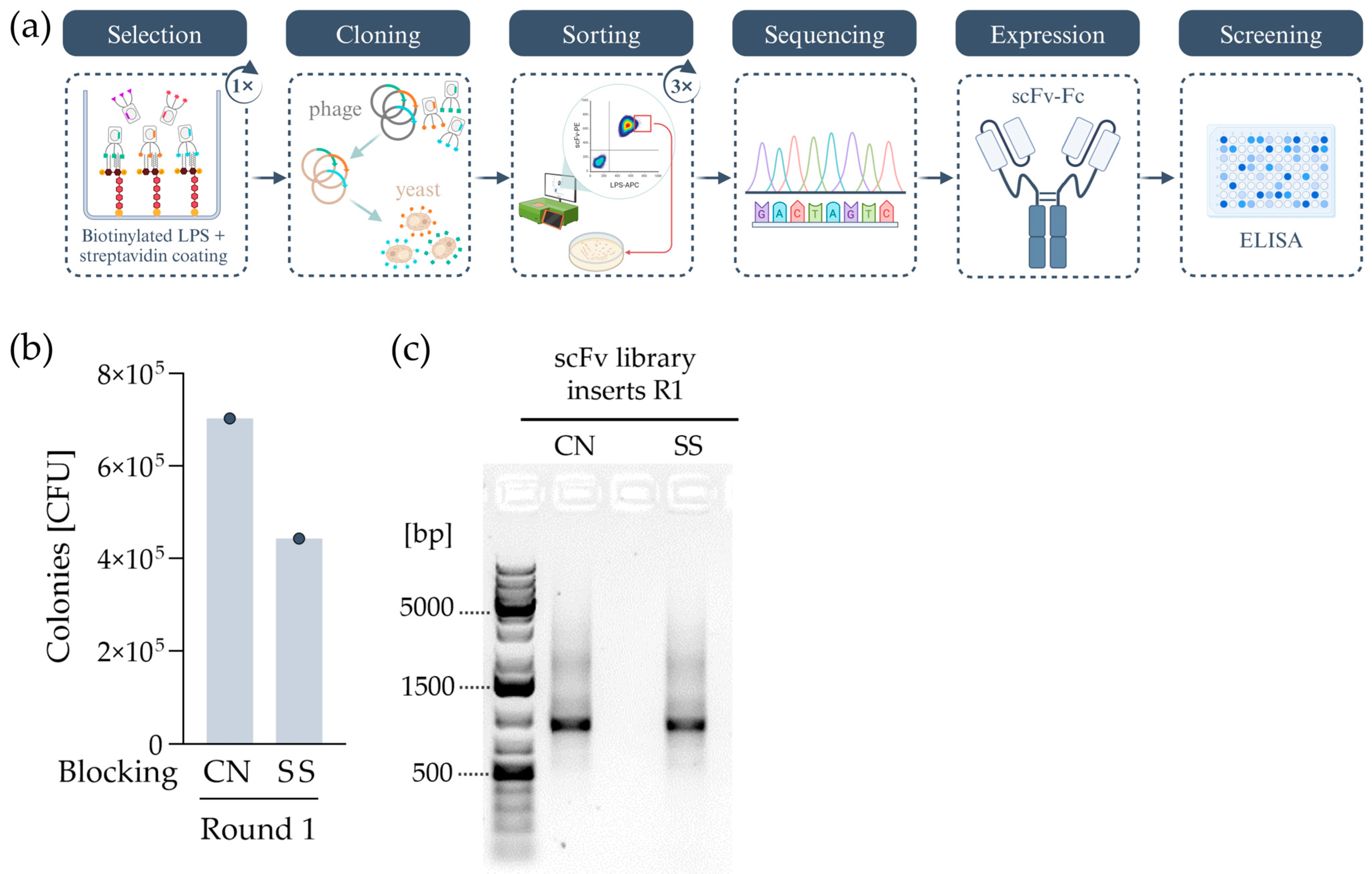
2.2.1. Combining Phage and Yeast Display
2.2.2. Selection for LPS-Specific scFv Clones via Cell Sorting
2.3. Conjugation of scFv-Fc and Anti-Lipid A on Polystyrene Beads
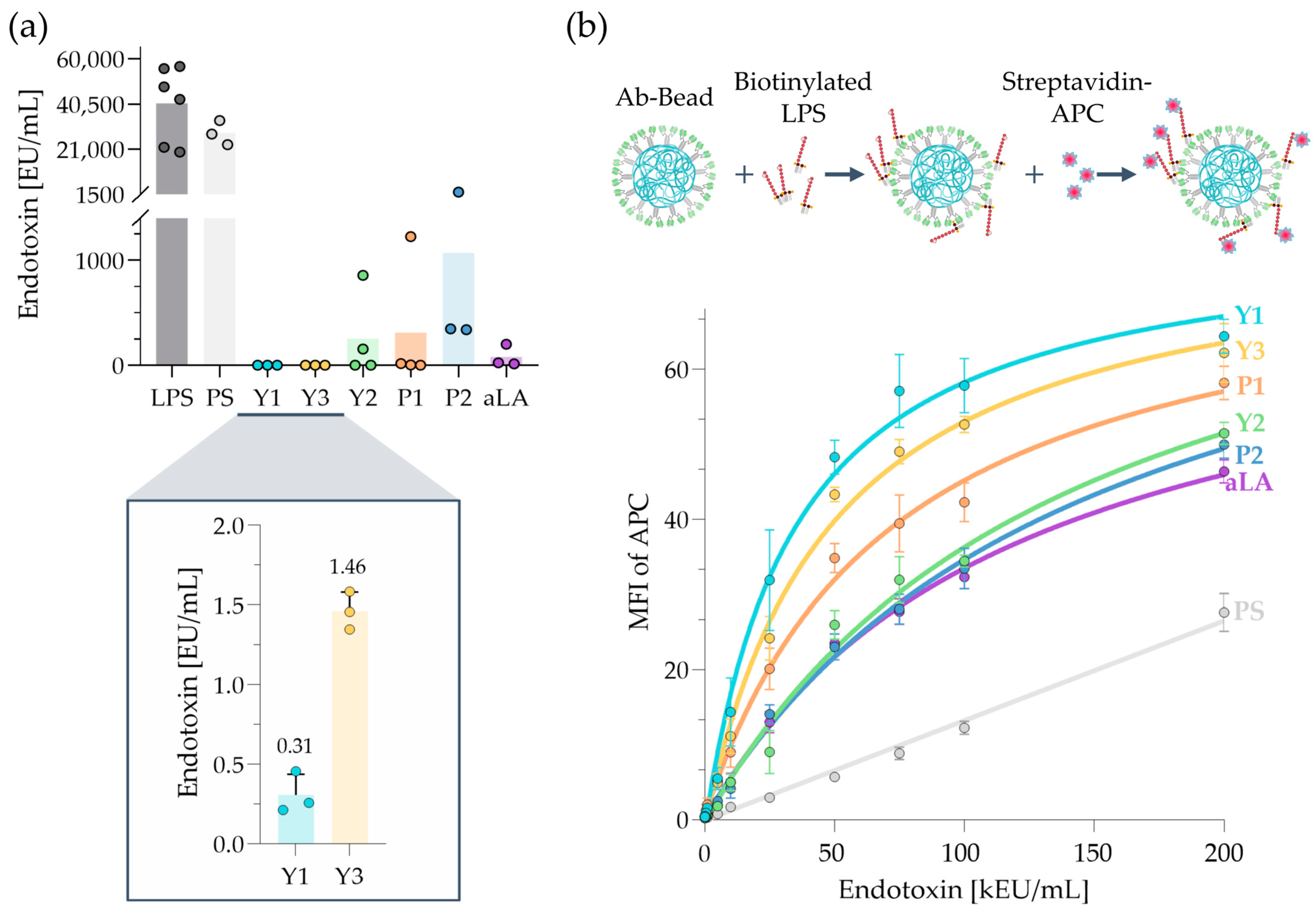
2.4. LPS-Removal Capacity of scFv-Fc Beads Versus Anti-Lipid A Beads
2.5. LPS Binding via Flow Cytometry
3. Materials and Methods
3.1. Materials
3.1.1. Lipopolysaccharides
3.1.2. Enzymes
3.1.3. Cell Lines
3.1.4. Plasmids
3.1.5. Primers
- Phage vector Fwd: CATGAAATACCTATTGCCTACGG
- Phage vector Rev: TGATGATGGTGATGATGGGA
- Yeast pYSDM2 Primer Fwd: TCATGCAGTTACTTCGCTGT
- Yeast pYSDM2 Primer Rev: CCTGCAGCAAGTCCTCTTC
3.1.6. Antibodies
3.1.7. Kits
3.1.8. Chemicals and Reagents
3.1.9. Devices and Software
3.2. Methods
3.2.1. Panning for LPS-Specific Binders via Phage Display
3.2.2. ELISA Screening of LPS-Specific Binders
3.2.3. Cloning into a Fc-Expression Plasmid and Purification
3.2.4. Generation of a scFv-Expressing Yeast Library
3.2.5. Sorting for LPS-Specific Clones via the MACS Quant Tyto Cell Sorter
- SD-CAA medium pH 4.5 sterile filtered (2% w/v dextrose, 0.6% w/v yeast nitrogen base, 0.5% casein hydrolysate, 0.74% w/v citric acid, and 1% sodium citrate).
- SD-CAA agar pH 6.0 (18.2% w/v sorbitol, 1.5% select agar, 0.54% w/v disodium phosphate, and 0.86% w/v monosodium phosphate—in 800 mL autoclaved) and added at 55 °C to sterile filtered 200 mL (0.67% w/v yeast nitrogen base, 0.5% w/v, and 2% w/v dextrose).
- SG-CAA medium pH 6.0 sterile filtered (1.8% w/v galactose, 0.2% w/v dextrose, 0.67% w/v yeast nitrogen base, 0.5% casein hydrolysate, 0.54% w/v disodium phosphate, and 0.86% w/v monosodium phosphate).
3.2.6. Titration of Biotinylated LPS on Yeast Cells
3.2.7. Antibody Production and Purification on a Larger Scale
3.2.8. ELISA Measurements of scFv-Fc
3.2.9. Bead Conjugation
3.2.10. LPS Removal Assay in Water
3.2.11. Conjugated Ab Beads and Biotinylated LPS Measured in Flow Cytometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Fux, A.C.; Casonato Melo, C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef]
- Raina, S. Lipopolysaccharides: Regulated Biosynthesis and Structural Diversity. Int. J. Mol. Sci. 2023, 24, 7498. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers. Available online: https://www.fda.gov/media/83477/download (accessed on 8 December 2023).
- Dawson, M. Endotoxin Limits For Parenteral Drug Products. In BET White Paper; Associates of Cape Cod, Inc.: East Falmouth, MA, USA, 2017; Volume 1, pp. 1–7. [Google Scholar]
- Basauri, A.; González-Fernández, C.; Fallanza, M.; Bringas, E.; Fernandez-Lopez, R.; Giner, L.; Moncalián, G.; de la Cruz, F.; Ortiz, I. Biochemical Interactions between LPS and LPS-Binding Molecules. Crit. Rev. Biotechnol. 2020, 40, 292–305. [Google Scholar] [CrossRef]
- 765€ per 10 µg Human LPS-Binding Protein (LBP) #A60974. Available online: https://www.antibodies.com/de/lbp-human-a60974 (accessed on 13 December 2023).
- Kimkes, T.E.P.; Heinemann, M. How Bacteria Recognise and Respond to Surface Contact. FEMS Microbiol. Rev. 2019, 44, 106–122. [Google Scholar] [CrossRef]
- Redeker, C.; Briscoe, W.H. Interactions between Mutant Bacterial Lipopolysaccharide (LPS-Ra) Surface Layers: Surface Vesicles, Membrane Fusion, and Effect of Ca2+ and Temperature. Langmuir 2019, 35, 15739–15750. [Google Scholar] [CrossRef]
- Santos, N.C.; Silva, A.C.; Castanho, M.A.R.B.; Martins-Silva, J.; Saldanha, C. Evaluation of Lipopolysaccharide Aggregation by Light Scattering Spectroscopy. ChemBioChem 2003, 4, 96–100. [Google Scholar] [CrossRef]
- Bergstrand, A.; Svanberg, C.; Langton, M.; Nydén, M. Aggregation Behavior and Size of Lipopolysaccharide from Escherichia Coli O55:B5. Colloids Surf. B Biointerfaces 2006, 53, 9–14. [Google Scholar] [CrossRef]
- Tani, T.; Shoji, H.; Guadagni, G.; Perego, A. Extracorporeal Removal of Endotoxin: The Polymyxin B-Immobilized Fiber Cartridge. In Contributions to Nephrology; Karger Publishers: Basel, Switzerland, 2010; Volume 167, pp. 35–44. ISBN 9783805594844. [Google Scholar]
- Farooqui, R.; Antharavally, B.; Alegria-Schaffer, A. A Highly Specific Affinity Resin Enables Endotoxin Removal from Various Protein Sources, Sizes and Charges. Available online: https://www.thermofisher.com/de/de/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/protein-biology-application-notes/eliminate-endotoxins-protein-antibody-samples.html (accessed on 25 January 2024).
- Petsch, D.; Anspach, F.B. Endotoxin Removal from Protein Solutions. J. Biotechnol. 2000, 76, 97–119. [Google Scholar] [CrossRef]
- Razdan, S.; Wang, J.C.; Barua, S. PolyBall: A New Adsorbent for the Efficient Removal of Endotoxin from Biopharmaceuticals. Sci. Rep. 2019, 9, 8867. [Google Scholar] [CrossRef]
- Lambadi, P.R.; Sharma, T.K.; Kumar, P.; Vasnani, P.; Thalluri, S.M.; Bisht, N.; Pathania, R.; Navani, N.K. Facile Biofunctionalization of Silver Nanoparticles for Enhanced Antibacterial Properties, Endotoxin Removal, and Biofilm Control. Int. J. Nanomed. 2015, 10, 2155–2171. [Google Scholar] [CrossRef]
- Casonato Melo, C.; Fux, A.C.; Himly, M.; Bastús, N.G.; Schlahsa, L.; Siewert, C.; Puntes, V.; Duschl, A.; Gessner, I.; Fauerbach, J.A. Recovering What Matters: High Protein Recovery after Endotoxin Removal from LPS-Contaminated Formulations Using Novel Anti-Lipid A Antibody Microparticle Conjugates. Int. J. Mol. Sci. 2023, 24, 13971. [Google Scholar] [CrossRef]
- Ansar, W.; Ghosh, S. Monoclonal Antibodies: A Tool in Clinical Research. Indian J. Clin. Med. 2013, 4, IJCM.S11968. [Google Scholar] [CrossRef]
- Unkauf, T.; Miethe, S.; Fühner, V.; Schirrmann, T.; Frenzel, A.; Hust, M. Generation of Recombinant Antibodies against Toxins and Viruses by Phage Display for Diagnostics and Therapy. Adv. Exp. Med. Biol. 2016, 917, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef] [PubMed]
- Barderas, R.; Benito-Peña, E. The 2018 Nobel Prize in Chemistry: Phage Display of Peptides and Antibodies. Anal. Bioanal. Chem. 2019, 411, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.K.; Rahumatullah, A.; Lai, J.Y.; Lim, T.S. Naïve Human Antibody Libraries for Infectious Diseases. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 1053, pp. 35–59. [Google Scholar]
- Bird, R.E.; Hardman, K.D.; Jacobson, J.W.; Johnson, S.; Kaufman, B.M.; Lee, S.-M.; Lee, T.; Pope, S.H.; Riordan, G.S.; Whitlow, M. Single-Chain Antigen-Binding Proteins. Science 1988, 242, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.T.; Kirmann, T.; Wenzel, E.V.; Grosch, J.H.; Polten, S.; Meier, D.; Becker, M.; Matejtschuk, P.; Hust, M.; Russo, G.; et al. Shelf-Life Extension of Fc-Fused Single Chain Fragment Variable Antibodies by Lyophilization. Front. Cell. Infect. Microbiol. 2021, 11, 717689. [Google Scholar] [CrossRef] [PubMed]
- Tout, N.L.; Lam, J.S. Phage Display and Bacterial Expression of a Recombinant Fab Specific for Pseudomonas Aeruginosa Serotype O6 Lipopolysaccharide. Clin. Diagn. Lab. Immunol. 1997, 4, 147–155. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X.; Yang, X.; Zhang, X.; Ma, F.; Gan, H.; Chen, J.; Wang, D.; Sun, W.; Wang, J.; et al. Specific Clearance of Lipopolysaccharide from Blood Based on Peptide Bottlebrush Polymer for Sepsis Therapy. Adv. Mater. 2023, 35, 2302560. [Google Scholar] [CrossRef]
- Matsumoto, M.; Horiuchi, Y.; Yamamoto, A.; Ochiai, M.; Niwa, M.; Takagi, T.; Omi, H.; Kobayashi, T.; Suzuki, M.-M. Lipopolysaccaride-Binding Peptides Obtained by Phage Display Method. J. Microbiol. Methods 2010, 82, 54–58. [Google Scholar] [CrossRef]
- Boder, E.T.; Wittrup, K.D. Yeast Surface Display for Screening Combinatorial Polypeptide Libraries. Nat. Biotechnol. 1997, 15, 553–557. [Google Scholar] [CrossRef]
- Paracini, N.; Schneck, E.; Imberty, A.; Micciulla, S. Lipopolysaccharides at Solid and Liquid Interfaces: Models for Biophysical Studies of the Gram-Negative Bacterial Outer Membrane. Adv. Colloid Interface Sci. 2022, 301, 102603. [Google Scholar] [CrossRef] [PubMed]
- Gioannini, T.L.; Zhang, D.; Teghanemt, A.; Weiss, J.P. An Essential Role for Albumin in the Interaction of Endotoxin with Lipopolysaccharide-Binding Protein and SCD14 and Resultant Cell Activation. J. Biol. Chem. 2002, 277, 47818–47825. [Google Scholar] [CrossRef] [PubMed]
- Péterfi, Z.; Kocsis, B. Comparison of Blocking Agents for an ELISA for LPS. J. Immunoass. 2000, 21, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Gätjen, D.; Tomszak, F.; Dettmann, J.C.; Droste, M.; Nölle, V.; Wieczorek, M. Design of a Novel Switchable Antibody Display System in Pichia Pastoris. Appl. Microbiol. Biotechnol. 2022, 106, 6209–6224. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Lau, W.L.; Hackel, B.J.; Sazinsky, S.L.; Lippow, S.M.; Wittrup, K.D. Isolating and Engineering Human Antibodies Using Yeast Surface Display. Nat. Protoc. 2006, 1, 755–768. [Google Scholar] [CrossRef]
- Bidlingmaier, S.; Su, Y.; Liu, B. Combining Phage and Yeast Cell Surface Antibody Display to Identify Novel Cell Type-Selective Internalizing Human Monoclonal Antibodies. In Yeast Surface Display: Methods, Protocols, and Applications; Liu, B., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1319, pp. 51–63. ISBN 978-1-4939-2747-0. [Google Scholar]
- Benatuil, L.; Perez, J.M.; Belk, J.; Hsieh, C.M. An Improved Yeast Transformation Method for the Generation of Very Large Human Antibody Libraries. Protein Eng. Des. Sel. 2010, 23, 155–159. [Google Scholar] [CrossRef]

| Ab Beads | Y1-PS | Y2-PS | Y3-PS | P1-PS | P2-PS | aLA-PS |
|---|---|---|---|---|---|---|
| LPS removal | 100% | 99.58% | 100% | 99.48% | 98.22% | 99.87% |
| Ab Beads | Y1-PS | Y2-PS | Y3-PS | P1-PS | P2-PS | aLA-PS |
|---|---|---|---|---|---|---|
| EC50 (EU/mL) | 34,958 | 141,571 | 48,538 | 72,319 | 150,713 | 117,854 |
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Initial denaturation | 94 °C | 5 min | |
| Denaturation Annealing Elongation | 94 °C 58 °C 72 °C | 10 s 20 s 20 s | |
| ×35 | |||
| Final extension | 72 °C | 5 min | |
| Cooling | 12 °C | ∞ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fux, A.C.; Casonato Melo, C.; Schlahsa, L.; Burzan, N.B.; Felsberger, A.; Gessner, I.; Fauerbach, J.A.; Horejs-Hoeck, J.; Droste, M.; Siewert, C. Generation of Endotoxin-Specific Monoclonal Antibodies by Phage and Yeast Display for Capturing Endotoxin. Int. J. Mol. Sci. 2024, 25, 2297. https://doi.org/10.3390/ijms25042297
Fux AC, Casonato Melo C, Schlahsa L, Burzan NB, Felsberger A, Gessner I, Fauerbach JA, Horejs-Hoeck J, Droste M, Siewert C. Generation of Endotoxin-Specific Monoclonal Antibodies by Phage and Yeast Display for Capturing Endotoxin. International Journal of Molecular Sciences. 2024; 25(4):2297. https://doi.org/10.3390/ijms25042297
Chicago/Turabian StyleFux, Alexandra C., Cristiane Casonato Melo, Laura Schlahsa, Nico B. Burzan, André Felsberger, Isabel Gessner, Jonathan A. Fauerbach, Jutta Horejs-Hoeck, Miriam Droste, and Christiane Siewert. 2024. "Generation of Endotoxin-Specific Monoclonal Antibodies by Phage and Yeast Display for Capturing Endotoxin" International Journal of Molecular Sciences 25, no. 4: 2297. https://doi.org/10.3390/ijms25042297
APA StyleFux, A. C., Casonato Melo, C., Schlahsa, L., Burzan, N. B., Felsberger, A., Gessner, I., Fauerbach, J. A., Horejs-Hoeck, J., Droste, M., & Siewert, C. (2024). Generation of Endotoxin-Specific Monoclonal Antibodies by Phage and Yeast Display for Capturing Endotoxin. International Journal of Molecular Sciences, 25(4), 2297. https://doi.org/10.3390/ijms25042297







