Autoimmunity and Autoinflammation: Relapsing Polychondritis and VEXAS Syndrome Challenge
Abstract
1. Introduction
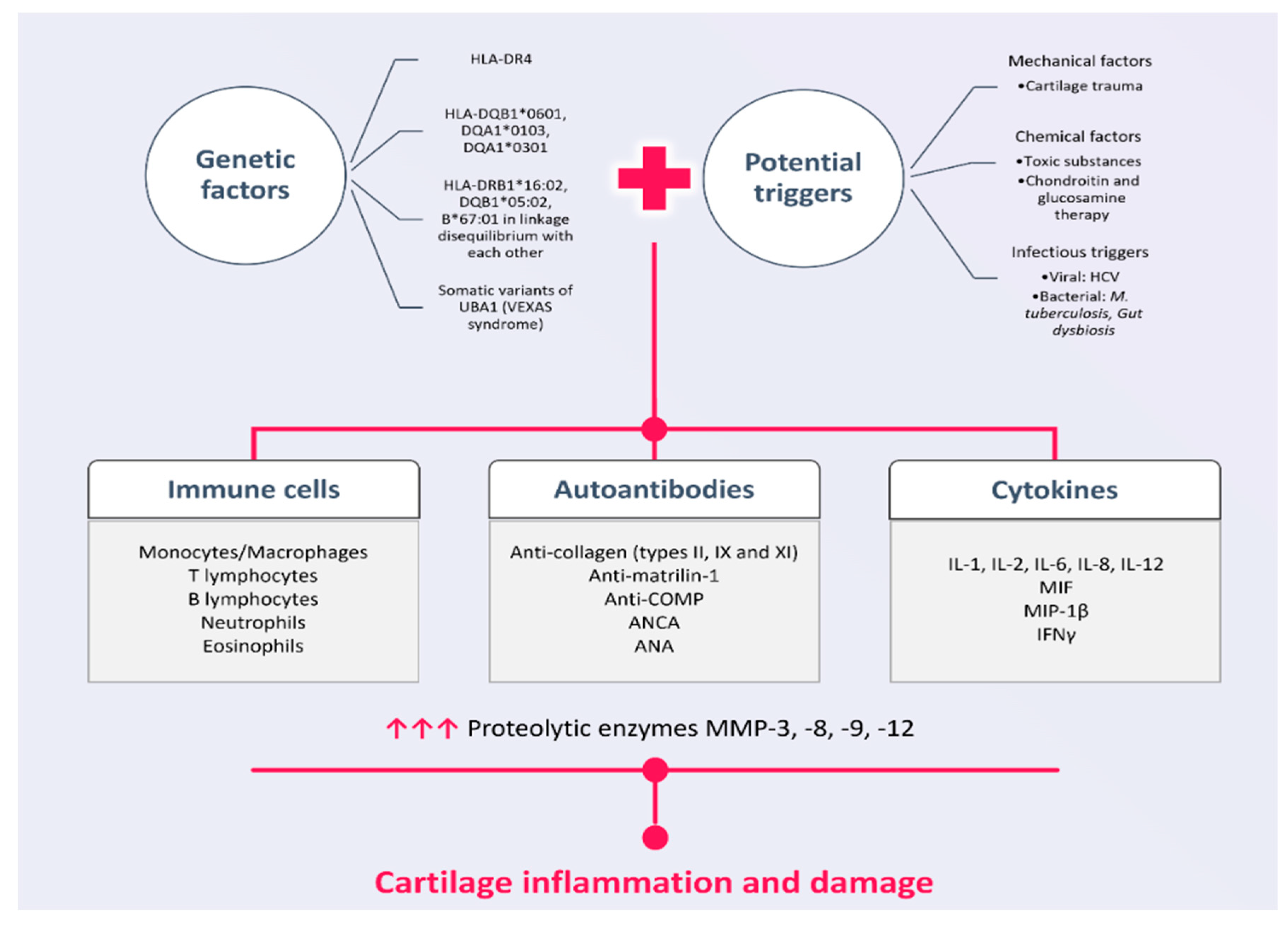
2. Pathogenetic Mechanisms in RP
2.1. Genetic Susceptibility in RP
2.2. External Triggers in RP
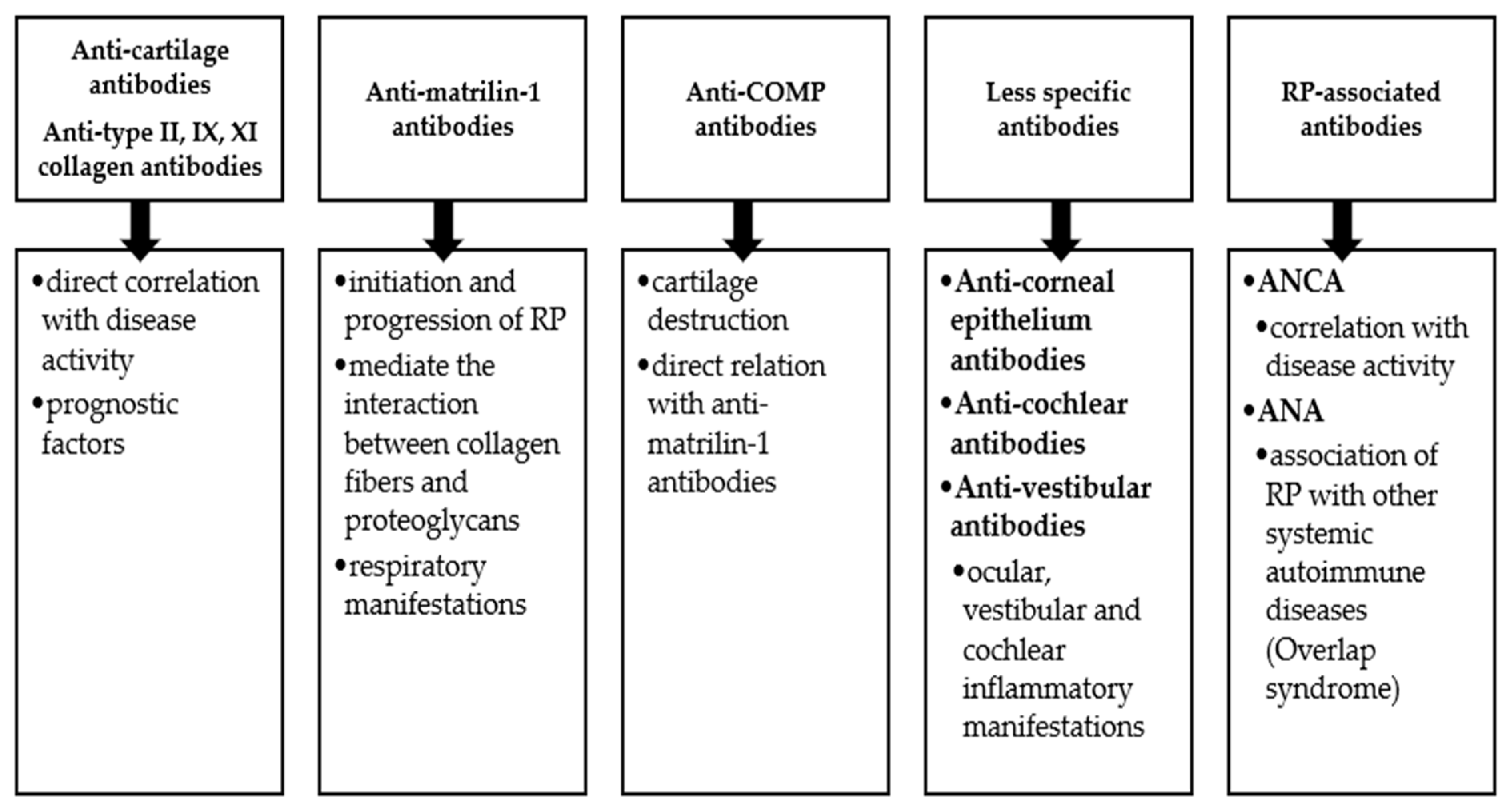
2.3. Humoral Mediators in RP
Autoantibodies in RP
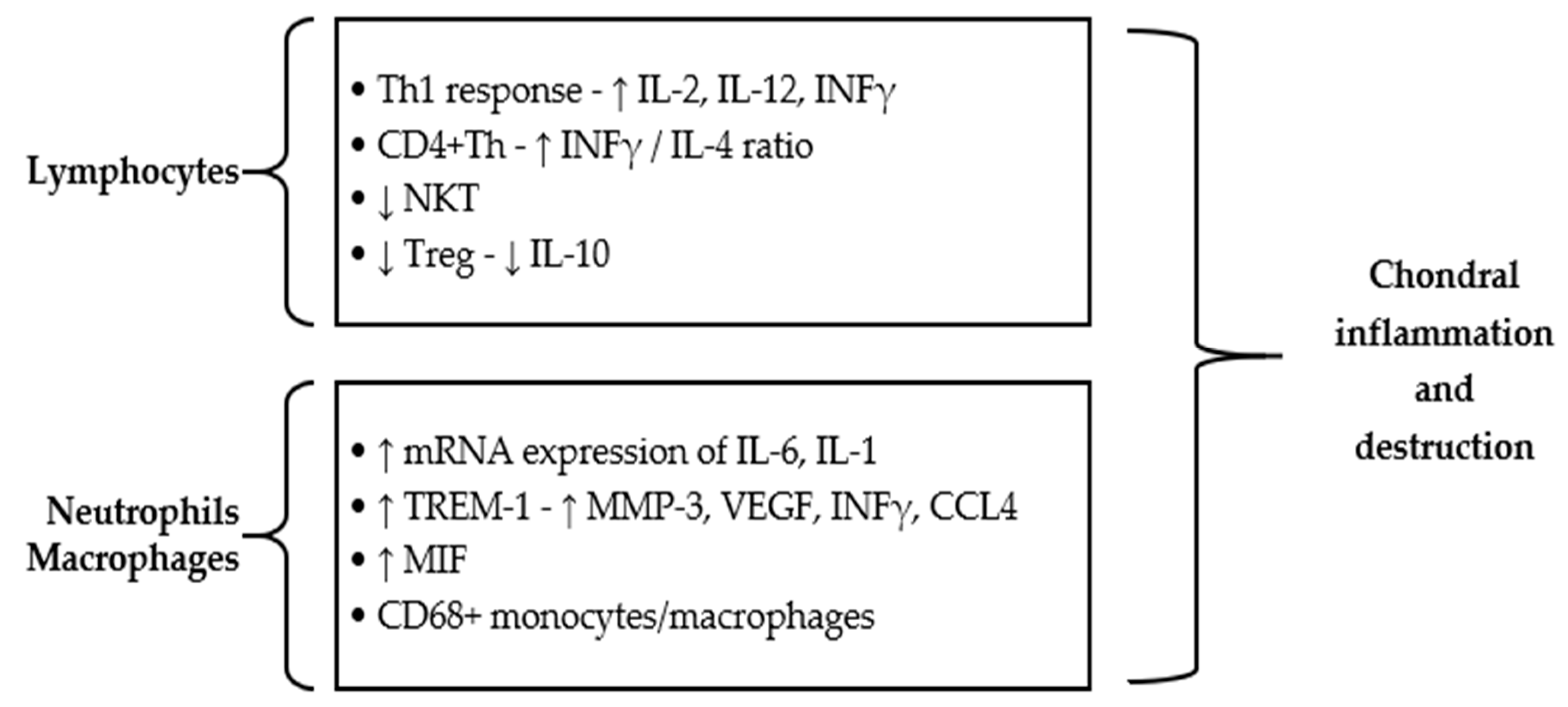
2.4. Cell-Mediated Immune Responses in RP
3. VEXAS Syndrome
4. Current Therapeutic Strategies in RP and VEXAS Syndrome
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borgia, F.; Giuffrida, R.; Guarneri, F.; Cannavò, S.P. Relapsing Polychondritis: An Updated Review. Biomedicines 2018, 6, 84. [Google Scholar] [CrossRef]
- Lahmer, T.; Treiber, M.; von Werder, A.; Foerger, F.; Knopf, A.; Heemann, U.; Thuermel, K. Relapsing polychondritis: An autoimmune disease with many faces. Autoimmun. Rev. 2010, 9, 540–546. [Google Scholar] [CrossRef]
- Sharma, A.; Gnanapandithan, K.; Sharma, K.; Sharma, S. Relapsing polychondritis: A review. Clin. Rheumatol. 2013, 32, 1575–1583. [Google Scholar] [CrossRef]
- Butterton, J.R.; Collier, D.S.; Romero, J.M.; Zembowicz, A. Case records of the Massachusetts General Hospital. Case 14-2007. A 59-year old man with fever and pain and swelling of both eyes and the right ear. N. Engl. J. Med. 2007, 356, 1980–1988. [Google Scholar] [CrossRef]
- Jaksch-Wartenhorst, R. Polychondropathia. Wien. Arch. Inn. Med. 1923, 6, 93–100. [Google Scholar]
- Pearson, C.M.; Kline, H.M.; Newcomer, V.D. Relapsing polychondritis. N. Engl. J. Med. 1960, 263, 51–58. [Google Scholar] [CrossRef]
- Lin, D.F.; Yang, W.Q.; Zhang, P.P.; Lv, Q.; Jin, O.; Gu, J.R. Clinical and prognostic characteristics of 158 cases of relapsing polychondritis in China and review of the literature. Rheumatol. Int. 2016, 36, 1003–1009. [Google Scholar] [CrossRef]
- Ostrowski, R.A.; Takagishi, T.; Robinson, J. Rheumatoid arthritis, spondyloarthropathies, and relapsing polychondritis. Handb. Clin. Neurol. 2014, 119, 449–461. [Google Scholar]
- Damiani, J.M.; Levine, H.L. Relapsing polychondritis—Report of ten cases. Laryngoscope 1979, 89, 929–946. [Google Scholar] [CrossRef]
- Cantarini, L.; Vitale, A.; Brizi, M.G.; Caso, F.; Frediani, B.; Punzi, L.; Galeazzi, M.; Rigante, D. Diagnosis and classification of relapsing polychondritis. J. Autoimmun. 2014, 48, 53–59. [Google Scholar] [CrossRef]
- D’Cruz, D.P.; Ferrada, M.A. Relapsing polychondritis and large-vessel vasculitis. J. Rheumatol. 2020, 47, 1732–1733. [Google Scholar] [CrossRef]
- Zeuner, M.; Straub, R.H.; Rauh, G.; Albert, E.D.; Scholmerich, J.; Lang, B. Relapsing polychondritis: Clinical and immunogenetic analysis of 62 patients. J. Rheumatol. 1997, 24, 96–101. [Google Scholar]
- Frances, C.; El Rassi, R.; Laporte, J.L.; Rybojad, M.; Papo, T.; Piette, J.C. Dermatologic manifestations of relapsing polychondritis: A study of 200 cases at a single center. Medicine 2000, 80, 173–179. [Google Scholar] [CrossRef]
- Dion, J.; Costedoat-Chalumeau, N.; Sene, D.; Cohen-Bittan, J.; Leroux, G.; Dion, C.; Francès, C.; Piette, J.-C. Relapsing polychondritis can be characterized by three different clinical phenotypes: Analysis of a recent series of 142 patients. Arthritis Rheumatol. 2016, 68, 2992–3001. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Cheng, L.; Zhan, H.; Huang, Y.; Li, H.; Li, Y. Progress and challenges in the use of blood biomarkers in relapsing polychondritis. Clin. Exp. Immunol. 2023, 212, 199–211. [Google Scholar] [CrossRef]
- Lang, B.; Rothenfusser, A.; Lanchbury, J.S.; Rauh, G.; Breedveld, F.C.; Urlacher, A.; Albert, E.D.; Peter, H.H.; Melchers, I. Susceptibility to relapsing polychondritis is associated with HLA-DR4. Arthritis Rheum. 1993, 36, 660–664. [Google Scholar] [CrossRef]
- Hewagama, A.; Richardson, B. The genetics and epigenetics of autoimmune diseases. J. Autoimmun. 2009, 33, 3–11. [Google Scholar] [CrossRef]
- Shimizu, J.; Murayama, M.A.; Mizukami, Y.; Arimitsu, N.; Takai, K.; Miyabe, Y. Innate immune responses in Behçet disease and relapsing polychondritis. Front. Med. 2023, 10, 1055753. [Google Scholar] [CrossRef]
- Bradley, D.S.; Das, P.; Griffiths, M.M.; Luthra, H.S.; David, C.S. HLA-DQ6/8 double transgenic mice develop auricular chondritis following type II collagen immunization: A model for human relapsing polychondritis. J. Immunol. 1998, 161, 5046–5053. [Google Scholar] [CrossRef]
- Hue-Lemoine, S.; Caillat-Zucman, S.; Amoura, Z.; Bach, J.F.; Piette, J.C. HLA-DQA1 AND DQB1 alleles are associated with susceptibility to relapsing polychondritis (RP): From transgenic mice to humans. Arthritis Rheum. 1999, 42, S261. [Google Scholar]
- Terao, C.; Yoshifuji, H.; Yamano, Y.; Kojima, H.; Yurugi, K.; Miura, Y.; Maekawa, T.; Handa, H.; Ohmura, K.; Saji, H.; et al. Genotyping of relapsing polychondritis identified novel susceptibility HLA alleles and distinct genetic characteristics from other rheumatic diseases. Rheumatology 2016, 55, 1686–1692. [Google Scholar] [CrossRef]
- Beck, D.B.; Ferrada, M.A.; Sikora, K.A.; Ombrello, A.K.; Collins, J.C.; Pei, W.; Balanda, N.; Ross, D.L.; Cardona, D.O.; Wu, Z.; et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 2020, 383, 2628–2638. [Google Scholar] [CrossRef]
- Tsuchida, N.; Kunishita, Y.; Uchiyama, Y.; Kirino, Y.; Enaka, M.; Yamaguchi, Y.; Taguri, M.; Yamanaka, S.; Takase-Minegishi, K.; Yoshimi, R.; et al. Pathogenic UBA1 variants associated with VEXAS syndrome in Japanese patients with relapsing polychondritis. Ann. Rheum. Dis. 2021, 80, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Ferrada, M.A.; Sikora, K.A.; Luo, Y.; Wells, K.V.; Patel, B.; Groarke, E.M.; Cardona, D.O.; Rominger, E.; Hoffmann, P.; Le, M.T.; et al. Somatic mutations in UBA1 define a distinct subset of relapsing polychondritis patients with VEXAS. Arthritis Rheumatol. 2021, 73, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Canas, C.A.; Abadía, F.B. Local cartilage trauma as a pathogenic factor in autoimmunity (one hypothesis based on patients with relapsing polychondritis triggered by cartilage trauma). Autoimmune Dis. 2012, 2012, 453698. [Google Scholar] [CrossRef] [PubMed]
- Alissa, H.; Kadanoff, R.; Adams, E. Does mechanical insult to cartilage trigger relapsing polychondritis? Scand. J. Rheumatol. 2001, 30, 311. [Google Scholar] [PubMed]
- Berger, R. Polychondritis resulting from intravenous substance abuse. Am. J. Med. 1988, 85, 415–417. [Google Scholar] [CrossRef]
- Furer, V.; Wieczorek, R.L.; Pillinger, M.H. Bilateral pinna chondritis preceded by glucosamine chondroitin supplement initiation. Scand. J. Rheumatol. 2010, 40, 241–243. [Google Scholar] [CrossRef]
- Mccluskey, R.T.; Thomas, L. The removal of cartilage matrix, in vivo, by papain: Identification of crystalline papain protease as the cause of the phenomenon. J. Exp. Med. 1958, 108, 371. [Google Scholar] [CrossRef] [PubMed]
- Zugel, U.; Kaufmann, S.H. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 1999, 12, 19–39. [Google Scholar] [CrossRef]
- Yang, C.L.; Brinckmann, J.; Rui, H.F.; Vehring, K.H.; Lehmann, H.; Kekow, J.; Wolff, H.H.; Gross, W.L.; Müller, P.K. Autoantibodies to cartilage collagens in relapsing polychondritis. Arch. Dermatol. Res. 1993, 285, 245–249. [Google Scholar] [CrossRef]
- Hemmati, I.; Yoshida, E.; Shojania, K. Relapsing polychondritis associated with hepatitis C virus infection. Clin. Rheumatol. 2012, 31, 391–394. [Google Scholar] [CrossRef]
- Herrera, I.; Concha, R.; Molina, E.G.; Schiff, E.R.; Altman, R.D. Relapsing polychondritis, chronic hepatitis C virus infection, and mixed cryoglobulemia. Semin. Arthritis Rheum. 2004, 33, 388–403. [Google Scholar] [CrossRef]
- Menge, T.; Rzepka, R.; Melchers, I. Monoclonal autoantibodies from patients with autoimmune diseases: Specificity, affinity and crossreactivity of MAbs binding to cytoskeletal and nucleolar epitopes, cartilage antigens and mycobacterial heat-shock protein 60. Immunobiology 2002, 205, 1–16. [Google Scholar] [CrossRef]
- Shimizu, J.; Takai, K.; Takada, E.; Fujiwara, N.; Arimitsu, N.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; Suzuki, N. Possible association of proinflammatory cytokines including IL1beta and TNFalpha with enhanced Th17 cell differentiation in patients with Behcet’s disease. Clin. Rheumatol. 2016, 35, 1857–1863. [Google Scholar] [CrossRef]
- Foidart, J.M.; Abe, S.; Martin, G.R.; Zizic, T.M.; Barnett, E.V.; Lawley, T.J.; Katz, S.J. Antibodies to type II collagen in relapsing polychondritis. N. Engl. J. Med. 1978, 299, 1203–1207. [Google Scholar] [CrossRef]
- Alsalameh, S.; Mollenhauer, J.; Scheuplein, F.; Stöss, H.; Kalden, J.R.; Burkhardt, H.; Burmester, G.R. Preferential cellular and humoral immune reactivities to native and denatured collagen types IX and XI in a patient with fatal relapsing polychondritis. J. Rheumatol. 1993, 20, 1419–1424. [Google Scholar]
- Ebringer, R.; Rook, G.; Swana, G.T.; Bottazzo, G.F.; Doniach, D. Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann. Rheum. Dis. 1981, 40, 473–479. [Google Scholar] [CrossRef]
- Buckner, J.H.; Van Landeghen, M.; Kwok, W.W.; Tsarknaridis, L. Identification of type II collagen peptide 261-273-specific T cell clones in a patient with relapsing polychondritis. Arthritis Rheum. 2002, 46, 238–244. [Google Scholar] [CrossRef]
- Wooley, P.H.; Luthra, H.S.; O’Duffy, J.D.; Bunch, T.W.; Moore, S.B.; Stuart, J.M. Anti-type II collagen antibodies in rheumatoid arthritis. The influence of HLA phenotype. Tissue Antigens 1984, 23, 263–269. [Google Scholar] [CrossRef]
- Terato, K.; Shimozuru, Y.; Katayama, K.; Takemitsu, Y.; Yamashita, I.; Miyatsu, M.; Fujii, K.; Sagara, M.; Kobayashi, S.; Goto, M.; et al. Specificity of antibodies to type II collagen in rheumatoid arthritis. Arthritis Rheum. 1990, 33, 1493–1500. [Google Scholar] [CrossRef]
- Taneja, V.; Griffiths, M.; Behrens, M.; Luthra, H.S.; David, C.S. Auricular chondritis in NOD.DQ8.Abetao (Ag7−/−) transgenic mice resembles human relapsing polychondritis. J. Clin. Investig. 2003, 112, 1843–1850. [Google Scholar]
- Mörgelin, M.; Paulsson, M.; Heinegård, D.; Aebi, U.; Engel, J. Evidence of a defined spatial arrangement of hyaluronate in the central filament of cartilage proteoglycan aggregates. Biochem. J. 1995, 307, 595–601. [Google Scholar] [CrossRef]
- Wu, J.-J.; Eyre, D.R. Matrilin-3 forms disulfide-linked oligomers with matrilin-1 in bovine epiphyseal cartilage. J. Biol. Chem. 1998, 273, 17433–17438. [Google Scholar] [CrossRef]
- Hansson, A.S.; Johannesson, M.; Svensson, L.; Nandakumar, K.S.; Heinegard, D.; Holmdahl, R. Relapsing polychondritis, induced in mice with matrilin 1, is an antibody- and complement-dependent disease. Am. J. Pathol. 2004, 164, 959–966. [Google Scholar] [CrossRef][Green Version]
- Lamoureux, J.L.; Buckner, J.H.; David, C.S.; Bradley, D.S. Mice expressing HLADQ6alpha8beta transgenes develop polychondritis spontaneously. Arthritis Res. Ther. 2006, 8, R134. [Google Scholar] [CrossRef]
- Paulsson, M.; Wagener, R. Matrilins. Methods Cell Biol. 2018, 143, 429–446. [Google Scholar]
- Saxne, T.; Heinegard, D. Serum concentrations of two cartilage matrix proteins reflecting different aspects of cartilage turnover in relapsing polychondritis. Arthritis Rheum. 1995, 38, 294–296. [Google Scholar] [CrossRef]
- Hansson, A.S.; Heinegard, D.; Piette, J.C.; Burkhardt, H.; Holmdahl, R. The occurrence of autoantibodies to matrilin 1 reflects a tissue-specific response to cartilage of the respiratory tract in patients with relapsing polychondritis. Arthritis Rheum. 2001, 44, 2402–2412. [Google Scholar] [CrossRef]
- Hedbom, E.; Antonsson, P.; Hjerpe, A.; Aeschlimann, D.; Paulsson, M.; Rosa-Pimentel, E.; Sommarin, Y.; Wendel, M.; Oldberg, A.; Heinegård, D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 1992, 267, 6132–6136. [Google Scholar] [CrossRef]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef]
- Hansson, A.S.; Heinegard, D.; Holmdahl, R. A new animal model for relapsing polychondritis, induced by cartilage matrix protein (matrilin-1). J. Clin. Investig. 1999, 104, 589–598. [Google Scholar] [CrossRef]
- Kempta, L.F.; Piette, J.C.; Bastuji-Garin, S.; Kraus, V.B.; Stabler, T.V.; Poole, A.R.; Marini-Portugal, A.; Chevalier, X. Serum cartilage oligomeric matrix protein (COMP) level is a marker of disease activity in relapsing polychondritis. Clin. Exp. Rheumatol. 2010, 28, 553–555. [Google Scholar]
- Papo, T.; Piette, J.C.; Le Thi, H.D.; Godeau, P.; Meyer, O.; Kahn, M.F.; Bourgeois, P. Antineutrophil cytoplasmic antibodies in polychondritis. Ann. Rheum. Dis. 1993, 52, 384–385. [Google Scholar] [CrossRef]
- Xuan, Y.Y.; Li, T.F.; Zhang, L.; Liu, S.Y. ANCA positive relapsing polychondritis, Graves disease, and suspected moyamoya disease: A case report. Medicine 2017, 96, e9378. [Google Scholar] [CrossRef]
- Piette, J.C.; El-Rassi, R.; Amoura, Z. Antinuclear antibodies in relapsing polychondritis. Ann. Rheum. Dis. 1999, 58, 656–657. [Google Scholar] [CrossRef]
- Albers, F.W.; Majoor, M.H.; Van der Gaag, R. Corneal autoimmunity in a patient with relapsing polychondritis. Eur. Arch. Otorhinolaryngol. 1992, 249, 296–299. [Google Scholar] [CrossRef]
- Issing, W.J.; Selover, D.; Schulz, P. Anti-labyrinthine antibodies in a patient with relapsing polychondritis. Eur. Arch. Otorhinolaryngol. 1999, 256, 163–166. [Google Scholar] [CrossRef]
- Kashihara, K.; Kawada, S.; Takahashi, Y. Autoantibodies to glutamate receptor GluRepsilon2 in a patient with limbic encephalitis associated with relapsing polychondritis. J. Neurol. Sci. 2009, 28, 275–277. [Google Scholar] [CrossRef]
- Kumakiri, K.; Sakamoto, T.; Karahashi, T.; Mineta, H.; Takebayashi, S. A case of relapsing polychondritis preceded by inner ear involvement. Auris Nasus Larynx 2005, 32, 71–76. [Google Scholar] [CrossRef]
- Kobayashi, T.; Moody, S.; Komori, M.; Jibatake, A.; Yaegashi, M. Early stage relapsing polychondritis diagnosed by nasal septum biopsy. Case Rep. Med. 2015, 2015, 307868. [Google Scholar] [CrossRef]
- Ouchi, N.; Uzuki, M.; Kamataki, A.; Miura, Y.; Sawai, T. Cartilage destruction is partly induced by the internal proteolytic enzymes and apoptotic phenomenon of chondrocytes in relapsing polychondritis. J. Rheumatol. 2011, 38, 730–737. [Google Scholar] [CrossRef]
- Arnaud, L.; Mathian, A.; Haroche, J.; Gorochov, G.; Amoura, Z. Pathogenesis of relapsing polychondritis: A 2013 update. Autoimmun. Rev. 2014, 13, 90–95. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, M.; Li, H.; Xu, D.; Li, M.; Zhang, X.; Zhang, F.; Hou, Y.; Zeng, X. Three new inflammatory markers C reactive protein to albumin ratio, neutrophil to lymphocyte ratio, and platelet to lymphocyte ratio correlated with relapsing polychondritis disease activity index. Clin. Rheumatol. 2021, 40, 4685–4691. [Google Scholar] [CrossRef]
- Yu, E.N.; Jurkunas, U.; Rubin, P.A.; Baltatzis, S.; Foster, C.S. Obliterative microangiopathy presenting as chronic conjunctivitis in a patient with relapsing polychondritis. Cornea 2006, 25, 621–622. [Google Scholar] [CrossRef]
- Shimizu, J.; Wakisaka, S.; Suzuki, T.; Suzuki, N. Serum MMP3 correlated with IL1beta messenger RNA expressions of peripheral blood mononuclear cells in patients with relapsing Polychondritis with respiratory involvement. ACR Open Rheumatol. 2021, 3, 636–641. [Google Scholar] [CrossRef]
- Tammaro, A.; Stroo, I.; Rampanelli, E.; Blank, F.; Butter, L.M.; Claessen, N.; Takai, T.; Colonna, M.; Leemans, J.C.; Florquin, S.; et al. Role of TREM1-DAP12 in renal inflammation during obstructive nephropathy. PLoS ONE 2013, 8, e82498. [Google Scholar] [CrossRef]
- Sato, T.; Yamano, Y.; Tomaru, U.; Shimizu, Y.; Ando, H.; Okazaki, T.; Nagafuchi, H.; Shimizu, J.; Ozaki, S.; Miyazawa, T.; et al. Serum level of soluble triggering receptor expressed on myeloid cells-1 as a biomarker of disease activity in relapsing polychondritis. Mod. Rheumatol. 2014, 24, 129–136. [Google Scholar] [CrossRef]
- Gomez-Pina, V.; Soares-Schanoski, A.; Rodriguez-Rojas, A.; Del, F.C.; Garcia, F.; Vallejo-Cremades, M.T.; Fernández-Ruiz, I.; Arnalich, F.; Fuentes-Prior, P.; López-Collazo, E. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J. Immunol. 2007, 179, 4065–4073. [Google Scholar] [CrossRef]
- Ohwatari, R.; Fukuda, S.; Iwabuchi, K.; Inuyama, Y.; Onoe, K.; Nishihira, J. Serum level of macrophage migration inhibitory factor as a useful parameter of clinical course in patients with Wegener’s granulomatosis and relapsing polychondritis. Ann. Otol. Rhinol. Laryngol. 2001, 110, 1035–1040. [Google Scholar] [CrossRef]
- Stabler, T.; Piette, J.C.; Chevalier, X.; Marini-Portugal, A.; Kraus, V.B. Serum cytokine profiles in relapsing polychondritis suggest monocyte/macrophage activation. Arthritis Rheum. 2004, 50, 3663–3667. [Google Scholar] [CrossRef]
- Kraus, V.B.; Stabler, T.; Le, E.T.; Saltarelli, M.; Allen, N.B. Urinary type II collagen neoepitope as an outcome measure for relapsing polychondritis. Arthritis Rheum. 2003, 48, 2942–2948. [Google Scholar] [CrossRef]
- Hu, F.-Y.; Wang, J.; Zhang, S.-X.; Su, R.; Yan, N.; Gao, C.; Li, X.-F.; Wang, C.-H. Absolute reduction of peripheral regulatory T cell in patients with relapsing polychondritis. Clin. Exp. Rheumatol. 2021, 39, 487–493. [Google Scholar] [CrossRef]
- Shimizu, J.; Kubota, T.; Takada, E.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Murayama, M.A.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; et al. Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis. PLoS ONE 2018, 13, e203657. [Google Scholar] [CrossRef]
- Takagi, D.; Iwabuchi, K.; Iwabuchi, C.; Nakamaru, Y.; Maguchi, S.; Ohwatari, R.; Furata, Y.; Fukada, S.; Joyce, S.; Onoé, K. Immunoregulatory defects of V alpha 24V+ beta 11+ NKT cells in development of Wegener’s granulomatosis and relapsing polychondritis. Clin. Exp. Immunol. 2004, 136, 591–600. [Google Scholar] [CrossRef]
- Evans, M.A.; Walsh, K. Clonal hematopoiesis, somatic mosaicism, and age-associated disease. Physiol. Rev. 2023, 103, 649–716. [Google Scholar] [CrossRef]
- Abbasi, A.; Alexandrov, L.B. Significance and limitations of the use of next-generation sequencing technologies for detecting mutational signatures. DNA Repair. 2021, 107, 103200. [Google Scholar] [CrossRef]
- Oh, E.; Akopian, D.; Rape, M. Principles of ubiquitin dependent signaling. Annu. Rev. Cell Dev. Biol. 2018, 34, 137–162. [Google Scholar] [CrossRef]
- Schulman, B.A.; Harper, J.W. Ubiquitin-like protein activation by E1 enzymes: The apex for downstream signalling pathways. Nat. Rev. Mol. Cell Biol. 2009, 10, 319–331. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Rape, M. Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 59–70. [Google Scholar] [CrossRef]
- Aksentijevich, I.; Zhou, Q. NF-kappaB pathway in autoinflammatory diseases: Dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front. Immunol. 2017, 8, 399. [Google Scholar] [CrossRef]
- Lacombe, V.; Prevost, M.; Bouvier, A.; Thépot, S.; Chabrun, F.; Kosmider, O.; Lacout, C.; Beucher, A.; Lavigne, C.; Geneviève, F.; et al. Vacuoles in neutrophil precursors in VEXAS syndrome: Diagnostic performances and threshold. Br. J. Haematol. 2021, 195, 286–289. [Google Scholar] [CrossRef]
- Arlet, J.B.; Terrier, B.; Kosmider, O. Mutant UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 2021, 384, 2163. [Google Scholar]
- Stubbins, R.J. Lost in translation: Cytoplasmic UBA1 and VEXAS syndrome. Blood 2022, 140, 1455–1457. [Google Scholar] [CrossRef]
- Shaukat, F.; Hart, M.; Burns, T.; Bansal, P. UBA1 and DNMT3A mutations in VEXAS syndrome. A case report and literature review. Mod. Rheumatol. Case Rep. 2022, 6, 134–139. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W.; Cai, W.; Liu, J.; Wang, H.; Qin, T.; Xu, Z.; Li, B.; Qu, S.; Pan, L.; et al. VEXAS syndrome in myelodysplastic syndrome with autoimmune disorder. Exp. Hematol. Oncol. 2021, 10, 23. [Google Scholar] [CrossRef]
- Jachiet, V.; Ricard, L.; Hirsch, P.; Malard, F.; Pascal, L.; Beyne-Rauzy, O.; Peterlin, P.; Maria, A.T.J.; Vey, N.; D’Aveni, M.; et al. MINHEMON: French Network of dysimmune disorders associated with hemopathies. Reduced peripheral blood dendritic cell and monocyte subsets in MDS patients with systemic inflammatory or dysimmune diseases. Clin. Exp. Med. 2023, 23, 803–813. [Google Scholar] [CrossRef]
- Lucchino, B.; Finucci, A.; Ghellere, F.; Bortolotti, M.E.; Tedesco, A.; Lombardi, S. Influence of HLA polymorphisms on clinical features of VEXAS syndrome: A potential epistatic mechanism. Rheumatology 2022, 62, e7–e8. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; Terrier, B.; Guedon, A.F.; Heiblig, M.; Comont, T.; Lazaro, E.; Lacombe, V.; Terriou, L.; Ardois, S.; Bouaziz, J.-D.; et al. Further characterization of clinical and laboratory features in VEXAS syndrome: Large-scale analysis of a multicentre case series of 116 French patients. Br. J. Dermatol. 2022, 186, 564–574. [Google Scholar] [CrossRef]
- Barba, T.; Jamilloux, Y.; Durel, C.-A.; Bourbon, E.; Mestrallet, F.; Sujobert, P.; Hot, A. VEXAS syndrome in a woman. Rheumatology 2021, 60, E402–E403. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.J.; McGinnis, E.; Johal, B.; Chen, L.Y.; Wilson, L.; Cardona, D.O.; Nevill, T.J. VEXAS syndrome in a female patient with constitutional 45,X (Turner syndrome). Haematologica 2022, 107, 1011. [Google Scholar] [CrossRef] [PubMed]
- Templé, M.; Kosmider, O. Syndrome: VEXAS. A Novelty in MDS Landscape. Diagnostics 2022, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bermúdez, C.A.; Cardona-Cardona, A.F.; Ariza-Parra, E.J.; Arostegui, J.I.; Mensa-Vilaro, A.; Yague, J.; Vásquez, G.; Muñoz-Vahos, C.H. Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic syndrome (VEXAS syndrome) with prominent supraglottic larynx involvement: A case-based review. Clin. Rheumatol. 2022, 41, 3565–3572. [Google Scholar] [CrossRef]
- Obiorah, I.E.; Patel, B.A.; Groarke, E.M.; Wang, W.; Trick, M.; Ombrello, A.K.; Ferrada, M.A.; Wu, Z.; Gutierrez-Rodrigues, F.; Lotter, J.; et al. Benign and malignant hematologic manifestations in patients with VEXAS syndrome due to somatic mutations in UBA1. Blood Adv. 2021, 5, 3203–3215. [Google Scholar] [CrossRef]
- D’Angelo, G. Hematopoietic cells vacuolation, not always a reactive event. The VEXAS syndrome. Int. J. Lab. Hematol. 2023, 45, e15–e16. [Google Scholar] [CrossRef]
- Matsumoto, H.; Asano, T.; Tsuchida, N.; Maeda, A.; Yoshida, S.; Yokose, K.; Fujita, Y.; Temmoku, J.; Matsuoka, N.; Yashiro-Furuya, M.; et al. Behçet’s disease with a somatic UBA1 variant: Expanding spectrum of autoinflammatory phenotypes of VEXAS syndrome. Clin. Immunol. 2022, 238, 108996. [Google Scholar] [CrossRef]
- Khitri, M.-Y.; Guedon, A.F.; Georgin-Lavialle, S.; Terrier, B.; Saadoun, D.; Seguier, J.; le Besnerais, M.; De Moreuil, C.; Denis, G.; Gerfaud-Valentin, M.; et al. Comparison between idiopathic and VEXAS-relapsing polychondritis: Analysis of a French case series of 95 patients. RMD Open 2022, 8, e002255. [Google Scholar] [CrossRef]
- Bourbon, E.; Heiblig, M.; Valentin, M.G.; Barba, T.; Durel, C.-A.; Lega, J.C.; Barraco, F.; Sève, P.; Jamilloux, Y.; Sujobert, P. Therapeutic options in VEXAS syndrome: Insights from a retrospective series. Blood 2021, 137, 3682–3684. [Google Scholar] [CrossRef]
- Sterling, D.; Duncan, M.E.; Philippidou, M.; Salisbury, J.R.; Kulasekararaj, A.G.; Basu, T.N. VEXAS syndrome (vacuoles, E1 enzyme, X-linked, autoinfammatory, somatic) for the dermatolo gist. J. Am. Acad. Dermatol. 2022, 89, 1209–1214. [Google Scholar] [CrossRef]
- Ferrada, M.A.; Savic, S.; Cardona, D.O.; Collins, J.C.; Alessi, H.; Gutierrez-Rodrigues, F.; Kumar, D.B.U.; Wilson, L.; Goodspeed, W.; Topilow, J.S.; et al. Translation of cytoplasmic UBA1 contributes to VEXAS syndrome pathogenesis. Blood 2022, 140, 1496–1506. [Google Scholar] [CrossRef]
- Comont, T.; Heiblig, M.; Rivière, E.; Terriou, L.; Rossignol, J.; Bouscary, D.; Rieu, V.; Le Guenno, G.; Mathian, A.; Aouba, A.; et al. Azacitidine for patients with Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic syndrome (VEXAS) and myelodysplastic syndrome: Data from the French VEXAS registry. Br. J. Haematol. 2022, 196, 969–974. [Google Scholar] [CrossRef]
- Mekinian, A.; Zhao, L.P.; Chevret, S.; Desseaux, K.; Pascal, L.; Comont, T.; Maria, A.; Peterlin, P.; Terriou, L.; Piney, A.; et al. A Phase II prospective trial of azacitidine in steroid-dependent or refractory systemic autoimmune/inflammatory disorders VEXAS syndrome associated with MDS and CMML. Leukemia 2022, 36, 2739–2742. [Google Scholar] [CrossRef]
- van der Made, C.I.; Potjewijd, J.; Hoogstins, A.; Willems, H.P.; Kwakernaak, A.J.; de Sevaux, R.G.; van Daele, P.L.; Simons, A.; Heijstek, M.; Beck, D.B.; et al. Adult-onset autoinflammation caused by somatic mutations in UBA1: A Dutch case series of patients with VEXAS. J. Allergy Clin. Immunol. 2022, 149, 432–439.e4. [Google Scholar] [CrossRef]
- Martín-Nares, E.; Vargas-Serafín, C.; Delgado-de la Mora, J.; Mont ante-Montes de Oca, D.; Grayson, P.C.; Larios, E.; Crispín, J.C. Orbital and periorbital infammation in VEXAS syndrome. Scand. J. Rheumatol. 2022, 51, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Himmelmann, A.; Brücker, R. The VEXAS syndrome: Uncontrolled inflammation and macrocytic anaemia in a 77-year old male patient. Eur. J. Case Rep. Intern. Med. 2021, 8, 002484. [Google Scholar] [CrossRef] [PubMed]
- Groarke, E.M.; Dulau-Florea, A.E.; Kanthi, Y. Thrombotic manifestations of VEXAS syndrome. Semin. Hematol. 2021, 58, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Khider, L.; Templé, M.; Bally, C.; Spaeth, A.; Darnige, L.; Sanchez, O.; Planquette, B.; Mortelette, H.; Messas, E.; Smadja, D.M.; et al. Systematic search for the UBA1 mutation in men after a first episode of venous thromboembolism: A monocentric study. J. Thromb. Haemost. 2022, 20, 2697–2699. [Google Scholar] [CrossRef] [PubMed]
- Bert-Marcaz, C.; Briantais, A.; Faucher, B.; Corazza, G.; Ebbo, M.; Attarian, S.; Delmont, E.; Fortanier, E. Expanding the spectrum of VEXAS syndrome: Association with acute-onset CIDP. J. Neurol. Neurosurg. Psychiatry 2021, 93, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Pieringer, H. Relapsing polychondritis: A chameleon among orphan diseases. Wien. Med. Wochenschr. 2017, 167, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Cuestas, D.; Peñaranda, E.; Mora, S.; Cortes, C.; Galvis, I.; Patiño, M.; Velasquez, O. Relapsing polychondritis, an underestimated dermatological urgency: Case report and literature review. Int. J. Dermatol. 2017, 56, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.A.; Franks, A.G., Jr. Colchicine and indomethacin for the treatment of relapsing polychondritis. J. Am. Acad. Dermatol. 2002, 46, S22–S24. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Sota, J.; Rigante, D.; Lopalco, G.; Molinaro, F.; Messina, M.; Iannone, F.; Cantarini, L. Relapsing Polychondritis: An Update on Pathogenesis, Clinical Features, Diagnostic Tools, and Therapeutic Perspectives. Curr. Rheumatol. Rep. 2016, 18, 3. [Google Scholar] [CrossRef]
- Sosada, B.; Loza, K.; Bialo-Wojcicka, E. Relapsing polychondritis. Case Rep. Dermatol. Med. 2014, 2014, 791951. [Google Scholar] [CrossRef]
- Mathian, A.; Miyara, M.; Cohen-Aubart, F.; Haroche, J.; Hie, M.; Pha, M.; Grenier, P.; Amoura, Z. Relapsing polychondritis: A 2016 update on clinical features, diagnostic tools, treatment and biological drug use. Best Pract. Res. Clin. Rheumatol 2016, 30, 316–333. [Google Scholar] [CrossRef]
- Hazra, N.; Dregan, A.; Charlton, J.; Gulliford, M.C.; D’Cruz, D.P. Incidence and mortality of relapsing polychondritis in the UK: A population-based cohort study. Rheumatology 2015, 54, 2181–2187. [Google Scholar] [CrossRef]
- Trentham, D.E.; Le, C.H. Relapsing polychondritis. Ann. Intern. Med. 1998, 129, 114–122. [Google Scholar] [CrossRef]
- Puéchal, X.; Terrier, B.; Mouthon, L.; Costedoat-Chalumeau, N.; Guillevin, L.; Le Jeunne, C. Relapsing polychondritis. Jt. Bone Spine 2014, 81, 118–124. [Google Scholar] [CrossRef]
- Letko, E.; Zafirakis, P.; Baltatzis, S.; Voudouri, A.; Livir-Rallatos, C.; Foster, C.S. Relapsing polychondritis: A clinical review. Semin. Arthritis Rheum. 2002, 31, 384–395. [Google Scholar] [CrossRef]
- Smylie, A.; Malhotra, N.; Brassard, A. Relapsing Polychondritis: A Review and Guide for the Dermatologist. Am. J. Clin. Dermatol. 2017, 18, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kemta Lekpa, F.; Kraus, V.B.; Chevalier, X. Biologics in relapsing polychondritis: A literature review. Semin. Arthritis Rheum. 2012, 41, 712–719. [Google Scholar] [CrossRef]
- Henes, J.C.; Xenitidis, T.; Horger, M. Tocilizumab for refractory relapsing polychondritis-long- term response monitoring by magnetic resonance imaging. Jt. Bone Spine 2016, 83, 365–366. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.M.; Cunnane, G. Treatment of relapsing polychondritis in the era of biological agents. Rheumatol. Int. 2010, 30, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Wendling, D.; Govindaraju, S.; Prati, C.; Toussirot, E.; Bertolini, E. Efficacy of anakinra in a patient with refractory relapsing polychondritis. Jt. Bone Spine 2008, 75, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Sailler, L.; Pugnet, G.; Astudillo, L.; Arlet, P. Biologics in relapsing polychondritis: A case series. Clin. Exp. Rheumatol. 2013, 31, 937–939. [Google Scholar] [PubMed]
- Moulis, G.; Sailler, L.; Astudillo, L.; Arlet, P. Abatacept for relapsing polychondritis. Rheumatology 2010, 49, 1019. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.L.; Rodriguez, D. Abatacept in relapsing polychondritis. Ann. Rheum. Dis. 2013, 72, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- Rapini, R.P.; Warner, N.B. Relapsing polychondritis. Clin. Dermatol. 2006, 24, 482–485. [Google Scholar] [CrossRef]
- Terrier, B.; Aouba, A.; Bienvenu, B.; Bloch-Queyrat, C.; Delair, E.; Mallet, J.; Mahr, A.; Guillevin, L. Complete remission in refractory relapsing polychondritis with intravenous immunoglobulins. Clin. Exp. Rheumatol. 2008, 26, 136–138. [Google Scholar]
- Kingdon, J.; Roscamp, J.; Sangle, S.; D’Cruz, D. Relapsing polychondritis: A clinical review for rheumatologists. Rheumatology 2017, 57, 1525–1532. [Google Scholar] [CrossRef]
- Zakine, E.; Schell, B.; Battistella, M.; Vignon-Pennamen, M.-D.; Chasset, F.; Mahévas, T.; Cordoliani, F.; Adès, L.; Sébert, M.; Delaleu, J.; et al. UBA1 Variations in neutrophilic dermatosis skin lesions of patients with VEXAS syndrome. JAMA Dermatol. 2021, 157, 1349–1354. [Google Scholar] [CrossRef]
- Al-Hakim, A.; Savic, S. An update on VEXAS syndrome. Expert. Rev. Clin. Immunol. 2023, 19, 203–215. [Google Scholar] [CrossRef]
- Heiblig, M.; Ferrada, M.A.; Koster, M.T.; Barba, T.; Gerfaud-Valentin, M.; Mékinian, A.; Coelho, H.; Fossard, G.; Barraco, F.; Galicier, L.; et al. Ruxolitinib is more effective than other JAK inhibitors to treat VEXAS syndrome: A retrospective multicenter study. Blood 2022, 140, 140. [Google Scholar] [CrossRef]
- Raaijmakers, M.H.G.P.; Hermans, M.; Aalbers, A.; Rijken, M.; Dalm, V.A.S.H.; van Daele, P.; Valk, P.J.M. Azacytidine treatment for VEXAS syndrome. HemaSphere 2021, 5, e661. [Google Scholar] [CrossRef]
- Patel, B.A.; Groarke, E.M.; Lotter, J.; Shalhoub, R.; Gutierrez-Rodrigues, F.; Rios, O.; Raffo, D.Q.; Wu, C.O.; Young, N.S. Long-term outcomes in patients with severe aplastic anemia treated with immunosuppression and eltrombopag: A phase 2 study. Blood 2022, 139, 34–43. [Google Scholar] [CrossRef]
- Vicente, A.; Patel, B.A.; Gutierrez-Rodrigues, F.; Groarke, E.; Giudice, V.; Lotter, J.; Feng, X.; Kajigaya, S.; Weinstein, B.; Barranta, E.; et al. Eltrombopag monotherapy can improve hematopoiesis in patients with low to intermediate risk-1 myelodysplastic syndrome. Haematologica 2020, 105, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Diarra, A.; Duployez, N.; Fournier, E.; Preudhomme, C.; Coiteux, V.; Magro, L.; Quesnel, B.; Heiblig, M.; Sujobert, P.; Barraco, F.; et al. Successful allogeneic hematopoietic stem cell transplantation in patients with VEXAS syndrome: A 2-center experience. Blood Adv. 2022, 6, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakim, A.; Poulter, J.A.; Mahmoud, D.; Rose, A.M.S.; Elcombe, S.; Lachmann, H.; Cargo, C.; Duncan, C.J.A.; Bishton, M.; Bigley, V.; et al. Allogeneic haematopoietic stem cell transplantation for VEXAS syndrome: UK experience. Br. J. Haematol. 2022, 199, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Mangaonkar, A.A.; Langer, K.J.; Lasho, T.L.; Finke, C.; Litzow, M.R.; Hogan, W.J.; Shah, M.V.; Go, R.S.; Bartoo, G.; Kutzke, J.; et al. Reduced intensity conditioning allogeneic hematopoietic stem cell trans plantation in VEXAS syndrome: Data from a prospective series of patients. Am. J. Hematol. 2022, 98, E28–E31. [Google Scholar] [PubMed]
- Staels, F.; Betrains, A.; Woei-A-Jin, S.; Boeckx, N.; Beckers, M.; Bervoets, A.; Willemsen, M.; Neerinckx, B.; Humblet-Baron, S.; Blockmans, D.E.; et al. Case report: VEXAS syndrome: From mild symptoms to life-threatening macrophage activation syndrome. Front. Immunol. 2021, 12, 678927. [Google Scholar] [CrossRef] [PubMed]
- Kirino, Y.; Takase-Minegishi, K.; Tsuchida, N.; Hirahara, L.; Kunishita, Y.; Yoshimi, R.; Nakajima, H. Tocilizumab in VEXAS relapsing polychondritis: A single-center pilot study in Japan. Ann. Rheum. Dis. 2021, 80, 1501–1502. [Google Scholar] [CrossRef] [PubMed]
- Guilpain, P. JAK inhibitors in autoinflammatory syndromes? The long road from drug development to daily clinical use. Rheumatology 2023, 62, 1368–1369. [Google Scholar] [CrossRef] [PubMed]
- Conway, R. Ruxolitinib takes center stage for VEXAS syndrome. Blood 2022, 140, 807–808. [Google Scholar] [CrossRef] [PubMed]
| Genetic Susceptibility in RP (HLA Class II) | The Main Clinical Manifestations | References |
|---|---|---|
| HLA-DR4 | Major risk of RP occurrence No predominance of a certain subtype | [16,17] |
| HLA-DRB1 HLA-DQ6/8 | Cartilaginous inflammatory manifestations (auricular chondritis) | [16,19] |
| HLA-DR6 | Negative correlations with organ damage A higher median age at disease onset | [17] |
| DQB1*0601 DQA1*0103 DQA1*0301 | Confers susceptibility to RP Severe experimental polychondritis, exhibiting both polyarthritis and auricular chondritis | [20] |
| HLA-DRB1*16:02 HLA-DQB1*05:02 HLA-B*67:01 | Associated with susceptibility to RP (in linkage disequilibrium with each other) Cartilaginous inflammatory manifestations | [21] |
| Trigger Type | Pathogenic Triggering Mechanism and Clinical Manifestations | References | |
|---|---|---|---|
| Mechanical triggers | Trauma to the cartilage | Exposure of cartilage matrix protein antigens | [25] |
| Autoimmune response | |||
| Ear piercing | Inflammatory changes in the nose, ears and upper respiratory tract | [26] | |
| Chemical triggers | Toxic substances (hydrochloric acid, carburetor fluid, waxy internal matrix of a mentholated nasal inhaler) | Nasal and auricular inflammation, peripheral and axial joint damage, scleritis and vestibular disorders | [27] |
| After chondroitin and glucosamine therapy initiation | Rapid onset of bilateral auricular chondritis | [28] | |
| Intravenous papain injection | Ear collapse, tracheal and bronchial damage, even acute respiratory distress | [29] | |
| Infectious triggers | Chronic hepatitis C virus infection Mycobacterium tuberculosis Gut dysbiosis (increase in Ruminococcus, Bacteroides, Veillonella and Eubacterium species) | Molecular mimicry | [30,31] |
| Structural similarity between own HSP and microbial HSP | [32,33] | ||
| Innate immune system activation through the TLR and NLR signaling pathways | [34] | ||
| Cartilaginous inflammatory manifestations | [35] | ||
| Organ Involvement | Percentage of Patients | Clinical Manifestations | References |
|---|---|---|---|
| Skin | 84% | Dermatitis | [22] |
| Cutaneous nodules | [90] | ||
| Vasculitis (medium-vessel arteritis, leukocytoclastic vasculitis) | [95] | ||
| Erytema nodosum | [99] | ||
| Urticaria | [100] | ||
| Musculoskeletal | Up to 50% | Arthralgia | [22,24] |
| Arthritis | [90,95] | ||
| Myalgia | [99] | ||
| Chondritis (cartilage, ear, nose) | [101,102,103] | ||
| Eyes | Up to 40.5% | Episcleritis | [22,23,24] |
| Scleritis | [90,95] | ||
| Uveitis | [99] | ||
| Orbital mass | [101,102,104] | ||
| Orbital and periorbital inflammation | [105] | ||
| Lungs | Up to 50% | Pulmonary infiltrates | [22,24] |
| Pleural effusion | [90,95] | ||
| Pulmonary fibrosis | [99] | ||
| Bronchiolitis obliterans | [101,102,103,104] | ||
| Cardiovascular | 11% | Pericarditis | [22,23,24] |
| Myocarditis | [90,95,99] | ||
| Aortitis | [101,102,103,106] | ||
| Arterial aneurysms | [107,108] | ||
| Venous and arterial thrombosis | |||
| Gastrointestinal | 13.8% | Abdominal pain | [22] |
| Diarrhea | [90,95] | ||
| Ulcerative lesions | [99,104] | ||
| Digestive obstruction/perforation | [102] | ||
| Neurological | Up to 5% | Headache | [22] |
| Minor/major cerebrovascular accidents | [90] | ||
| Meningitis | [104] | ||
| Sensory neuropathy | [102] | ||
| Inflammatory demyelinating polyradiculoneuropathy | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoneanu, A.; Rezus, I.I.; Burlui, A.M.; Richter, P.; Bratoiu, I.; Mihai, I.R.; Macovei, L.A.; Rezus, E. Autoimmunity and Autoinflammation: Relapsing Polychondritis and VEXAS Syndrome Challenge. Int. J. Mol. Sci. 2024, 25, 2261. https://doi.org/10.3390/ijms25042261
Cardoneanu A, Rezus II, Burlui AM, Richter P, Bratoiu I, Mihai IR, Macovei LA, Rezus E. Autoimmunity and Autoinflammation: Relapsing Polychondritis and VEXAS Syndrome Challenge. International Journal of Molecular Sciences. 2024; 25(4):2261. https://doi.org/10.3390/ijms25042261
Chicago/Turabian StyleCardoneanu, Anca, Ioana Irina Rezus, Alexandra Maria Burlui, Patricia Richter, Ioana Bratoiu, Ioana Ruxandra Mihai, Luana Andreea Macovei, and Elena Rezus. 2024. "Autoimmunity and Autoinflammation: Relapsing Polychondritis and VEXAS Syndrome Challenge" International Journal of Molecular Sciences 25, no. 4: 2261. https://doi.org/10.3390/ijms25042261
APA StyleCardoneanu, A., Rezus, I. I., Burlui, A. M., Richter, P., Bratoiu, I., Mihai, I. R., Macovei, L. A., & Rezus, E. (2024). Autoimmunity and Autoinflammation: Relapsing Polychondritis and VEXAS Syndrome Challenge. International Journal of Molecular Sciences, 25(4), 2261. https://doi.org/10.3390/ijms25042261






