Abstract
Brain metastases represent a significant clinical challenge in the treatment of non-small-cell lung cancer (NSCLC), often leading to a severe decline in patient prognosis and survival. Recent advances in imaging and systemic treatments have increased the detection rates of brain metastases, yet clinical outcomes remain dismal due to the complexity of the metastatic tumor microenvironment (TME) and the lack of specific biomarkers for early detection and targeted therapy. The intricate interplay between NSCLC tumor cells and the surrounding TME in brain metastases is pivotal, influencing tumor progression, immune evasion, and response to therapy. This underscores the necessity for a deeper understanding of the molecular underpinnings of brain metastases, tumor microenvironment, and the identification of actionable biomarkers that can inform multimodal treatment approaches. The goal of this review is to synthesize current insights into the TME and elucidate molecular mechanisms in NSCLC brain metastases. Furthermore, we will explore the promising horizon of emerging biomarkers, both tissue- and liquid-based, that hold the potential to radically transform the treatment strategies and the enhancement of patient outcomes.
1. Introduction
Non-small-cell lung cancer (NSCLC), the predominant form of lung cancer, continues to be a profound health concern across the globe and a leading cause of morbidity and mortality in the United States [1]. This concern is magnified by NSCLC’s tendency to metastasize, most notably to the brain, an attribute that significantly influences its mortality statistics. In NSCLC patients, the development of brain metastases is witnessed in around 40% cases, making up nearly half of all brain metastases diagnoses and indicating a particularly severe prognosis [2]. Brain metastases often appear as multiple lesions, although one-third of these patients present with single lesions [3]. Untreated, the median survival for those with brain metastases from NSCLC ranges from just 1 to 2 months, underscoring the urgency and complexity of this clinical problem [4].
The key prognostic factors in NSCLC include the patient’s age, extracranial tumor activity, number and nature of brain metastases, and molecular subtype of the primary tumor [5]. The emergence of brain metastases consistently signals a bleak prognosis, with median survival times averaging between 3 and 6 months [6]. The tumor microenvironment (TME) is a key factor in driving the complex pathophysiology of NSCLC, starting at the primary site to involving lymphovascular channels to arriving and culminating in the brain [7]. The TME, a multifaceted network comprising both cellular and non-cellular constituents such as blood vessels, fibroblasts, immune cells, bone marrow-derived inflammatory cells, signaling molecules, and the extracellular matrix (ECM), facilitates a symbiotic relationship with tumor cells [8,9]. This interaction modulates tumor progression by endorsing angiogenesis and impeding immune response. Remarkably, the brain’s exclusive microenvironment retains its distinct characteristics following brain metastases, differentiating it from other organs [10]. Furthermore, the unique structure of the blood–brain barrier (BBB) restricts most chemotherapeutic agents from reaching intracranial lesions, limiting their therapeutic application [11]. Traditional treatment for brain metastases from NSCLC, such as surgical resection, is often constrained by risks associated with lesion numbers and locations in the brain. Other approaches such as whole-brain and stereotactic radiotherapy, despite their efficacy, have potential adverse effects like radionecrosis, leading to neurotoxicity [12]. In this comprehensive review, we explore the current state of knowledge and understanding of the TME’s role in NSCLC brain metastasis, focusing on underlying molecular mechanisms and predictive biomarkers. Moreover, we investigate the promising potential of these biomarkers in paving the way for innovative diagnostic and therapeutic strategies, offering hope for a more nuanced and targeted approach to NSCLC.
2. Tumor Microenvironment in Brain Metastases of NSCLC
2.1. Overview of TME in Brain Metastases
Brain metastases in NSCLC represent a complex and dynamic interplay between tumor cells, immune cells, and the unique brain TME. Metastasis is not an inherent trait of a specific tumor but rather multi-step and multidimensional processes influenced by factors such as mutations, epigenetic changes, and the presence of growth factors [3,13]. As illustrated in Figure 1, starting from the invasion of the primary tumor’s basement membrane, the metastatic process involves epithelial-to-mesenchymal transition (EMT) and intravasation into local blood and lymphatic vessels, eventually leading to circulating tumor cells (CTCs) surviving defensive mechanisms in the circulatory system. CTCs then face the considerable challenge of crossing the blood–brain barrier (BBB), a tightly regulated structure. Many brain metastases show BBB deterioration, suggesting an active role of CTCs in compromising these junctions. The mechanism of BBB crossing mimics aspects of leukocyte extravasation and involves adhesion and interactions with endothelial markers such as CD15 and CD15s [14]. These CTCs further release factors, such as CXCL12/CXCR4, MMP-1, and VEGF, that reciprocally degrade the BBB and facilitate their invasion [15]. Within the TME of NSCLC, hypoxia can induce HIF-1a, which elevates midkine (MDK) levels, thus promoting cell proliferation and further inducing EMT through Notch signaling. Other molecular players such as zinc transporter 4 also regulate EMT via the Snail–N-cadherin signaling axis, offering potential avenues for therapeutic interventions [16]. Upon invading the brain, tumor cells must alter the brain TME in a fashion that promotes tumor invasion and growth. To do this, tumor cells phenotypically modify local cells. These modified local cells can then go on to further stimulate tumor cells and the TME in a reciprocal fashion [17]. The brain TME is enriched with various local brain cells such as microglia and astrocytes that have been shown to demonstrate this reciprocal interaction. Initially, microglia adopt an anti-tumor M1 phenotype but gradually transition to a pro-tumor M2 phenotype under the influence of tumor-derived cytokines, specifically STAT3 [18]. This M1-to-M2 shift has substantial implications, as a higher M2:M1 ratio is strongly associated with brain metastases. Astrocytes also transform, evolving from an initial anti-tumor role to a pro-tumor one [19]. This transition is mediated by the formation of gap junctions between tumor cells and astrocytes which facilitate the transfer of messenger molecules that induce pro-tumor phenotypes in astrocytes. Additionally, the brain TME is further characterized by reduced T cell infiltration and changed diversity in T cell receptor (TCR) sequences compared to primary tumors, highlighting a state of immunosuppression [20,21]. Other factors that remain poorly understood include the influence of cancer-associated fibroblasts (CAFs) and vascular interactions, specifically vascular co-option, which are thought to be integral to tumor growth within the brain. Vascular co-option, the process where tumor cells physically adhere to pre-existing vessels, often precedes angiogenesis and is critical for tumor establishment and growth. Understanding these complex interactions between tumor cells and the TME in brain metastases from NSCLC holds both significant prognostic and therapeutic implications as it could pave the way for more targeted and effective therapeutic strategies in managing NSCLC brain metastases.
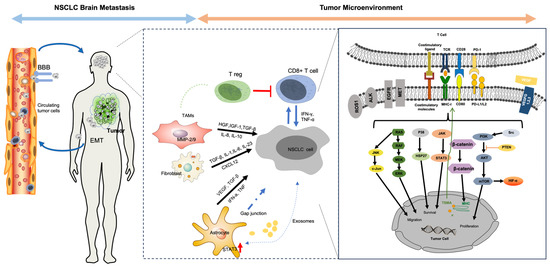
Figure 1.
Schema of interactions between TME and NSCLC brain metastases. Abbreviations: ALK: anaplastic lymphoma kinase; AKT: protein kinase B; BBB: blood–brain barrier; EGFR: epidermal growth factor receptor; EMT: epithelial-mesenchymal transition; HGF: hepatocyte growth factor; HIF-α: hypoxia-inducible factor-alpha; HSP27: heat shock protein 27; JAK: Janus kinase; JNK: c-Jun N-terminal kinase; TGF-β: transforming growth factor-beta; IFN-α: interferon-alpha; IL: interleukin; PI3K: phosphoinositide 3-kinase; PTEN: phosphatase and tensin homolog; NSCLC: non-small-cell lung cancer; STAT3: signal transducer and activator of transcription 3; TAMs: tumor-associated macrophages; TCR: T cell receptor; TNF: tumor necrosis factor; MAPK: mitogen-activated protein kinase; MMP: matrix metalloproteinase; MET: mesenchymal–epithelial transition factor; ROS1: c-ros oncogene 1, receptor tyrosine kinase; VEGF: vascular endothelial growth factor.
2.2. Cellular Components
The TME of brain metastases originating from NSCLC exhibits distinct characteristics divergent from those of the primary NSCLC site. This TME is composed of a constellation of cellular elements, including not only tumor cells but also a milieu of stromal components such as fibroblasts, astrocytes, and an array of immune cells, including microglia, macrophages, and lymphocytes [22]. Concurrently, non-cellular elements, predominantly the extracellular matrix (ECM), significantly contribute to the architecture of this environment [23]. The brain’s microenvironment is subject to transformative changes following tumor colonization, which implicates both the ECM and the resident tissue cells. Given the brain microenvironment’s critical role in shaping the trajectory of brain metastases, it becomes imperative to delineate the interactions between metastatic tumor cells and the diverse constituents of the brain’s ecological niche.
In general, brain metastases are characterized by an immunosuppressive tumor microenvironment (TME). This is evidenced by the suppression of immune-related signaling pathways, diminished expression of immune checkpoints, reduced infiltration by CD8+ T cells and cytotoxic lymphocytes, and a higher proportion of immunosuppressive M2 macrophages [3,10,24,25]. Kudo et al. [26] conducted a thorough immune profiling and sequencing analysis of NSCLC, focusing on both primary tumors and their paired brain metastases. Their findings revealed a predominant sharing of somatic hot spot mutations across these tumor sites. Notably, they observed a more pronounced inhibition of certain signaling pathways in brain metastases, namely those related to dendritic cell maturation, Th1 response, and leukocyte extravasation, compared to primary tumors. Moreover, the proinflammatory cell adhesion molecule, vascular cell adhesion protein 1, was markedly downregulated in brain metastases relative to primary tumors. The study also highlighted a distinct immune landscape in brain metastases, characterized by a reduced T cell presence and increased macrophage infiltration, in contrast to primary tumors. Furthermore, an expansion of T cell clones was observed in 64% of brain metastases when compared to their corresponding primary tumors. A notable decrease in the interferon-γ-related gene signature and an upregulation of anti-inflammatory markers such as TOLLIP and HLA g in brain metastases indicate a shift in the inflammatory profile of these tumors [27]. Furthermore, while TCR repertoires were largely shared between paired brain metastases and primary tumors, T cell densities were sparse in the metastases. Zhang et al. [28] provided further insight into the brain TME, highlighting an extensive remodeling process that fosters an immunosuppressive and fibrogenic niche conducive to brain metastasis establishment. This altered brain TME is typified by attenuated antigen presentation, compromised B/T cell functionality, an uptick in neutrophils and M2-type macrophages, along with immature microglia and reactive astrocytes. They pinpoint fibrosis as a hallmark of the brain tumor microenvironment. Differential gene expression and network analyses have illuminated that fibrosis and immune regulation represent the major disrupted functional modules within both lung and brain TMEs. Recent studies applying single-cell transcriptomic sequencing were able to unlock novel key molecular features of individual metastatic cells in brain metastases [29]. The researchers delineated pronounced disparities in the immune landscapes of primary and metastatic brain tumors, uncovering a distinctive gene signature linked to metabolic functions and cell adhesion in the metastases. Notably, there was a marked increase in the prevalence and concentration of CD16+ NK cells, neutrophils, macrophages, classical monocytes, and T cells in brain metastases compared to glioblastoma, coupled with a reduction in dendritic cells and non-classical monocytes. The study also revealed greater heterogeneity among the immune cells of different patients than within the cells from individual patients.
2.2.1. T Cells
Despite the distinct genetic and immunological landscape of brain metastases, the composition and function of T cells, particularly regulatory T cells (Tregs), are largely unexplored. In NSCLC-associated brain metastases, Tregs contribute to an immunosuppressive tumor microenvironment, often appearing in increased numbers alongside elevated levels of myeloid-derived suppressor cells (MDSCs), monocytes PD-L1 and IL-6 in the peripheral blood of affected patients [30,31]. Interestingly, Wainwright et al. [32] identified Helios+ thymus-derived natural Tregs as the predominant subset infiltrating brain tumors, further accumulating in the cervical lymph nodes through meningeal lymphatic drainage from the CNS. Notably, a high ratio of infiltrating Tregs to CD8+ T cells in tumor tissues is correlated with poor prognoses [33]. In contrast, brain metastases resulting from NSCLC show a reduced abundance of CD8+ T cells, CD4+ T cells, and Tregs compared to primary lung tumors and an increase in CD204+ cells [20]. Recent studies by Dykema et al. [34] have integrated single-cell TCRseq/RNAseq data from human NSCLC patients and murine tumors to reveal distinct Treg subclusters within the TME. An “activated” Treg subcluster, defined by the expression of TNFR superfamily genes OX40 and GITR, was highly suppressive and associated with resistance to immune checkpoint inhibitors. In murine models, most tumor-reactive Tregs evolved into a proinflammatory Th1-like phenotype, also prevalent in human tumors responding to anti-PD-1 therapy. These findings highlight tumor-associated Treg heterogeneity and suggest that targeting specific Treg subclusters may improve ICI responses. Furthermore, research by another team has demonstrated that T cell-mediated tumor reactivity manifests in brain metastases, characterized by the enrichment of CD39+ T cells expressing CXCL13, which are potential tumor-reactive T cells (pTRT) [35].
2.2.2. Cancer-Associated Fibroblasts
Cancer-associated fibroblasts (CAFs) represent a pivotal cellular component in the tumor microenvironment of non-small-cell lung cancer, contributing significantly to disease progression and brain metastases. Activated by factors such as TGF-β and exosomes secreted from NSCLC cells, CAFs adopt a pro-tumorigenic role, secreting a myriad of cytokines, chemokines, and growth factors [36,37]. These secretory products modulate the behavior of various immune cells, including CD8+ T cells, regulatory T cells, and macrophages, further contributing to immune suppression and pro-angiogenic processes. A critical mechanism through which CAFs exert immunosuppression is via the release of the chemokine CXCL12, which engages the receptor CXCR4 on cancer cells and leads to T cell exclusion [38,39]. This interaction emphasizes the role of fibroblast activation protein-a (FAP-a) in immune evasion. Several studies underscore the functional diversity within the CAF population. For instance, Hu et al. [40] identified an abundance of CAFs not only in primary tumors but also in brain metastases, while Akanda et al. linked the elevated expression of CAF-related biomarkers such as PDGFR-β and α-SMA to poor prognosis and recurrence in NSCLC brain metastases [41]. On the molecular level, CD10+GPR77+ and CD44+ CAF subsets have been implicated in maintaining tumor stemness. Furthermore, novel regulatory pathways have been proposed. Kim et al. [42] reported that apoptotic NSCLC cells reprogram CAFs through Notch1-WISP-1 signaling, which inhibits the migratory and invasive properties of both cancer cells and CAFs. Zhang et al. [43] demonstrated that CAFs protect NSCLC cells from irradiation-induced DNA damage by promoting DNA repair and cell cycle arrest in the radioresistant S phase. Through these multifaceted interactions and secretory functions, CAFs substantially alter the TME, rendering it more conducive for NSCLC progression and brain metastases. Thus, understanding the specific roles and functional heterogeneity of CAFs is crucial for the development of targeted therapies for NSCLC brain metastases.
2.2.3. Tumor-Associated Macrophages
Tumor-associated macrophages (TAMs), constituting approximately 30% of the immune infiltrate, are prominent players in the TME of NSCLC and its corresponding brain metastases. Exhibiting remarkable plasticity, TAMs can differentiate into distinct phenotypes—M1, which are pro-inflammatory and anti-tumorigenic, and M2, which are immunosuppressive and pro-tumorigenic—based on specific cues within the TME [44]. Notably, M2-polarized TAMs are associated with more aggressive tumor phenotypes, facilitating invasion and metastasis through the secretion of cytokines like IL-6, IL-8, and IL-10. These M2 TAMs also modulate the expression of programmed cell death-ligand 1 (PD-L1) on both tumor cells and infiltrating immune cells, contributing to immune evasion and disease progression in NSCLC patients [44,45]. Recent studies have elucidated the molecular underpinnings of TAM functionality in NSCLC brain metastases. Xiao et al. [24] reported that EGFR-positive and ALK-positive brain metastases are characterized by an immunosuppressive milieu, with decreased CD8+ T cells and increased regulatory T cells (Tregs) or M2 macrophages, respectively. Xu et al. [46] demonstrated that extracellular vesicle (EV)-derived long non-coding RNA LINC00482 modulates the miR-142-3p/TGF-β1 axis, inducing M2 polarization in microglia and fostering a pre-metastatic niche for NSCLC brain metastases. Other research has implicated exosomal miR-155, miR-196a-5p, and miR 942 from M2 TAMs in promoting NSCLC metastasis [47,48]. Moreover, Tiong et al. established that exosomal miR-21 drives lung-to-brain metastases through the DGKB/ERK axis within the TME [49]. Intriguingly, single-cell RNA sequencing identified IL6 as a critical regulator in brain metastatic NSCLC cells, inducing anti-inflammatory microglia via JAK2/STAT3 signaling and promoting colonization, as indicated by Jin et al. [50]. Targeting this IL6/JAK2/STAT3 pathway in activated microglia offers a promising avenue for inhibiting brain metastases in NSCLC. Recent advancements in imaging technologies, like fluorine isotope 19 magnetic resonance imaging (19F MRI), have even enabled the non-invasive tracking of TAMs over time, as demonstrated by Croci et al. [51]. Through these complex interactions and regulatory mechanisms, TAMs are shaping the immunosuppressive landscape of NSCLC brain metastases, offering multiple avenues for targeted therapies.
2.2.4. Astrocytes
Astrocytes are the most abundant mesenchymal cell type within the central nervous system (CNS) and play a pivotal role in maintaining homeostasis. In the context of brain metastases from NSCLC, they form a crucial component of the tumor microenvironment (TME) and exhibit a complex and dual behavior [52]. Initially, astrocytes act defensively against invading cancer cells. They produce plasminogen activator (PA), converting plasminogen into plasmin, activating the pro-apoptotic cytokine Fas ligand, which serves as a defense mechanism against exudative metastatic cells and inhibits the spread of tumor cells along intracerebral capillaries. However, tumor cells develop several convoluted countermeasures against these defensive mechanisms. For instance, they secrete anti-PA serine protease inhibitors such as neuroserpin and serpin B2, thereby resisting Fas-mediated apoptosis. Adler et al. [53] demonstrated that lipocalin-2 (LCN2), primarily secreted by granulocytes, induces the inflammatory activation of astrocytes, subsequently attracting myeloid cells to the brain and promoting neuroinflammation. Interestingly, the study found that the genetic targeting of LCN2 or bone marrow transplantation from LCN2−/− mice attenuated neuroinflammation and inhibited brain metastasis. Astrocytes also interact with tumor cells by assembling connexin 43 gap junctions, mediated by intracellular pro-calmodulin 7 [54]. This interplay induces innate immune responses that paradoxically promote tumor growth and chemoresistance via intracellular STAT1 and NF-kB pathways [55]. Ma et al. [56] discovered that IFN signaling in astrocytes activates the production of C-C motif chemokine ligand 2 (CCL2), recruiting monocytic myeloid cells and thus facilitating brain metastasis, despite IFN’s traditionally considered anti-tumor effects. Ye et al. [57] further contribute by showing that exosomes from NSCLC cells can induce apoptosis in astrocytes and promote the secretion of cytokines that facilitate an inflammatory and immunosuppressive microenvironment, thus setting the stage for the formation of a pre-metastatic niche in lung cancer brain metastases. Recent studies have shown that activated astrocytes can be identified by phosphorylated STAT3 (pSTAT3) expression, instrumental in altering the TME to facilitate brain metastasis [58]. Astrocytes also secrete miRNA-142-3p, inhibiting the transient receptor potential ankyrin-1-mediated activation of fibroblast growth factor 2, offering another avenue for brain metastasis mitigation [59]. Astrocytes exhibit a complex relationship with metastatic NSCLC cells, initially acting as a defense but eventually facilitating tumor growth due to intricate molecular interactions and their relationship with various immune cells [15,52]. These findings underscore the complexity of targeting astrocyte interactions in seeking effective therapies against NSCLC brain metastases.
2.3. Molecular Landscape of Brain Metastases
The molecular intricacies of NSCLC brain metastases are complex. NSCLC itself demonstrates an augmented tendency to give rise to brain metastases, especially in the context of ALK fusions and EGFR mutations [60,61]. This propensity remains pronounced even when systemic malignancy is controlled through targeted therapeutics or immunotherapeutic strategies. The inefficacy of early generation ALK inhibitors to sufficiently penetrate the central nervous system further amplifies the risk of CNS-based progression. In a comprehensive genomic study, Huang et al. [62] highlighted the brain metastases-specific increase in ALK fusions, KRAS G12C mutations, and MET amplifications compared to their primary NSCLC counterparts. Nonetheless, the frequency of MET exon14 skipping mutations was significantly reduced. Whole-exome sequencing of lung adenocarcinoma brain metastases further revealed that the prevalence of pathogenic genomic alterations, such as MYC, YAP1, and MMP13 amplifications and CDKN2A/B deletions [63]. In the context of primary squamous cell carcinoma (SCC), the aberrant PI3K pathway is linked to worse survival and increased brain metastasis incidence [64]. Other investigations also detected the aberrations in the PI3K/AKT pathway in both primary and brain metastases tissues, underscoring the pivotal role of this pathway in BM evolution [65]. A wide-angled whole-genome sequencing (WGS) approach revealed a homogenous pattern of heterozygous PTEN loss across all brain metastases in patients with SCC [65,66]. These findings highlight the unique genomic basis of brain metastases and the intricate clonal heterogeneity compared to primary tumors.
Recent studies have significantly advanced our understanding of brain metastases on a molecular basis. A comparative genomic study that investigates the difference between brain metastases and extracranial metastases revealed unique BCL6 and NOTCH2 variants as brain metastases-specific markers [67]. Additionally, a panel of 20 genes, including TP53, SMAD4, SF3B1, NOTCH2, and others, presented as prevalent variants in a significant fraction of patients with brain metastases, suggesting a potential genetic signature for brain metastases susceptibility that requires further validation [62,67]. Using Illumina RNA sequencing, a cohort study involving 91 NSCLC patients (32 developed brain metastases) identified a panel of 22 genes that were distinctly associated with brain metastases, exhibiting no correlation with other metastatic sites. The next-generation sequencing (NGS) of 416 cancer-associated genes from primary lung tumors and paired brain metastases reported over 80% concordance for mutations in EGFR, KRAS, TP53, and ALK, underscoring the parallel evolution of primary tumors and brain metastases [68]. For instance, synchronous brain metastases were found to possess a greater burden of cancer-associated mutations compared to primary tumors, suggesting an accelerated genomic evolution driven by the activation of supplementary oncogenic mechanisms. Conversely, this observation was not mirrored in follow-up studies. Others suggest that protein levels of TYMS, CDK1, CEP55, HJURP, and KIF11 could potentially forecast brain metastases incidence in lung adenocarcinoma patients [69]. Additionally, using single-cell transcriptomic sequencing uncovered substantial disparities in immune landscapes between primary tumors and brain metastases, and a unique metastatic gene signature rich in metabolism and cell adhesion-related genes. Zhang et al. explored gene expression differences between primary lung adenocarcinomas and their corresponding brain metastases using two patient-derived xenograft models. The research highlights CKAP4, SERPINA1, SDC2, and GNG11 as genes that are differentially expressed in these malignancies, proposing their utility as potential biomarkers. These biomarkers could significantly enhance prognostic assessments and inform therapeutic strategies for lung cancer patients with brain metastatic involvement [70]. A variety of gene classes associated with metastasis present modified DNA methylation patterns, correlating with altered gene expression in metastatic samples, including hypermethylation of the CDH1 promoter and subsequent reduction in expression [71]. Karlow et al. [72] revealed that lung cancer brain metastases are characterized by altered DNA methylation patterns. This change is associated with a reduction in EZH2 binding at genes crucial for development, potentially facilitating the emergence of stem-like traits that allow cancer cells to spread and thrive in diverse microenvironments. Specifically, an increase in methylation was identified at a locus overlapping the promoter of the ZNF154 gene in metastatic samples when compared to primary tumors. Higher methylation levels at this promoter correlate with a poorer patient prognosis. Furthermore, the most consistent epigenetic alterations observed as the cancer progresses to metastasis include methylation increases within DNA methylation valleys (DMVs). These valleys are marked by constitutive heterochromatin indicator, H3K9me3, alongside the bivalent chromatin modifications H3K27me3 and H3K4me1. Overall, genetic and epigenetic alterations play a crucial role in the development and characterization of brain metastases from NSCLC, offering potential biomarkers for prognosis and targets for therapeutic intervention.
3. Translational Biomarkers in NSCLC Brain Metastases
3.1. Tissue-Based Molecular Biomarkers
Recently, several FDA-approved therapies have been developed that specifically target genomic alterations in tyrosine kinase-related genes, including EGFR, ALK, ROS1, MET, RET, KRAS, BRAF, and NTRK1-3 [73]. These therapies, accompanied by their respective companion diagnostics, are now utilized in the treatment of NSCLC. The National Comprehensive Cancer Network (NCCN) guidelines currently endorse the use of next-generation sequencing for the detection of a broad spectrum of potential biomarkers in patients with NSCLC [74]. This includes testing for mutations in ERBB2 and amplifications in MET, which are increasingly recognized as actionable targets for personalized therapy. The landscape of NSCLC has been significantly advanced by biomarker testing, particularly in the challenging field of brain metastases. This complex issue affects an ethnic range, with 10–20% of Caucasians and up to 60% of Asians exhibiting molecular alterations at diagnosis [75]. Moreover, mutations in the EGFR gene, along with fusions in the ALK and RET genes, serve as critical driving factors for the progression of brain metastasis in patients with advanced NSCLC [76]. EGFR mutations, present in around 40% of Asian NSCLC patients, emphasize the necessity for routine testing, especially as a reported discordance rate up to 27% exists between extracranial and intracranial specimens in brain metastases patients. In NSCLC brain metastases, EGFR and ALK/ROS1 inhibitors show promising results in patients harboring these genomic alterations [77]. The third-generation EGFR-TKI osimertinib has shown remarkable intracranial response rates over 80% in metastatic cases, a significant step in treating brain metastasis [78]. The importance of EML4-ALK fusion protein, expressed in 2–9% of lung adenocarcinomas, has led to the recommendation of ALK testing in non-squamous histology if EGFR mutations are not detected. Next-generation ALK inhibitors, including alectinib and lorlatinib, have been associated with considerable intracranial response rates over 65%, showcasing their effectiveness in treating brain metastases [79]. ROS1 gene rearrangements, although not routinely screened, are present in 1–2% of non-squamous NSCLC patients, and their significant intracranial response rates with TKIs should prompt regular ROS1 testing, particularly in brain metastases patients [80]. BRAF mutations, detected in 2–4% of NSCLC patients, have revealed the clinical efficacy of targeted treatments like dabrafenib and trametinib, even in brain metastases, though routine testing is not universally recommended. Additionally, MET amplification emerges as an independent predictor of poor survival in brain metastases [81]. New insights into tissue-based biomarkers for NSCLC brain metastasis have emerged from recent studies. For instance. RAC1, notably enriched in metastatic LUAD tissues, is implicated in promoting brain metastases, with high expression levels indicating a poorer prognosis [82]. The increased expression of activated leukocyte cell adhesion molecule (ALCAM) is significantly correlated with upregulation in NSCLC brain metastases, leading to reduced survival times [83]. Brain-derived neurotrophic factor (BDNF) levels in primary LUAD have been linked to a heightened risk of brain metastasis development, with brain metastases expressing higher BDNF levels than their primary lesion counterparts [84]. Furthermore, other tissue markers, including cholecystokinin A receptor (CCKAR) and WD repeat domain 5 (WDR5), are significantly associated with brain metastasis and have emerged as independent risk factors [85,86].
The advent of PD-1 and PD-L1 immune checkpoint inhibitors has significantly reshaped the treatment landscape for metastatic NSCLC, particularly in managing brain metastases [87]. These therapies, as monotherapies or combined with chemotherapy, have presented substantial intracranial responses. Especially in NSCLC patients with high PD-L1 levels (≥50% for first-line and ≥1% for second-line treatments), significant benefits have been observed [88]. However, PD-L1 expression in NSCLC varies widely, ranging from 50% to 70%, reflecting the complex heterogeneity between tumors. Notably, a strong correlation between PD-L1 expression in primary NSCLC and brain metastases was found, for which the concordant rate ranged from 70 to 90%, whereas CD8+ TILs density was concordant in only about 50% of paired samples [89]. Immune checkpoint inhibitor-based treatment yielded similar intracranial and extracranial response rates. Interestingly, PD-L1-positive brain metastases patients exhibited shorter brain-specific disease-free survival than the PD-L1-negative resected brain metastatic group, and those with at least 1% PD-L1 expression had longer overall survival [90,91]. Factors like sex, age, and brain metastases status have been identified as predictive parameters for treatment responses. However, the predictive role of PD-L1 remains suboptimal due to discordant therapeutic responses, varying survival prognosis, non-standardized techniques, and the dynamic and heterogenous expression of PD-L1 during disease progression [92]. As such, the definitive potential of PD-L1 expression in guiding immune checkpoint inhibitor-based therapy in the brain metastases of NSCLC requires further clarification and standardized methodologies. Given this, the poor prognosis of NSCLC brain metastases, and the numerous approved therapies for metastatic NSCLC, the presence of the associated biomarkers for these approved agents within NSCLC brain metastases warrant further study. Figure 2 summarizes the predictive biomarkers in brain metastases of NSCLC.
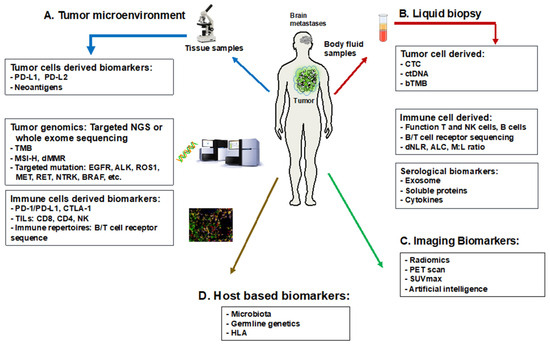
Figure 2.
Predictive translational biomarkers for brain metastases of NSCLC. Abbreviations: ALK: anaplastic lymphoma kinase; ALC: absolute lymphocyte count; BRAF: B-Raf proto-oncogene, serine/threonine kinase; ctDNA: circulating tumor DNA; bTMB: blood tumor mutational burden; dMMR: deficient mismatch repair; dNLR: derived neutrophil-to-lymphocyte ratio; CTC: circulating tumor cells; CTLA-4: cytotoxic T-lymphocyte-associated protein 4; EGFR: epidermal growth factor receptor; HLA: human leukocyte antigen; MET: mesenchymal–epithelial transition factor; M:L ratio: monocyte-to-lymphocyte ratio; MSI-H: microsatellite instability—high; NK: natural killer cells; NGS: next-generation sequencing; PD-L1: programmed death-ligand 1; RET: rearranged during transfection; TILs: tumor-infiltrating lymphocytes; TMB: tumor mutational burden; ROS1: c-ros oncogene 1; SUVmax: maximum standardized uptake value.
3.2. Liquid Biopsy Biomarkers: Circulating Tumor Cells and ctDNA
While tissue biopsy remains the gold standard for cancer diagnosis, it comes with certain constraints, including the size of the lesion, tumor heterogeneity, the patient’s ability to undergo the procedure, and the tumor’s accessibility. Liquid biopsy, on the other hand, is gaining interest in the scientific community as a minimally invasive technique that permits repeated sampling with minimal risk. Brain metastases in NSCLC are believed to originate from circulating tumor cells (CTCs), which constitute a significant component of liquid biopsies [93]. As most cancers are of epithelial origin, the most common marker used for CTCs is EpCAM. EpCAM expressions are commonly used to detect CTCs, but due to the EMT activity of NSCLC cells, detecting only EpCAM-positive CTCs probably underestimates the actual total CTC population and misses important biological information of EpCAM-negative CTCs [94]. It was even found that the quantity of EpCAM-negative CTCs was significantly larger than EpCAM-positive CTCs in NSCLC [95]. Using the CellSearch® system, one group found that CTCs were detected in only a few NSCLC patients with brain metastases and even fewer in oligo brain metastases. However, oligo-brain NSCLC metastatic patients with CTCs exhibited a very poor prognosis [96]. Scharpenseel et al. [97] found that by combining EpCAM enrichment with EGFR- and HER3-based enrichment, CTCs can be detected in a large proportion of NSCLC patients, including brain metastatic patients who were previously found to have only very few EpCAM positive CTCs.
Circulating tumor DNA (ctDNA) has emerged as a vital biomarker with profound implications in the diagnosis and prognosis of lung cancer brain metastases. Distinct from circulating tumor cells (CTCs), ctDNA allows for a unique exploration of genomic alterations, encompassing changes in tumor suppressor genes, oncogenes, microsatellite instability, and epigenetic modifications [98]. The accessibility of ctDNA eliminates the need for enrichment of rare cell types, enabling more efficient genotyping and assessment of drug responses, relapse screening, and survival prognosis [99,100]. The origins of ctDNA in brain metastases can be traced to neurons, oligodendrocytes, or astrocytes, exhibiting distinct methylation patterns and marked upregulation in serum samples when compared to other extracranial metastases [101]. Remarkably, ctDNA levels are not solely dictated by tumor volume but are also influenced by genetic factors, including mutations in genes such as EGFR or KRAS in non-small-cell lung cancer [102]. Studies on NSCLC brain metastases patients have demonstrated a 70-90% concordance of EGFR mutation status between tissue and serum samples [103]. Monitoring clonal evolution through ctDNA genotyping post-clinical interventions has further cemented its significance. Its role in therapy is also expanding, where ctDNA assessment post-surgery or chemotherapy can stratify patients and identify high-risk candidates for aggressive treatment, even targeting minimal residual disease, thus augmenting cure possibilities.
A deeper understanding of ctDNA also illuminates the complex nature of the metastatic cascade, discerning between monoclonal and polyclonal seeding. Recent research has proposed plasma ctDNA circulating tumor fraction (TF cut-off 10) as an independent prognostic biomarker across advanced cancers [104]. Although blood-based liquid biopsy offers non-invasive biomarker screening, cerebrospinal fluid (CSF) is an alternative biopsy type to evaluate the genetic landscape of intracranial tumors, particularly in cases of brain metastases. Li et al. [105] found that a higher number of unique copy number variations was detected in CSF-ctDNA than in plasma, and ctDNA positivity of CSF samples at baseline was associated with poor-outcome NSCLC brain metastases. Patients who achieved a reduction of 50% or greater in CSF ctDNA levels following an 8-week treatment regimen experienced notably extended intracranial progression-free survival (PFS) compared to those with reductions of less than 50%. Studies by Wu et al. [106] have underscored CSF ctDNA’s superior ability over plasma ctDNA at representing the mutational landscape of brain metastases, detecting all brain metastases mutations in 83.33% of patients. Mutant allele frequency (MAF) in CSF ctDNA has further shown a strong correlation with brain metastases tumor size. The utilization of targeted next-generation sequencing analysis of ctDNA and exosomal RNA in CSF and plasma has revealed substantial overlaps in variants between primary lung tumors and brain metastases. Specific in-frame deletions and missense mutations were identified, and correlations with brain metastases were unveiled. Detection frequencies of up to 90% in CSF and around 60% in blood were noted [107,108]. Mutations in genes like KEAP1, NRF2, P300, ALK, and RET were discovered in brain metastases, contributing insights into cellular survival during circulation [109]. ctDNA in the context of lung cancer brain metastases presents a multifaceted and promising field for enhanced diagnosis, prognosis, and tailored therapeutic strategies. In addition, the introduction of CSF as a new form of liquid biopsy has enabled higher detection rates of specific EGFR mutations in NSCLC brain metastases patients, contributing to remarkable response rates with EGFR-TKI treatment [110]. However, certain aspects of its mechanism and characteristics necessitate further exploration, underscoring the ongoing importance of research in this domain.
3.3. Exosomes and Non-Code RNA
Exosomes, extracellular vesicles ranging between 50 and 200 nm in diameter, are integral components of intercellular communication, immune response, and various pathological states, including inflammation, neurodegeneration, and particularly cancer [111]. Within the context of lung cancer’s propensity to metastasize to the brain, a notable increase in exosomal release has been observed [112]. These vesicles harbor critical components such as DNA, RNA, microRNA, proteins, and metabolites, all of which play a pivotal role in cancer proliferation and metastatic progression. Consequently, they hold significant promise for both diagnostic applications and targeted therapy [113]. In the intricate landscape of lung cancer, exosomes actively shape the tumor microenvironment, fostering tumor growth and facilitating distant metastasis by promoting the formation of pre-metastatic niche in brain metastases and induce astrocyte apoptosis by affecting cytokine secretion, protein expression, and energy metabolism [57,114]. The presence of unique surface proteins in exosomes provides valuable clues to their cellular origin, thus assisting in pinpointing preferred metastatic destinations [115]. Lung cancer-derived exosomes have been observed to compromise the integrity of the blood–brain barrier, instigating microglia activation [116]. This underscores their significance beyond mere diagnostic or prognostic markers. Emerging isolation techniques have broadened the scope of employing exosomes in diagnostics, therapy, and continuous treatment monitoring. By isolating and characterizing these vesicles, we can unravel complex challenges pertaining to drug efficiency or resistance in brain metastases.
Profiling the proteins within exosomes allows for precise differentiation between tumorous and normal cells, with multi-panel profiling demonstrating effectiveness in predicting tumor origin [117]. Exosomes enriched with specific proteins can condition the brain microenvironment to facilitate cancer cell proliferation, as evidenced by the elevated colonization and invasiveness observed in brain tissues treated with exosomes derived from brain metastatic cells [118]. Exosome integrins, known for their interaction with extracellular matrix proteins and regulation of cell survival and metastasis, present in varying amounts in different metastatic sites. Recent studies utilizing brain-tropic cell exosomes have revealed an upregulation of integrins, including ITGβ3, in brain metastases NSCLC models [115,119]. Our research group has also successfully applied the high-affinity peptide ligand LXY30 to target α3β1 integrin in NSCLC’s exosomes [120].
Non-coding RNAs (ncRNAs), including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are vital in the biology of lung cancer brain metastasis. MiRNA-378, for example, promotes brain metastases in NSCLC by modulating the genes involved in angiogenesis and extracellular matrix invasion [121], while the downregulation of miRNA-145 leads to brain metastases in lung adenocarcinoma [122]. PTEN regulation through microRNAs, downregulated in cancer cells but restored when leaving the brain microenvironment, demonstrates microRNAs’ critical role in brain metastases [123]. lncMMP-2 is highly expressed in TGF-β-mediated exosomes. It increases blood–brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis and leads to NSCLC brain metastases [124]. MiR-335-5p and miR-34b-3p were identified as unique to NSCLC brain metastasis, with others such as miR-330-3p having a diagnostic potential [125,126]. Comparative studies identified specific miRNAs upregulated and downregulated in brain metastases compared to primary NSCLC tumors, highlighting the role of miRNAs in controlling tumor cell proliferation, migration, and invasion [127]. Notable findings include miR-596-3p’s role in inhibiting brain metastases by modulating YAP1 and IL-8 and the significant downregulation of microRNA-375 in patients with brain metastases [128,129]. This intricate network of ncRNAs reveals promising diagnostic possibilities and emphasizes the need for continued exploration to enhance early detection and develop tailored therapies against metastatic lung cancer.
3.4. Imaging Biomarkers
The emerging evidence indicates that NSCLC with different features exhibits variability in imaging characteristics of the primary tumor and metastatic locations. The procurement of high-quality medical imaging through computed tomography and magnetic resonance imaging (MRI) is essential in the diagnostic process and treatment stratification for brain metastases [130]. Radiomics, a cutting-edge approach that utilizes advanced computational techniques to extract high-throughput quantitative features from medical images, has garnered considerable attention for its utility in characterizing various types of brain metastases, especially those originating from NSCLC [131]. Notably, work by Kneipp et al. [132] established that machine learning algorithms could be employed in conjunction with radiomic features to predict the histological origin of brain metastases with significant accuracy. Their study validated that quantitative metrics gleaned from routine magnetic resonance imaging (MRI) scans, when processed by machine learning classifiers, could offer a high level of discriminatory accuracy in pinpointing the tumor origin of these metastases. In a parallel vein, Zhou et al. [133] found that when radiomic features are amalgamated with machine learning methodologies, there is a powerful discriminatory capacity to distinguish between primary lung lesions and metastatic lung lesions in the brain. Intriguingly, emerging datasets also indicate that NSCLCs harboring different targetable oncogenic driver mutations (e.g., ALK) present unique imaging features, both in the primary tumor and in metastatic tumors [134,135]. Specifically, the utility of contrast-enhanced T1-weighted (CET1W) images in predicting EGFR mutation status in small-sized brain metastases (<10 mm) from lung cancer has been highlighted [136]. Moreover, our group has made pivotal contributions in elucidating the synergistic potential of combining radiomic imaging biomarkers with conventional molecular markers. Our team found that the LXY30-biotin/streptavidin-Cy5.5 complex shows preferential uptake in intracranial xenografts of α3β1 integrin-expressing lung adenocarcinomas while sparing the surrounding normal tissues, suggesting its viability as a robust and specific biomarker for lung–brain metastases [120]. To transition these groundbreaking findings from the research phase to clinical application, it is paramount that the diagnostic and prognostic accuracy of these imaging biomarkers be substantiated through rigorous validation in multiple clinical settings to ensure both reproducibility and generalizability.
3.5. Immune Cells and Serological Biomarkers
Peripheral immune biomarkers in NSCLC present a multifaceted landscape, comprising many components that undergo alterations during natural tumor progression and therapeutic interventions. Our prior investigations have highlighted that anti-cancer agents, including small molecule tyrosine kinase inhibitors and immune checkpoint inhibitors (ICIs), exert modulatory effects on the immune milieu and peripheral blood immune cells of advanced NSCLC patients [137]. For instance, the emergence of post-treatment lymphopenia was discernibly linked with suboptimal clinical outcomes in this patient cohort [138]. Diving deeper into the prognostic potential of immune parameters, several groups uncovered that the neutrophil–lymphocyte ratio (NLR) and serum albumin levels served as independent harbingers of enhanced survival in NSCLC patients with brain metastasis [139]. When NLR and albumin metrics were integrated to conceive the modified systemic inflammation score (mSIS), it outperformed the discriminative capability of the AJCC’s seventh T + N staging system [140]. In parallel, another research contingent identified the prognostic relevance of the prognostic nutritional index (PNI) and systemic immune inflammation index (SII) for NSCLC patients manifesting brain metastases [141]. Exploiting multicolor spectrum flow cytometry, our team recently pinpointed that ICIs notably influenced the prevalence of blood cytotoxic CD16+CD56dim, CD16+CD56- natural killer cells, and CD14+HLDRhigh monocytes [142]. These subsets bore significant prognostic associations in advanced NSCLC, indicating their prospective utility as monitoring tools in NSCLC brain metastases therapeutic paradigms. On the serological front, proteomics emerges as a promising avenue for the early detection and surveillance of NSCLC brain metastases patients [143]. A few previous studies have drawn correlations between pretreatment serum carcinoembryonic antigen (CEA) levels and brain metastasis in advanced NSCLC [144]. In a recent endeavor, Wei et al. [145] spotlighted the upregulated profiles of cathepsin F (CTSF) and fibulin-1 (FBLN1) in serum and tissue specimens of NSCLC brain metastasis patients. Intriguingly, serum alterations in CTSF mirrored the therapeutic trajectories of these patients, even preceding detectable changes in magnetic resonance imaging. Elevated tissue levels of CTSF correlated with diminished progression-free survival (PFS), establishing its stature as an independent prognostic marker. Concurrently, Chen et al. noted that elevated serum S100B levels were directly proportional to the burden of brain metastases in NSCLC, portending an adverse prognosis [146]. Such serological markers undeniably warrant exhaustive validation in subsequent studies.
3.6. Microbiota
The complex interplay between the microbiota and carcinogenesis is increasingly evident, particularly in NSCLC pathogenesis. The lung and gut microbiota have been intricately associated with NSCLC, yet their distinct contributions, especially regarding brain metastases, remain elucidated [147]. Lu et al. [148] reported a notable enrichment of Pseudomonas aeruginosa in the sputum of patients with brain metastases, a bacterium classically linked to wound infections. Remarkably, this species was predominantly observed in the sputum of brain metastases patients compared to other forms of NSCLC or distant metastases. Notably, the activation of toll-like receptor 4 by Gram-negative bacteria enhances metastasis in non-small-cell lung cancer through the phosphorylation of mitogen-activated protein kinases [149]. In a parallel investigation, Huang et al. [150] conducted a sequencing analysis of bronchoalveolar lavage fluid in 40 NSCLC cases. Their findings indicated a marked reduction in Streptococcus content in metastatic lung adenocarcinoma compared to its non-metastatic counterpart. Conversely, in lung squamous cell carcinoma, the proportions of Veillonella and Rothia were considerably elevated in metastatic cases compared to non-metastatic ones. The gut–brain axis (GBA) is a critical communication pathway where the gut microbiota can influence brain disorders. Within this framework, metabolites play a pivotal role as mediators, traversing into the bloodstream to exert their functional impact. Significantly, there is evidence to suggest that the activation of microglia has a positive correlation with brain tumor progression [151]. Li and colleagues further illuminated the intricate relationship between gut microbiota and NSCLC brain metastasis [152]. They highlighted the role of a specific bacterium, intestinimonas, and its associated plasma metabolite, spermine, in augmenting brain metastasis in NSCLC. This research elucidated a novel intestinimonas–spermine model, which offers potential as a biomarker for evaluating brain metastasis risk in NSCLC and sheds light on the mechanistic pathway involving STAT3 signaling. Notably, this signaling pathway accentuates the polarization of microglia towards a pro-tumor M2 phenotype, subsequently propelling brain tumor growth. While an association between microbiota dysbiosis and NSCLC has been established, precise mechanisms warrant further investigation, particularly in brain metastasis.
4. Conclusions and Future Therapeutic Implications
The treatment of brain metastases in NSCLC presents a complex clinical challenge. Guided by comprehensive guidelines, decisions often require a multidisciplinary approach, incorporating key factors such as the patient’s Karnofsky performance status and the primary cancer’s origin. Local interventions like stereotactic radiosurgery (SRS) and surgical resection remain valuable options, particularly for limited metastatic burden within the brain [153]. Advanced surgical techniques, such as cortical mapping, are increasingly employed to minimize perioperative morbidity. The advent of molecular diagnostics and targeted therapies has profoundly impacted brain metastases management in NSCLC. Broad molecular profiling is essential, especially for patients with metastatic non-squamous NSCLC. TKIs, for instance, have shown significant efficacy in controlling CNS metastases, frequently obviating the immediate need for local therapies like surgery or whole-brain radiation therapy. Table 1 outlines recent clinical studies on NSCLC brain metastasis treatments. In asymptomatic patients with limited brain metastases burden, first-line TKIs can often defer local treatment and mitigate the risk of CNS progression. Liquid biopsy offers a non-invasive, timely means of identifying actionable mutations and evaluating TKI resistance mechanisms. While immune checkpoint inhibitors have ushered in a new era in NSCLC treatment, their role in managing brain metastases remains an area of ongoing research, with current evidence indicating modest intracranial effectiveness [154,155]. A summary of recent clinical trials evaluating NSCLC brain metastases treatment can be found in Table 2. Future investigations should prioritize phase II and III trials that explicitly assess intracranial efficacy as a secondary endpoint. Concurrently, there is an imperative need to develop companion diagnostic and prognostic biomarkers for more nuanced risk stratification and treatment planning. In the era of precision therapy for NSCLC brain metastases, management mandates a synergistic collaboration among surgeons, medical oncologists, neurologists, radiologists, and pathologists, each bringing unique insights to revolutionize treatment approaches. There is an urgent need to optimize biomarker-guided treatment selection and precisely define treatment durations to significantly improve survival outcomes in NSCLC brain metastases. Future research should focus on developing innovative biomarkers, understanding basic molecular mechanisms, and enhancing diagnostic approaches.

Table 1.
Summary of reported clinical efficacy of drugs in NSCLC brain metastases.

Table 2.
Summary of ongoing clinical trials for lung cancer (NSCLC) brain metastases.
Author Contributions
Conceptualization, original draft preparation, writing and review of the manuscript, W.M.; resources and data curation, J.W., J.-Z.Y. and W.M.; review and editing, J.W., C.L. and A.A.O.O.I.; final review and supervision, W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- El Rassy, E.; Botticella, A.; Kattan, J.; Le Pechoux, C.; Besse, B.; Hendriks, L. Non-small cell lung cancer brain metastases and the immune system: From brain metastases development to treatment. Cancer Treat. Rev. 2018, 68, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef]
- Getman, V.; Devyatko, E.; Dunkler, D.; Eckersberger, F.; End, A.; Klepetko, W.; Marta, G.; Mueller, M.R. Prognosis of patients with non-small cell lung cancer with isolated brain metastases undergoing combined surgical treatment. Eur. J. Cardiothorac. Surg. 2004, 25, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Ruda, R. Management of brain metastases according to molecular subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients with Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef]
- Lin, A.; Wei, T.; Meng, H.; Luo, P.; Zhang, J. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol. Cancer 2019, 18, 139. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 53. [Google Scholar] [CrossRef]
- Schulz, M.; Salamero-Boix, A.; Niesel, K.; Alekseeva, T.; Sevenich, L. Microenvironmental Regulation of Tumor Progression and Therapeutic Response in Brain Metastasis. Front. Immunol. 2019, 10, 1713. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Ruda, R. Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef]
- Srinivasan, E.S.; Deshpande, K.; Neman, J.; Winkler, F.; Khasraw, M. The microenvironment of brain metastases from solid tumors. Neurooncol. Adv. 2021, 3, v121–v132. [Google Scholar] [CrossRef]
- Guan, Z.; Lan, H.; Cai, X.; Zhang, Y.; Liang, A.; Li, J. Blood-Brain Barrier, Cell Junctions, and Tumor Microenvironment in Brain Metastases, the Biological Prospects and Dilemma in Therapies. Front. Cell Dev. Biol. 2021, 9, 722917. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Wa, Y.; Ding, S.; Yang, Y.; Liao, J.; Tong, L.; Xiao, G. Tumor Immune Microenvironment and Immunotherapy in Brain Metastasis From Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 829451. [Google Scholar] [CrossRef]
- Wilhelm, I.; Molnar, J.; Fazakas, C.; Hasko, J.; Krizbai, I.A. Role of the blood-brain barrier in the formation of brain metastases. Int. J. Mol. Sci. 2013, 14, 1383–1411. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Caffarel, M.M.; Braza, M.S. Microglia and metastases to the central nervous system: Victim, ravager, or something else? J. Exp. Clin. Cancer Res. 2022, 41, 327. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, D.; Okimoto, T.; Shukuya, T.; Onagi, H.; Hayashi, T.; Sinicropi-Yao, S.L.; Amann, J.M.; Nakatsura, T.; Kitano, S.; Carbone, D.P. Comparison of Tumor Microenvironments between Primary Tumors and Brain Metastases in Patients with NSCLC. JTO Clin. Res. Rep. 2021, 2, 100230. [Google Scholar] [CrossRef] [PubMed]
- Porciello, N.; Franzese, O.; D’Ambrosio, L.; Palermo, B.; Nistico, P. T-cell repertoire diversity: Friend or foe for protective antitumor response? J. Exp. Clin. Cancer Res. 2022, 41, 356. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Li, L.; Tanzhu, G.; Liu, Z.; Gao, X.; Wan, X.; Xiao, D.; Chen, L.; Xia, X.; Zhou, R. Heterogeneity of tumor immune microenvironment of EGFR/ALK-positive tumors versus EGFR/ALK-negative tumors in resected brain metastases from lung adenocarcinoma. J. Immunother. Cancer 2023, 11, e006243. [Google Scholar] [CrossRef] [PubMed]
- Vilarino, N.; Bruna, J.; Bosch-Barrera, J.; Valiente, M.; Nadal, E. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat. Rev. 2020, 89, 102067. [Google Scholar] [CrossRef]
- Kudo, Y.; Haymaker, C.; Zhang, J.; Reuben, A.; Duose, D.Y.; Fujimoto, J.; Roy-Chowdhuri, S.; Solis Soto, L.M.; Dejima, H.; Parra, E.R.; et al. Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1521–1530. [Google Scholar] [CrossRef]
- Song, S.G.; Kim, S.; Koh, J.; Yim, J.; Han, B.; Kim, Y.A.; Jeon, Y.K.; Chung, D.H. Comparative analysis of the tumor immune-microenvironment of primary and brain metastases of non-small-cell lung cancer reveals organ-specific and EGFR mutation-dependent unique immune landscape. Cancer Immunol. Immunother. 2021, 70, 2035–2048. [Google Scholar] [CrossRef]
- Zhang, Q.; Abdo, R.; Iosef, C.; Kaneko, T.; Cecchini, M.; Han, V.K.; Li, S.S. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat. Commun. 2022, 13, 5983. [Google Scholar] [CrossRef]
- Karimi, E.; Yu, M.W.; Maritan, S.M.; Perus, L.J.M.; Rezanejad, M.; Sorin, M.; Dankner, M.; Fallah, P.; Dore, S.; Zuo, D.; et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 2023, 614, 555–563. [Google Scholar] [CrossRef]
- Huppert, L.A.; Green, M.D.; Kim, L.; Chow, C.; Leyfman, Y.; Daud, A.I.; Lee, J.C. Tissue-specific Tregs in cancer metastasis: Opportunities for precision immunotherapy. Cell Mol. Immunol. 2022, 19, 33–45. [Google Scholar] [CrossRef]
- Li, Y.D.; Lamano, J.B.; Lamano, J.B.; Quaggin-Smith, J.; Veliceasa, D.; Kaur, G.; Biyashev, D.; Unruh, D.; Bloch, O. Tumor-induced peripheral immunosuppression promotes brain metastasis in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2019, 68, 1501–1513. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Sengupta, S.; Han, Y.; Lesniak, M.S. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011, 13, 1308–1323. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef]
- Dykema, A.G.; Zhang, J.; Cheung, L.S.; Connor, S.; Zhang, B.; Zeng, Z.; Cherry, C.M.; Li, T.; Caushi, J.X.; Nishimoto, M.; et al. Lung tumor-infiltrating T(reg) have divergent transcriptional profiles and function linked to checkpoint blockade response. Sci. Immunol. 2023, 8, eadg1487. [Google Scholar] [CrossRef]
- Wischnewski, V.; Maas, R.R.; Aruffo, P.G.; Soukup, K.; Galletti, G.; Kornete, M.; Galland, S.; Fournier, N.; Lilja, J.; Wirapati, P.; et al. Phenotypic diversity of T cells in human primary and metastatic brain tumors revealed by multiomic interrogation. Nat. Cancer 2023, 4, 908–924. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Huang, H.; Ye, M.; Li, X.; Wu, R.; Liu, H.; Song, Y. Metastasis-associated fibroblasts: An emerging target for metastatic cancer. Biomark. Res. 2021, 9, 47. [Google Scholar] [CrossRef]
- Liu, W.; Powell, C.A.; Wang, Q. Tumor microenvironment in lung cancer-derived brain metastasis. Chin. Med. J. (Engl.) 2022, 135, 1781–1791. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: New opportunities in cancer immunotherapy and advances in clinical trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef]
- Ghorani, E.; Swanton, C.; Quezada, S.A. Cancer cell-intrinsic mechanisms driving acquired immune tolerance. Immunity 2023, 56, 2270–2295. [Google Scholar] [CrossRef]
- Hu, H.; Piotrowska, Z.; Hare, P.J.; Chen, H.; Mulvey, H.E.; Mayfield, A.; Noeen, S.; Kattermann, K.; Greenberg, M.; Williams, A.; et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 2021, 39, 1531–1547.e10. [Google Scholar] [CrossRef]
- Akanda, M.R.; Ahn, E.J.; Kim, Y.J.; Salam, S.M.A.; Noh, M.G.; Kim, S.S.; Jung, T.Y.; Kim, I.Y.; Kim, C.H.; Lee, K.H.; et al. Different Expression and Clinical Implications of Cancer-Associated Fibroblast (CAF) Markers in Brain Metastases. J. Cancer 2023, 14, 464–479. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, K.; Kim, K.; Lee, Y.J.; Lee, S.; Ahn, S.Y.; Ahn, Y.H.; Kang, J.L. Reprogramming of cancer-associated fibroblasts by apoptotic cancer cells inhibits lung metastasis via Notch1-WISP-1 signaling. Cell Mol. Immunol. 2022, 19, 1373–1391. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, K.; Qiu, L.; Yue, J.; Jiang, H.; Deng, Q.; Zhou, R.; Yin, Z.; Ma, S.; Ke, Y. Cancer-associated fibroblasts facilitate DNA damage repair by promoting the glycolysis in non-small cell lung cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166670. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Xu, W.; Patel, N.; Deng, Y.; Ding, S.; Wang, T.; Zhang, H. Extracellular vesicle-derived LINC00482 induces microglial M2 polarization to facilitate brain metastasis of NSCLC. Cancer Lett. 2023, 561, 216146. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Ni, Y.; Bian, C.; Huang, J.; Chen, L.; Xie, X.; Wang, J. Tumor-associated macrophages secret exosomal miR-155 and miR-196a-5p to promote metastasis of non-small-cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 1338–1354. [Google Scholar] [CrossRef]
- Wei, K.; Ma, Z.; Yang, F.; Zhao, X.; Jiang, W.; Pan, C.; Li, Z.; Pan, X.; He, Z.; Xu, J.; et al. M2 macrophage-derived exosomes promote lung adenocarcinoma progression by delivering miR-942. Cancer Lett. 2022, 526, 205–216. [Google Scholar] [CrossRef]
- Tiong, T.Y.; Chan, M.L.; Wang, C.H.; Yadav, V.K.; Pikatan, N.W.; Fong, I.H.; Yeh, C.T.; Kuo, K.T.; Huang, W.C. Exosomal miR-21 determines lung-to-brain metastasis specificity through the DGKB/ERK axis within the tumor microenvironment. Life Sci. 2023, 329, 121945. [Google Scholar] [CrossRef]
- Jin, Y.; Kang, Y.; Wang, M.; Wu, B.; Su, B.; Yin, H.; Tang, Y.; Li, Q.; Wei, W.; Mei, Q.; et al. Targeting polarized phenotype of microglia via IL6/JAK2/STAT3 signaling to reduce NSCLC brain metastasis. Signal Transduct. Target. Ther. 2022, 7, 52. [Google Scholar] [CrossRef]
- Croci, D.; Santalla Mendez, R.; Temme, S.; Soukup, K.; Fournier, N.; Zomer, A.; Colotti, R.; Wischnewski, V.; Flogel, U.; van Heeswijk, R.B.; et al. Multispectral fluorine-19 MRI enables longitudinal and noninvasive monitoring of tumor-associated macrophages. Sci. Transl. Med. 2022, 14, eabo2952. [Google Scholar] [CrossRef]
- Wasilewski, D.; Priego, N.; Fustero-Torre, C.; Valiente, M. Reactive Astrocytes in Brain Metastasis. Front. Oncol. 2017, 7, 298. [Google Scholar] [CrossRef]
- Adler, O.; Zait, Y.; Cohen, N.; Blazquez, R.; Doron, H.; Monteran, L.; Scharff, Y.; Shami, T.; Mundhe, D.; Glehr, G.; et al. Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2. Nat. Cancer 2023, 4, 401–418. [Google Scholar] [CrossRef]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Jacob, L.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 533, 493–498. [Google Scholar] [CrossRef]
- Tamai, S.; Ichinose, T.; Tsutsui, T.; Tanaka, S.; Garaeva, F.; Sabit, H.; Nakada, M. Tumor Microenvironment in Glioma Invasion. Brain Sci. 2022, 12, 505. [Google Scholar] [CrossRef]
- Ma, W.; Oliveira-Nunes, M.C.; Xu, K.; Kossenkov, A.; Reiner, B.C.; Crist, R.C.; Hayden, J.; Chen, Q. Type I interferon response in astrocytes promotes brain metastasis by enhancing monocytic myeloid cell recruitment. Nat. Commun. 2023, 14, 2632. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Y.; Zhou, J.; Xie, M.; Zhang, Z.; Su, C. Influence of Exosomes on Astrocytes in the Pre-Metastatic Niche of Lung Cancer Brain Metastases. Biol. Proced. Online 2023, 25, 5. [Google Scholar] [CrossRef]
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martinez, L.; Martinez-Saez, E.; Ramon, Y.C.S.; et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 2018, 24, 1024–1035. [Google Scholar] [CrossRef]
- Pahlavan, Y.; Mohammadi Nasr, M.; Dalir Abdolahinia, E.; Pirdel, Z.; Razi Soofiyani, S.; Siahpoush, S.; Nejati, K. Prominent roles of microRNA-142 in cancer. Pathol. Res. Pract. 2020, 216, 153220. [Google Scholar] [CrossRef]
- Shin, D.Y.; Na, I., II; Kim, C.H.; Park, S.; Baek, H.; Yang, S.H. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J. Thorac. Oncol. 2014, 9, 195–199. [Google Scholar] [CrossRef]
- Johung, K.L.; Yeh, N.; Desai, N.B.; Williams, T.M.; Lautenschlaeger, T.; Arvold, N.D.; Ning, M.S.; Attia, A.; Lovly, C.M.; Goldberg, S.; et al. Extended Survival and Prognostic Factors for Patients with ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J. Clin. Oncol. 2016, 34, 123–129. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Harries, L.; Decker, B.; Hiemenz, M.C.; Murugesan, K.; Creeden, J.; Tolba, K.; Stabile, L.P.; Ramkissoon, S.H.; Burns, T.F.; et al. Clinicopathologic and Genomic Landscape of Non-Small Cell Lung Cancer Brain Metastases. Oncologist 2022, 27, 839–848. [Google Scholar] [CrossRef]
- Shih, D.J.H.; Nayyar, N.; Bihun, I.; Dagogo-Jack, I.; Gill, C.M.; Aquilanti, E.; Bertalan, M.; Kaplan, A.; D’Andrea, M.R.; Chukwueke, U.; et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat. Genet. 2020, 52, 371–377. [Google Scholar] [CrossRef]
- Paik, P.K.; Shen, R.; Won, H.; Rekhtman, N.; Wang, L.; Sima, C.S.; Arora, A.; Seshan, V.; Ladanyi, M.; Berger, M.F.; et al. Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discov. 2015, 5, 610–621. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef]
- Vidotto, T.; Melo, C.M.; Lautert-Dutra, W.; Chaves, L.P.; Reis, R.B.; Squire, J.A. Pan-cancer genomic analysis shows hemizygous PTEN loss tumors are associated with immune evasion and poor outcome. Sci. Rep. 2023, 13, 5049. [Google Scholar] [CrossRef]
- Wilson, G.D.; Johnson, M.D.; Ahmed, S.; Cardenas, P.Y.; Grills, I.S.; Thibodeau, B.J. Targeted DNA sequencing of non-small cell lung cancer identifies mutations associated with brain metastases. Oncotarget 2018, 9, 25957–25970. [Google Scholar] [CrossRef]
- Wang, H.; Ou, Q.; Li, D.; Qin, T.; Bao, H.; Hou, X.; Wang, K.; Wang, F.; Deng, Q.; Liang, J.; et al. Genes associated with increased brain metastasis risk in non-small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non-small cell lung cancer (Guangdong Association Study of Thoracic Oncology 1036). Cancer 2019, 125, 3535–3544. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Gao, Z.; Zhao, Y.; Wen, Z.; Han, H.; Li, Y.; Chen, H. Development and validation of a five-gene model to predict postoperative brain metastasis in operable lung adenocarcinoma. Int. J. Cancer 2020, 147, 584–592. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, F.; Zhou, M.; Wu, S.; Zou, Q.; Gao, B. Single-cell RNA Sequencing Analysis Identifies Key Genes in Brain Metastasis from Lung Adenocarcinoma. Curr. Gene Ther. 2021, 21, 338–348. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Eccles, M.R. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol. 2018, 51, 149–159. [Google Scholar] [CrossRef]
- Karlow, J.A.; Devarakonda, S.; Xing, X.; Jang, H.S.; Govindan, R.; Watson, M.; Wang, T. Developmental Pathways Are Epigenetically Reprogrammed during Lung Cancer Brain Metastasis. Cancer Res. 2022, 82, 2692–2703. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, P.; Wei, J.; Zhang, X.; Guo, J.; Lin, Y. Recent progress in targeted therapy for non-small cell lung cancer. Front. Pharmacol. 2023, 14, 1125547. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Ha, S.Y.; Choi, S.J.; Cho, J.H.; Choi, H.J.; Lee, J.; Jung, K.; Irwin, D.; Liu, X.; Lira, M.E.; Mao, M.; et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015, 6, 5465–5474. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, G.; Zhang, M.; Zhang, X.; Li, H.; Zheng, X.; Ma, Z. Driver genes as predictive indicators of brain metastasis in patients with advanced NSCLC: EGFR, ALK, and RET gene mutations. Cancer Med. 2020, 9, 487–495. [Google Scholar] [CrossRef]
- Lamberti, G.; Andrini, E.; Sisi, M.; Rizzo, A.; Parisi, C.; Di Federico, A.; Gelsomino, F.; Ardizzoni, A. Beyond EGFR, ALK and ROS1: Current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit. Rev. Oncol. Hematol. 2020, 156, 103119. [Google Scholar] [CrossRef]
- Remon, J.; Steuer, C.E.; Ramalingam, S.S.; Felip, E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol. 2018, 29, i20–i27. [Google Scholar] [CrossRef]
- Ceddia, S.; Codacci-Pisanelli, G. Treatment of brain metastases in ALK-positive non-small cell lung cancer. Crit. Rev. Oncol. Hematol. 2021, 165, 103400. [Google Scholar] [CrossRef]
- Drilon, A.; Jenkins, C.; Iyer, S.; Schoenfeld, A.; Keddy, C.; Davare, M.A. ROS1-dependent cancers—Biology, diagnostics and therapeutics. Nat. Rev. Clin. Oncol. 2021, 18, 35–55. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Zhang, H.; Chang, Q.; Fang, M.; Lu, C.; Tian, P.; Mei, F. Molecular characteristics and prognostic factors of leptomeningeal metastasis in non-small cell lung cancer. Clin. Neurol. Neurosurg. 2023, 225, 107572. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Xu, X.; Bao, X.; Xue, L.; Ai, X.; Xu, J.; Xu, M.; Shi, Y.; Zhen, T.; et al. Identification of RAC1 in promoting brain metastasis of lung adenocarcinoma using single-cell transcriptome sequencing. Cell Death Dis. 2023, 14, 330. [Google Scholar] [CrossRef]
- Munsterberg, J.; Loreth, D.; Brylka, L.; Werner, S.; Karbanova, J.; Gandrass, M.; Schneegans, S.; Besler, K.; Hamester, F.; Robador, J.R.; et al. ALCAM contributes to brain metastasis formation in non-small-cell lung cancer through interaction with the vascular endothelium. Neuro Oncol. 2020, 22, 955–966. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Yi, G.; Yang, W.; Chen, K.; Tan, X.; Zhang, X.; Xu, Z.; Yang, Z.; Peng, Y. BDNF is a prognostic biomarker involved in immune infiltration of lung adenocarcinoma and is associated with brain metastasis. Immunology 2023, 168, 320–330. [Google Scholar] [CrossRef]
- Li, Z.; Liang, N.; Wang, N.; Jia, Y.; Tian, C. WDR5 is a prognostic biomarker of brain metastasis from non-small cell lung cancer. Front. Oncol. 2022, 12, 1023776. [Google Scholar] [CrossRef]
- Liang, N.; Sun, S.; Li, Z.; Wu, T.; Zhang, C.; Xin, T. CCKAR is a biomarker for prognosis and asynchronous brain metastasis of non-small cell lung cancer. Front. Oncol. 2022, 12, 1098728. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, J.; Huang, Z.; Deng, L.; Wu, L.; Yu, J.; Meng, X. Anti-PD-(L)1 immunotherapy for brain metastases in non-small cell lung cancer: Mechanisms, advances, and challenges. Cancer Lett. 2021, 502, 166–179. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Tonse, R.; Rubens, M.; Appel, H.; Tom, M.C.; Hall, M.D.; Odia, Y.; McDermott, M.W.; Ahluwalia, M.S.; Mehta, M.P.; Kotecha, R. Systematic review and meta-analysis of PD-L1 expression discordance between primary tumor and lung cancer brain metastasis. Neurooncol. Adv. 2021, 3, vdab166. [Google Scholar] [CrossRef]
- Takamori, S.; Toyokawa, G.; Okamoto, I.; Takada, K.; Kinoshita, F.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Mukae, N.; et al. Clinical Significance of PD-L1 Expression in Brain Metastases from Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 553–557. [Google Scholar] [CrossRef]
- Zhou, D.; Gong, Z.; Wu, D.; Ma, C.; Hou, L.; Niu, X.; Xu, T. Harnessing immunotherapy for brain metastases: Insights into tumor-brain microenvironment interactions and emerging treatment modalities. J. Hematol. Oncol. 2023, 16, 121. [Google Scholar] [CrossRef]
- Wang, S.; Hu, C.; Xie, F.; Liu, Y. Use of Programmed Death Receptor-1 and/or Programmed Death Ligand 1 Inhibitors for the Treatment of Brain Metastasis of Lung Cancer. Onco Targets Ther. 2020, 13, 667–683. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, Y.; Xu, J.; He, J.; Gao, W. Progress and application of circulating tumor cells in non-small cell lung cancer. Mol. Ther. Oncolytics 2021, 22, 72–84. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tang, C.; Liu, Y.; Sun, J.; Mu, X.; Zhang, L.; Dai, B.; Li, X.; Zhuo, H.; et al. Label-Free Isolation and mRNA Detection of Circulating Tumor Cells from Patients with Metastatic Lung Cancer for Disease Diagnosis and Monitoring Therapeutic Efficacy. Anal. Chem. 2015, 87, 11893–11900. [Google Scholar] [CrossRef]
- Hanssen, A.; Riebensahm, C.; Mohme, M.; Joosse, S.A.; Velthaus, J.L.; Berger, L.A.; Bernreuther, C.; Glatzel, M.; Loges, S.; Lamszus, K.; et al. Frequency of Circulating Tumor Cells (CTC) in Patients with Brain Metastases: Implications as a Risk Assessment Marker in Oligo-Metastatic Disease. Cancers 2018, 10, 527. [Google Scholar] [CrossRef]
- Scharpenseel, H.; Hanssen, A.; Loges, S.; Mohme, M.; Bernreuther, C.; Peine, S.; Lamszus, K.; Goy, Y.; Petersen, C.; Westphal, M.; et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci. Rep. 2019, 9, 7406. [Google Scholar] [CrossRef]
- Rehman, A.U.; Khan, P.; Maurya, S.K.; Siddiqui, J.A.; Santamaria-Barria, J.A.; Batra, S.K.; Nasser, M.W. Liquid biopsies to occult brain metastasis. Mol. Cancer 2022, 21, 113. [Google Scholar] [CrossRef]
- Li, M.; Hou, X.; Zheng, L.; Ma, Y.; Li, D.; Lv, Y.; Chen, J.; Zheng, W.; Shao, Y.; Mou, Y.; et al. Utilizing phenotypic characteristics of metastatic brain tumors to improve the probability of detecting circulating tumor DNA from cerebrospinal fluid in non-small-cell lung cancer patients: Development and validation of a prediction model in a prospective cohort study. ESMO Open 2022, 7, 100305. [Google Scholar] [CrossRef]
- Aldea, M.; Hendriks, L.; Mezquita, L.; Jovelet, C.; Planchard, D.; Auclin, E.; Remon, J.; Howarth, K.; Benitez, J.C.; Gazzah, A.; et al. Circulating Tumor DNA Analysis for Patients with Oncogene-Addicted NSCLC with Isolated Central Nervous System Progression. J. Thorac. Oncol. 2020, 15, 383–391. [Google Scholar] [CrossRef]
- Lubotzky, A.; Zemmour, H.; Neiman, D.; Gotkine, M.; Loyfer, N.; Piyanzin, S.; Ochana, B.L.; Lehmann-Werman, R.; Cohen, D.; Moss, J.; et al. Liquid biopsy reveals collateral tissue damage in cancer. JCI Insight 2022, 7, e153559. [Google Scholar] [CrossRef]
- Lam, V.K.; Zhang, J.; Wu, C.C.; Tran, H.T.; Li, L.; Diao, L.; Wang, J.; Rinsurongkawong, W.; Raymond, V.M.; Lanman, R.B.; et al. Genotype-Specific Differences in Circulating Tumor DNA Levels in Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Ye, X.; Hu, Z.; Peng, K.; Song, Y.; Yin, X.; Zhu, G.; Ji, Q.; Peng, Y. EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumour Biol. 2014, 35, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Reichert, Z.R.; Morgan, T.M.; Li, G.; Castellanos, E.; Snow, T.; Dall’Olio, F.G.; Madison, R.W.; Fine, A.D.; Oxnard, G.R.; Graf, R.P.; et al. Prognostic value of plasma circulating tumor DNA fraction across four common cancer types: A real-world outcomes study. Ann. Oncol. 2023, 34, 111–120. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Zhang, B.; Yu, J.; Wang, N.; Li, D.; Shao, Y.; Zhu, D.; Liang, C.; Ma, Y.; et al. Dynamic monitoring of cerebrospinal fluid circulating tumor DNA to identify unique genetic profiles of brain metastatic tumors and better predict intracranial tumor responses in non-small cell lung cancer patients with brain metastases: A prospective cohort study (GASTO 1028). BMC Med. 2022, 20, 398. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Huang, T.; Wang, Y.; Song, M.M.; Song, T.; Long, G.; Zhang, X.; Li, X.; Zhang, L. Cerebrospinal fluid circulating tumor DNA depicts profiling of brain metastasis in NSCLC. Mol. Oncol. 2023, 17, 810–824. [Google Scholar] [CrossRef]
- Tsakonas, G.; Tadigotla, V.; Chakrabortty, S.K.; Stragliotto, G.; Chan, D.; Lewensohn, R.; Yu, W.; Skog, J.K.; Hydbring, P.; Ekman, S. Cerebrospinal fluid as a liquid biopsy for molecular characterization of brain metastasis in patients with non-small cell lung cancer. Lung Cancer 2023, 182, 107292. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martinez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 2015, 6, 8839. [Google Scholar] [CrossRef]
- Aljohani, H.M.; Aittaleb, M.; Furgason, J.M.; Amaya, P.; Deeb, A.; Chalmers, J.J.; Bahassi, E.M. Genetic mutations associated with lung cancer metastasis to the brain. Mutagenesis 2018, 33, 137–145. [Google Scholar] [CrossRef]
- Ma, C.; Yang, X.; Xing, W.; Yu, H.; Si, T.; Guo, Z. Detection of circulating tumor DNA from non-small cell lung cancer brain metastasis in cerebrospinal fluid samples. Thorac. Cancer 2020, 11, 588–593. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, X.; Qian, Z.; Zhang, C.; Niu, Y.; Wang, Z.; Sun, J.; Zhang, X.; Yu, Y. The biological functions and clinical applications of exosomes in lung cancer. Cell Mol. Life Sci. 2019, 76, 4613–4633. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Cheng, Z.; Qin, W.; Jiang, L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer 2018, 116, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.D.; Castanho, M.; Neves, V. Exosomes and Brain Metastases: A Review on Their Role and Potential Applications. Int. J. Mol. Sci. 2021, 22, 10899. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Y.; Liu, R.; Xie, Y.; Jin, Y.; Wang, M.; Yu, Z.; Wang, W.; Luo, X. Non-small cell lung cancer-derived exosomes promote proliferation, phagocytosis, and secretion of microglia via exosomal microRNA in the metastatic microenvironment. Transl. Oncol. 2023, 27, 101594. [Google Scholar] [CrossRef]
- Li, S.; Qu, Y.; Liu, L.; Zhang, X.; He, Y.; Wang, C.; Guo, Y.; Yuan, L.; Ma, Z.; Bai, H.; et al. Comparative proteomic profiling of plasma exosomes in lung cancer cases of liver and brain metastasis. Cell Biosci. 2023, 13, 180. [Google Scholar] [CrossRef]
- Rodrigues, G.; Hoshino, A.; Kenific, C.M.; Matei, I.R.; Steiner, L.; Freitas, D.; Kim, H.S.; Oxley, P.R.; Scandariato, I.; Casanova-Salas, I.; et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat. Cell Biol. 2019, 21, 1403–1412. [Google Scholar] [CrossRef]
- Chen, G.Y.; Cheng, J.C.; Chen, Y.F.; Yang, J.C.; Hsu, F.M. Circulating Exosomal Integrin beta3 Is Associated with Intracranial Failure and Survival in Lung Cancer Patients Receiving Cranial Irradiation for Brain Metastases: A Prospective Observational Study. Cancers 2021, 13, 380. [Google Scholar] [CrossRef]
- Xiao, W.; Ma, W.; Wei, S.; Li, Q.; Liu, R.; Carney, R.P.; Yang, K.; Lee, J.; Nyugen, A.; Yoneda, K.Y.; et al. High-affinity peptide ligand LXY30 for targeting alpha3beta1 integrin in non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 56. [Google Scholar] [CrossRef]
- Chen, L.T.; Xu, S.D.; Xu, H.; Zhang, J.F.; Ning, J.F.; Wang, S.F. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med. Oncol. 2012, 29, 1673–1680. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, Y.; Zhang, Y.; Tan, W.; Xue, J.; Yang, Z.; Zhang, Y.; Lu, Y.; Hu, X. Downregulation of miR-145 contributes to lung adenocarcinoma cell growth to form brain metastases. Oncol. Rep. 2013, 30, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Deng, S.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; Xu, Y. TGF-beta1-mediated exosomal lnc-MMP2-2 increases blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis. 2021, 12, 721. [Google Scholar] [CrossRef]
- Im, J.H.; Kim, T.H.; Lee, K.Y.; Gwak, H.S.; Lin, W.; Park, J.B.; Kim, J.H.; Yoo, B.C.; Park, S.M.; Kwon, J.W.; et al. Exploratory Profiling of Extracellular MicroRNAs in Cerebrospinal Fluid Comparing Leptomeningeal Metastasis with Other Central Nervous System Tumor Statuses. J. Clin. Med. 2021, 10, 4860. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, R.; Cai, Q.; Gao, X.; Tong, F.; Dong, J.; Hu, Y.; Wu, G.; Dong, X. MicroRNA-330-3p promotes brain metastasis and epithelial-mesenchymal transition via GRIA3 in non-small cell lung cancer. Aging 2019, 11, 6734–6761. [Google Scholar] [CrossRef]
- Tsakonas, G.; Koulouris, A.; Kazmierczak, D.; Botling, J.; Ortiz-Villalon, C.; Nord, H.; Lindskog, M.; Sandelin, M.; Micke, P.; Hydbring, P.; et al. Matched Analyses of Brain Metastases versus Primary Non-Small Cell Lung Cancer Reveal a Unique microRNA Signature. Int. J. Mol. Sci. 2022, 24, 193. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, X.Y.; Zhao, Y.Q.; Liu, W.J.; Wu, H.J.; Liu, J.; Mu, X.Q.; Wu, H.B. Down-regulated microRNA-375 expression as a predictive biomarker in non-small cell lung cancer brain metastasis and its prognostic significance. Pathol. Res. Pract. 2017, 213, 882–888. [Google Scholar] [CrossRef]
- Li, C.; Zheng, H.; Xiong, J.; Huang, Y.; Li, H.; Jin, H.; Ai, S.; Wang, Y.; Su, T.; Sun, G.; et al. miR-596-3p suppresses brain metastasis of non-small cell lung cancer by modulating YAP1 and IL-8. Cell Death Dis. 2022, 13, 699. [Google Scholar] [CrossRef]
- Nowakowski, A.; Lahijanian, Z.; Panet-Raymond, V.; Siegel, P.M.; Petrecca, K.; Maleki, F.; Dankner, M. Radiomics as an emerging tool in the management of brain metastases. Neurooncol. Adv. 2022, 4, vdac141. [Google Scholar] [CrossRef]
- Chu, X.; Gong, J.; Yang, X.; Ni, J.; Gu, Y.; Zhu, Z. A “Seed-and-Soil” Radiomics Model Predicts Brain Metastasis Development in Lung Cancer: Implications for Risk-Stratified Prophylactic Cranial Irradiation. Cancers 2023, 15, 307. [Google Scholar] [CrossRef]
- Kniep, H.C.; Madesta, F.; Schneider, T.; Hanning, U.; Schonfeld, M.H.; Schon, G.; Fiehler, J.; Gauer, T.; Werner, R.; Gellissen, S. Radiomics of Brain MRI: Utility in Prediction of Metastatic Tumor Type. Radiology 2019, 290, 479–487. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, X.L.; Zhang, T.; Wang, J.; Zhang, T.; Tian, R. Use of radiomics based on (18)F-FDG PET/CT and machine learning methods to aid clinical decision-making in the classification of solitary pulmonary lesions: An innovative approach. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2904–2913. [Google Scholar] [CrossRef]
- Mendoza, D.P.; Piotrowska, Z.; Lennerz, J.K.; Digumarthy, S.R. Role of imaging biomarkers in mutation-driven non-small cell lung cancer. World J. Clin. Oncol. 2020, 11, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.Z.; Li, W.H.; Han, Y.; Li, Q.; Ye, Z. Pretreatment Thoracic CT Radiomic Features to Predict Brain Metastases in Patients with ALK-Rearranged Non-Small Cell Lung Cancer. Front. Genet. 2022, 13, 772090. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Kwon, H.; Yang, J.J.; Park, M.; Cha, Y.J.; Suh, S.H.; Lee, J.M. Contrast-enhanced T1-weighted image radiomics of brain metastases may predict EGFR mutation status in primary lung cancer. Sci. Rep. 2020, 10, 8905. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zeng, J.; Chen, S.; Lyu, Y.; Toomey, K.A.; Phan, C.T.; Yoneda, K.Y.; Li, T. Small molecule tyrosine kinase inhibitors modulated blood immune cell counts in patients with oncogene-driven NSCLC. Biomark. Res. 2021, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Park, S.; Byun, H.K.; Lee, C.G.; Cho, J.; Hong, M.H.; Kim, H.R.; Cho, B.C.; Kim, S.; Park, J.; et al. Impact of Treatment-Related Lymphopenia on Immunotherapy for Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wu, J.; Liu, Y.; Zhou, J.; Wang, W.; Wang, X.; Guo, J.; Wang, Q.; Zhang, X.; Li, D.; et al. Relationship Between Neutrophil-To-Lymphocyte Ratio and Brain Metastasis in Non-Small Cell Lung Cancer Patients. Cancer Control 2022, 29, 10732748221076805. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Wang, Z.; Xu, Q.; Huang, H.; Wang, H.; Li, X.; Yu, M.; Chen, J.; Lin, F.; et al. Prognostic value of the modified systemic inflammation score in non-small-cell lung cancer with brain metastasis. Cancer Cell Int. 2022, 22, 320. [Google Scholar] [CrossRef]
- Li, W.; Qu, Y.; Wen, F.; Yu, R.; He, X.; Jia, H.; Liu, H.; Yu, H. Prognostic nutritional index and systemic immune-inflammation index are prognostic biomarkers for non-small-cell lung cancer brain metastases. Biomark. Med. 2021, 15, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wei, S.; Long, S.; Tian, E.C.; McLaughlin, B.; Jaimes, M.; Montoya, D.J.; Viswanath, V.R.; Chien, J.; Zhang, Q.; et al. Dynamic evaluation of blood immune cells predictive of response to immune checkpoint inhibitors in NSCLC by multicolor spectrum flow cytometry. Front. Immunol. 2023, 14, 1206631. [Google Scholar] [CrossRef] [PubMed]
- Woldmar, N.; Schwendenwein, A.; Kuras, M.; Szeitz, B.; Boettiger, K.; Tisza, A.; Laszlo, V.; Reiniger, L.; Bago, A.G.; Szallasi, Z.; et al. Proteomic analysis of brain metastatic lung adenocarcinoma reveals intertumoral heterogeneity and specific alterations associated with the timing of brain metastases. ESMO Open 2023, 8, 100741. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Saavedra-Perez, D.; Kuri, R.; Aviles-Salas, A.; Martinez, L.; Mendoza-Posada, D.; Castillo, P.; Astorga, A.; Guzman, E.; De la Garza, J. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: A prospective analysis. BMC Cancer 2009, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, W.; Xu, M.; Qin, H.; Liu, C.; Zhang, R.; Zhou, S.; Li, E.; Liu, Z.; Wang, Q. Cathepsin F and Fibulin-1 as novel diagnostic biomarkers for brain metastasis of non-small cell lung cancer. Br. J. Cancer 2022, 126, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, X.; Wu, H.; Jia, Y.; Liu, J.; Mu, X.; Wu, H.; Zhao, Y. Over-expression of S100B protein as a serum marker of brain metastasis in non-small cell lung cancer and its prognostic value. Pathol. Res. Pract. 2019, 215, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wen, M.; Liu, Y.; Wang, Y.; Jing, P.; Gu, Z.; Jiang, T.; Wang, W. The human microbiome: A promising target for lung cancer treatment. Front. Immunol. 2023, 14, 1091165. [Google Scholar] [CrossRef]
- Lu, H.; Gao, N.L.; Tong, F.; Wang, J.; Li, H.; Zhang, R.; Ma, H.; Yang, N.; Zhang, Y.; Wang, Y.; et al. Alterations of the Human Lung and Gut Microbiomes in Non-Small Cell Lung Carcinomas and Distant Metastasis. Microbiol. Spectr. 2021, 9, e0080221. [Google Scholar] [CrossRef]
- Chow, S.C.; Gowing, S.D.; Cools-Lartigue, J.J.; Chen, C.B.; Berube, J.; Yoon, H.W.; Chan, C.H.; Rousseau, M.C.; Bourdeau, F.; Giannias, B.; et al. Gram negative bacteria increase non-small cell lung cancer metastasis via Toll-like receptor 4 activation and mitogen-activated protein kinase phosphorylation. Int. J. Cancer 2015, 136, 1341–1350. [Google Scholar] [CrossRef]
- Huang, D.; Su, X.; Yuan, M.; Zhang, S.; He, J.; Deng, Q.; Qiu, W.; Dong, H.; Cai, S. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am. J. Cancer Res. 2019, 9, 2047–2063. [Google Scholar]
- Wu, S.Y.; Watabe, K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front. Biosci. (Landmark. Ed.) 2017, 22, 1805–1829. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Bin, Y.; Zeng, H.; Wang, J.; Zhang, R.; Tong, F.; Yang, N.; Dong, X. Microbiota-modulated spermine promotes brain metastasis in non-small cell lung cancer by regulating microglia M2 polarization via the STAT3 pathway. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ernani, V.; Stinchcombe, T.E. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2019, 15, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Brozos-Vazquez, E.M.; Rodriguez-Lopez, C.; Cortegoso-Mosquera, A.; Lopez-Landrove, S.; Muinelo-Romay, L.; Garcia-Gonzalez, J.; Lopez-Lopez, R.; Leon-Mateos, L. Immunotherapy in patients with brain metastasis: Advances and challenges for the treatment and the application of circulating biomarkers. Front. Immunol. 2023, 14, 1221113. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, Y.; Wang, M. Immune checkpoint inhibitors for the treatment of non-small cell lung cancer brain metastases. Chin. Med. J. (Engl.) 2023, 136, 1523–1531. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, C.; Wu, G.; Lin, W.; Xie, Z.; Zhang, H.; Yi, J.; Peng, Z.; Yin, L.; Ma, C.; et al. Efficacy, Safety, and Health-Related Quality of Life with Camrelizumab Plus Pemetrexed and Carboplatin as First-Line Treatment for Advanced Nonsquamous NSCLC with Brain Metastases (CAP-BRAIN): A Multicenter, Open-Label, Single-Arm, Phase 2 Study. J. Thorac. Oncol. 2023, 18, 769–779. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wakuda, K.; Fukuda, M.; Kenmotsu, H.; Mukae, H.; Ito, K.; Chibana, K.; Inoue, K.; Miura, S.; Tanaka, K.; et al. A Phase II Study of Osimertinib for Radiotherapy-Naive Central Nervous System Metastasis From NSCLC: Results for the T790M Cohort of the OCEAN Study (LOGIK1603/WJOG9116L). J. Thorac. Oncol. 2021, 16, 2121–2132. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, S.; Huo, L.; Luo, Q.; Wang, D.; Guo, J.; Liu, B.; Zhang, J.; Wang, B.; Qiu, B.; et al. Effect and safety of combining bevacizumab and fractionated stereotactic radiotherapy for brain metastases in patients with non-small cell lung cancer: A phase II trial. J. Clin. Oncol. 2023, 41, e21132. [Google Scholar] [CrossRef]
- Hong, M.H.; Choi, Y.J.; Ahn, H.K.; Keam, B.; Kim, Y.J.; Kim, J.W.; Kim, H.R.; Kang, J.-H. Phase II trial of lazertinib in patients with epidermal growth factor receptor (EGFR) mutation-positive (M+), metastatic non-small cell lung cancer (NSCLC) with asymptomatic or mild symptomatic brain metastases after failure of EGFR tyrosine kinase inhibitor (TKI) (KCSG LU20-15). J. Clin. Oncol. 2023, 41, 9054. [Google Scholar] [CrossRef]
- Nadal, E.; Rodriguez-Abreu, D.; Massuti, B.; Juan-Vidal, O.; Huidobro Vence, G.; Lopez, R.; de Castro Carpeño, J.; Estival, A.; Campelo, R.G.; Sullivan, I.; et al. Updated analysis from the ATEZO-BRAIN trial: Atezolizumab plus carboplatin and pemetrexed in patients with advanced nonsquamous non–small cell lung cancer with untreated brain metastases. J. Clin. Oncol. 2022, 40, 9010. [Google Scholar] [CrossRef]
- Zhang, L.; You, Y.; Liu, X.; Liu, F.; Nie, K.; Ji, Y. Osimertinib combined with bevacizumab as the first-line treatment in non-small cell lung cancer patients with brain metastasis harboring epidermal growth factor receptor mutations. Thorac. Cancer 2023, 14, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, Q.; Yang, N.; Liu, L.; Yu, Y.; Wang, Q.; Zhao, Y.; Yang, Y.; Jiang, W.; Tan, L.; et al. Tislelizumab plus chemotherapy in patients with advanced brain metastases of non-squamous non-small cell lung cancer: A multicenter, prospective, open-label phase 2 study. J. Clin. Oncol. 2023, 41, 9080. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhou, Q.; Wang, J.; Yu, Y.; Xing, L.; Wang, Y.; Cheng, Y.; Pan, Y.; Fan, Y.; Shi, J.; et al. Randomized phase 3 study of first-line AZD3759 (zorifertinib) versus gefitinib or erlotinib in EGFR-mutant (EGFRm+) non–small-cell lung cancer (NSCLC) with central nervous system (CNS) metastasis. J. Clin. Oncol. 2023, 41, 9001. [Google Scholar] [CrossRef]
- Paik, P.K.; Garassino, M.C.; Le, X.; Thomas, M.; Sakai, H.; Veillon, R.; Smit, E.F.; Mazieres, J.; Cortot, A.B.; Raskin, J.; et al. Long-term outcomes of tepotinib in patients with MET exon 14 skipping NSCLC from the VISION study. J. Clin. Oncol. 2023, 41, 9060. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liu, Z.; Wang, L.; Yao, Y.; Liu, Y.; Hao, X.Z.; Wang, J.; Xing, P.; Li, J. Efficacy of dacomitinib in patients with EGFR-mutated NSCLC and brain metastases. Thorac. Cancer 2021, 12, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Park, S.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Sun, J.M. Dacomitinib in EGFR-mutant non-small-cell lung cancer with brain metastasis: A single-arm, phase II study. ESMO Open 2023, 8, 102068. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Han, J.Y.; Kim, S.W.; Lee, K.H.; Cho, E.K.; Lee, Y.G.; Kim, D.W.; Kim, J.H.; Lee, G.W.; Lee, J.S.; et al. A Phase 1/2 Study of Lazertinib 240 mg in Patients with Advanced EGFR T790M-Positive NSCLC After Previous EGFR Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2022, 17, 558–567. [Google Scholar] [CrossRef]
- Cho, B.C.; Ahn, M.-J.; Kang, J.H.; Soo, R.A.; Reungwetwattana, T.; Yang, J.C.-H.; Cicin, I.; Kim, D.-W.; Wu, Y.-L.; Lu, S.; et al. Lazertinib Versus Gefitinib as First-Line Treatment in Patients with EGFR-Mutated Advanced Non–Small-Cell Lung Cancer: Results From LASER301. J. Clin. Oncol. 2023, 41, 4208–4217. [Google Scholar] [CrossRef]
- Crino, L.; Bronte, G.; Bidoli, P.; Cravero, P.; Minenza, E.; Cortesi, E.; Garassino, M.C.; Proto, C.; Cappuzzo, F.; Grossi, F.; et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2019, 129, 35–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).