Does Telotristat Have a Role in Preventing Carcinoid Heart Disease?
Abstract
1. Introduction
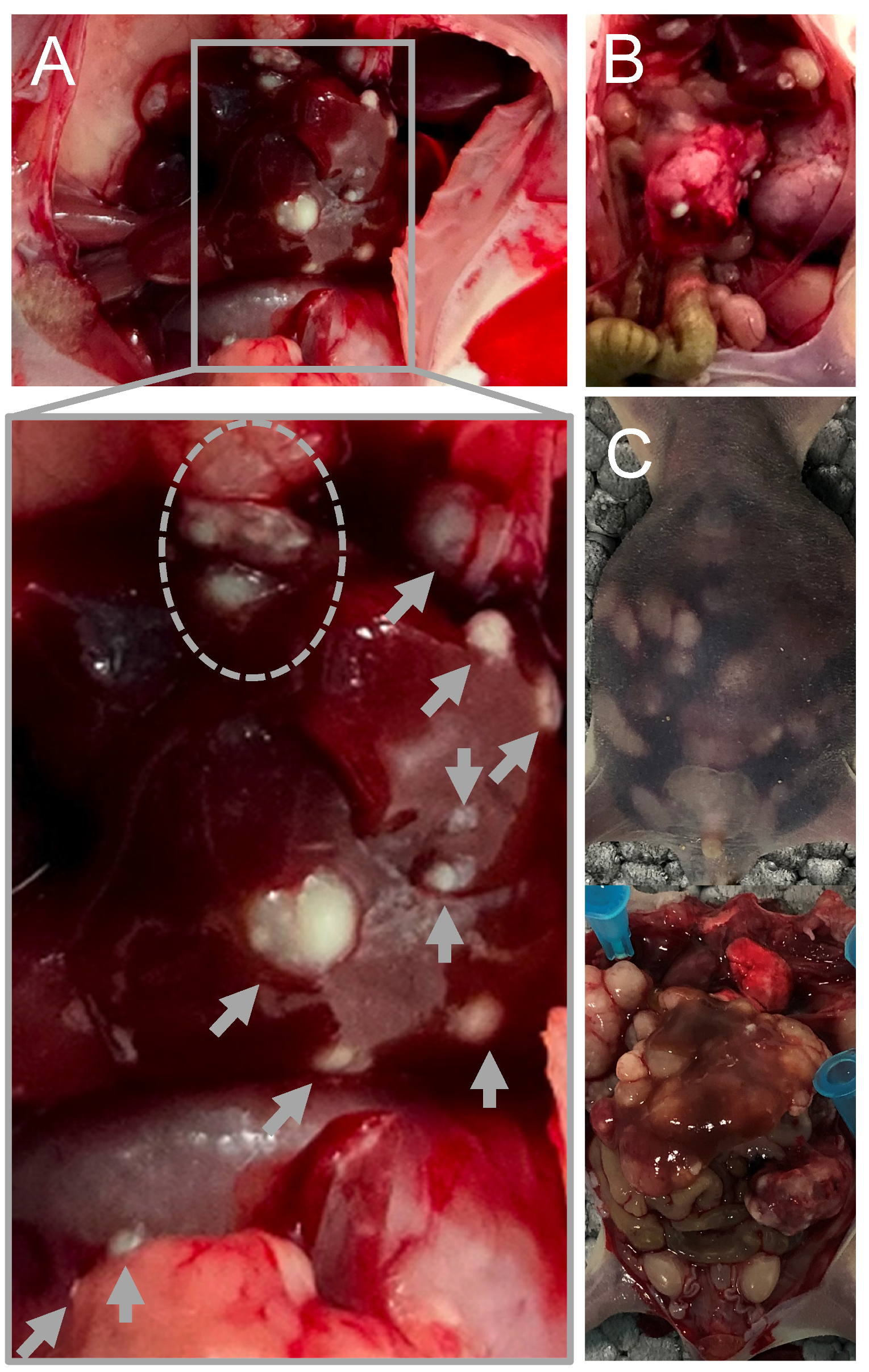
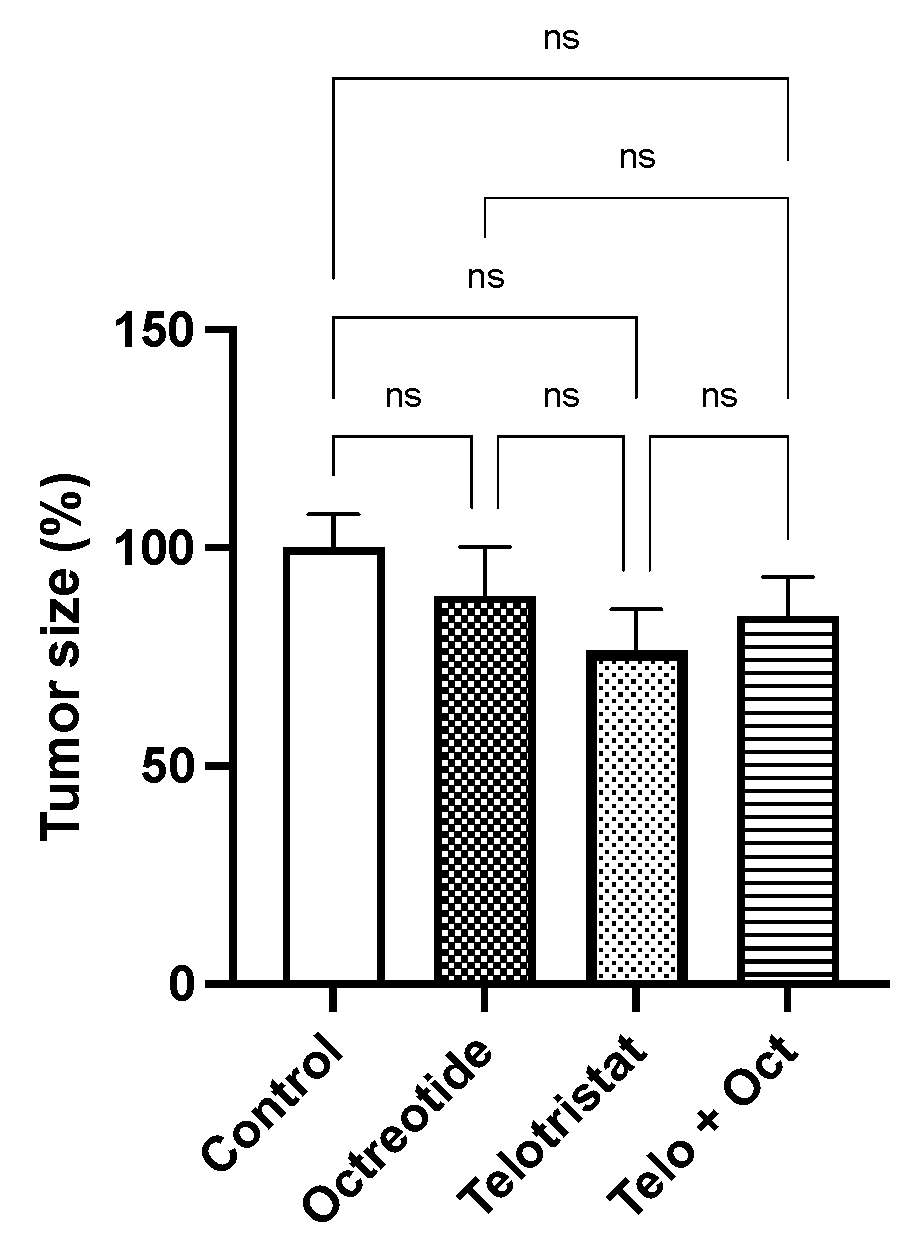
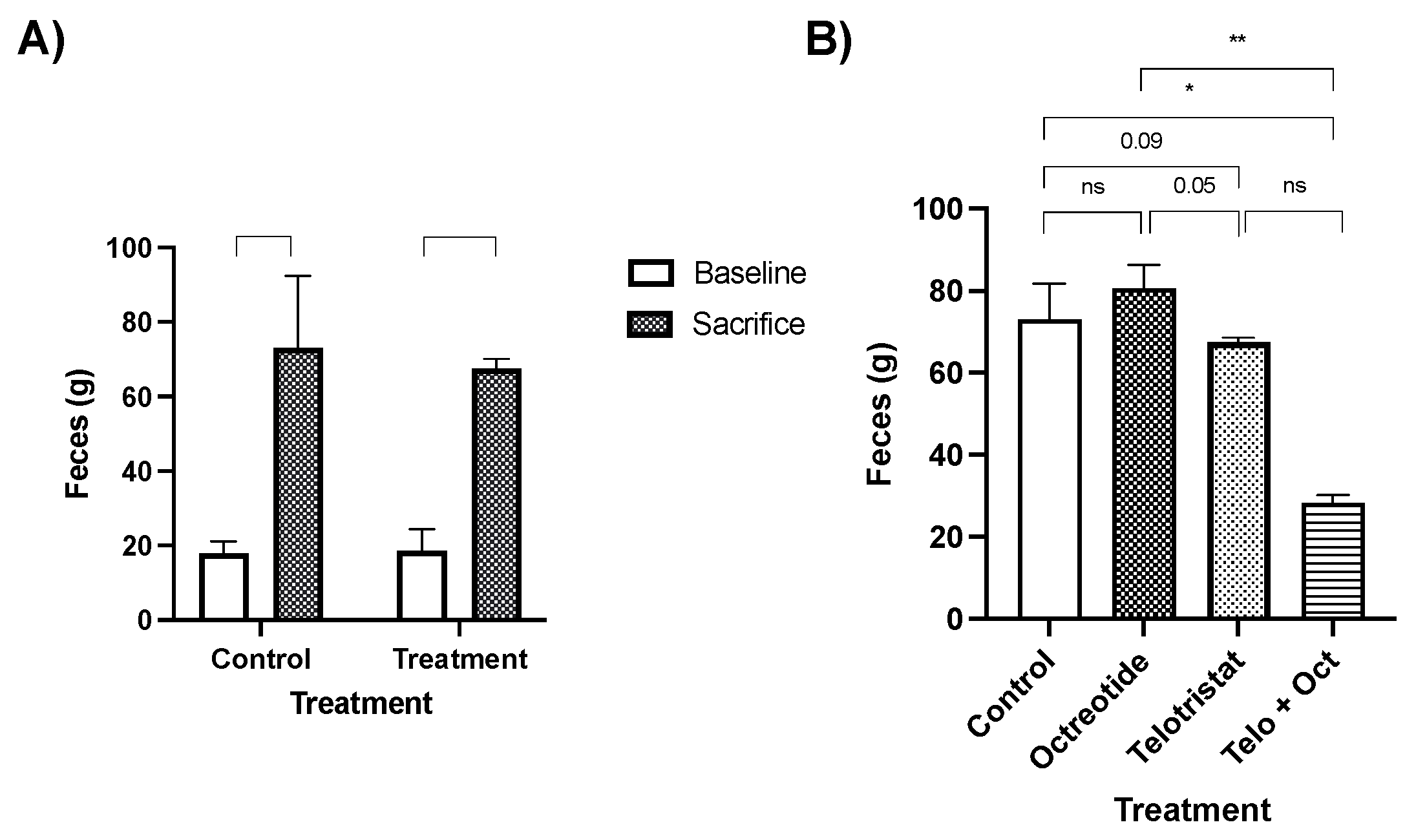
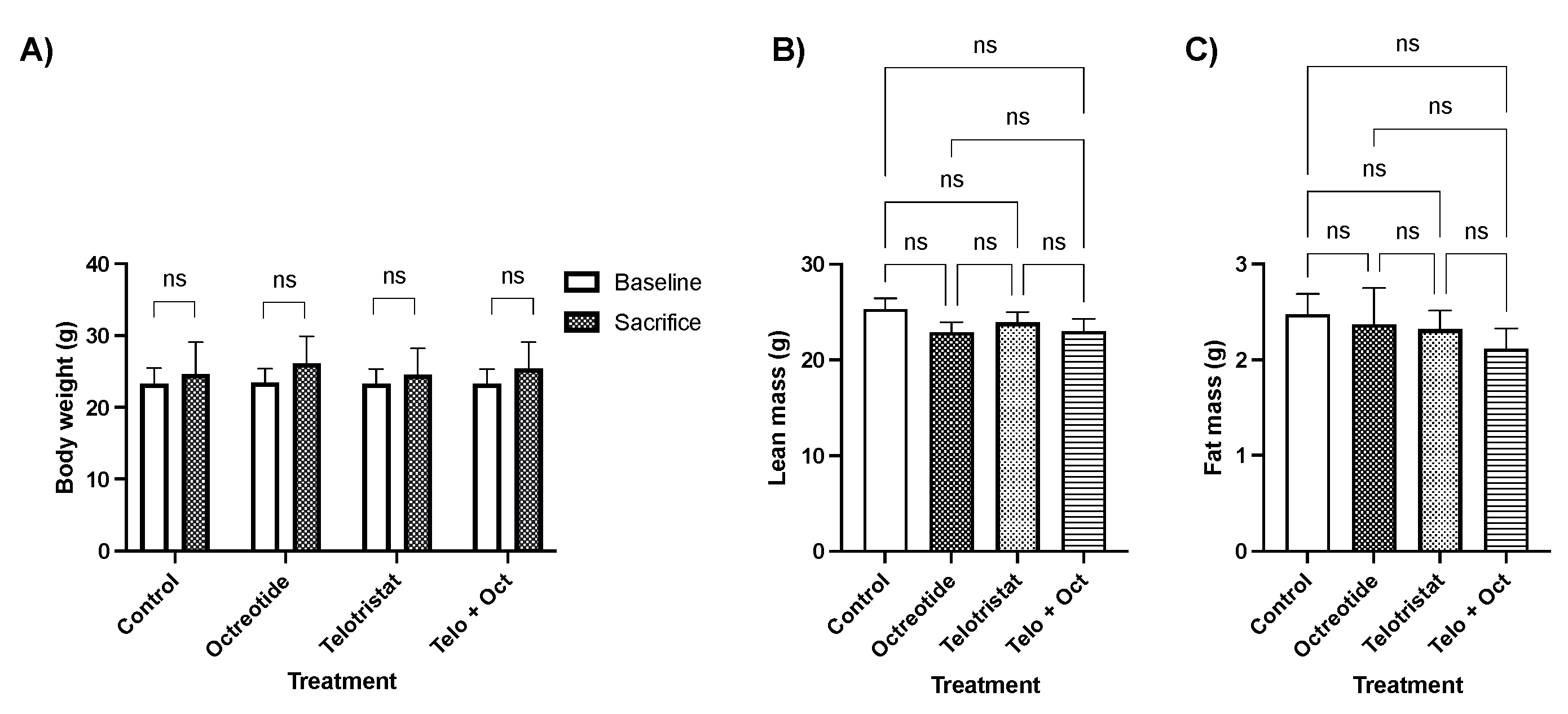
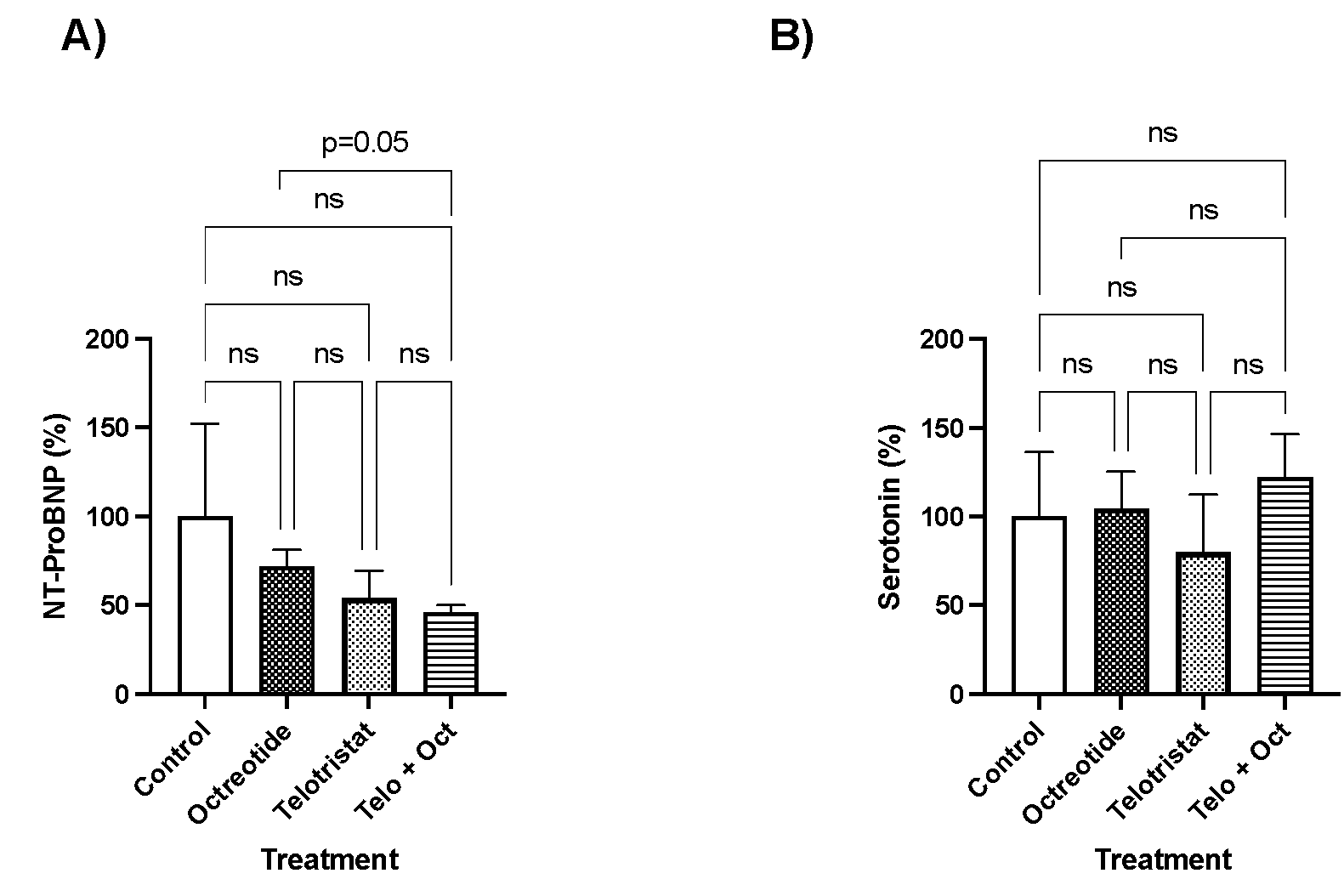
2. Results
3. Discussion
4. Materials and Methods
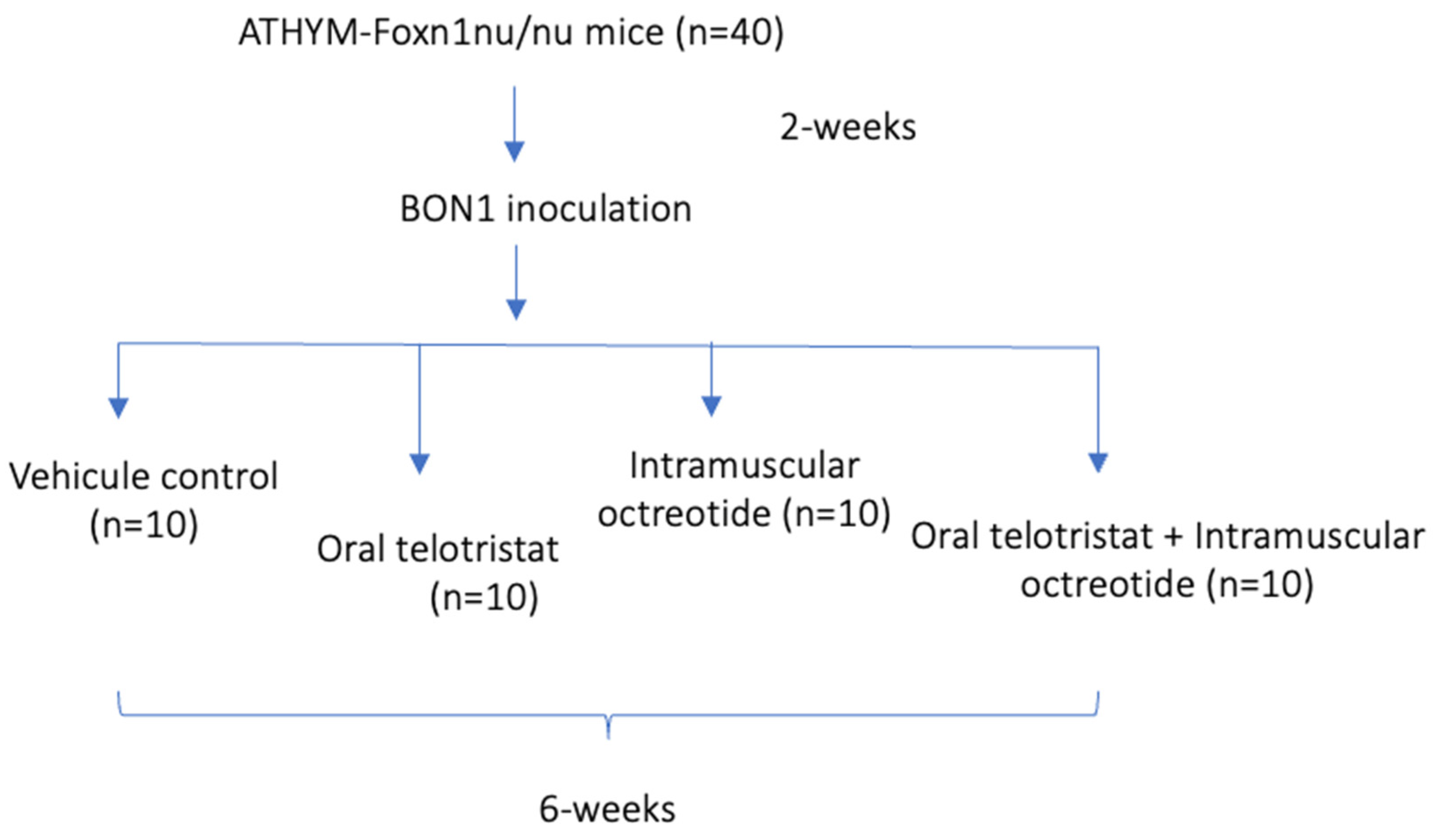
4.1. Establishment of the Mouse Model of Serotonin-Secreting NEN
4.2. Drugs, Reagents, and Treatment Groups
4.3. Determination of Whole Body Composition
4.4. Assessment of Plasma NT-proBNP and Serotonin
4.5. Heart Valve Histology
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Herrera-Martínez, A.D.; Hofland, L.J.; Moreno, M.A.G.; Castaño, J.P.; de Herder, W.W.; A Feelders, R. Neuroendocrine neoplasms: Current and potential diagnostic, predictive and prognostic markers. Endocr.-Relat. Cancer 2019, 26, R157–R179. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, S.G.; Gaztambide, D.S. Diagnosis and characteristics of intestinal carcinoid tumors. Carcinoid syndrome. Endocrinol. Nutr. 2007, 54, 9–14. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Davar, J.; Hofland, J.; Dobson, R.; Prasad, V.; Pascher, A.; Denecke, T.; Tesselaar, M.E.T.; Panzuto, F.; Albåge, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2022 Guidance Paper for Carcinoid Syndrome and Carcinoid Heart Disease. J. Neuroendocr. 2022, 34, e13146. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Hofland, J.; Hofland, L.J.; Brabander, T.; Eskens, F.A.L.M.; Moreno, M.A.G.; Luque, R.M.; Castaño, J.P.; de Herder, W.W.; Feelders, R.A. Targeted Systemic Treatment of Neuroendocrine Tumors: Current Options and Future Perspectives. Drugs 2018, 79, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Hofland, J.; Herrera-Martínez, A.D.; Zandee, W.T.; de Herder, W.W. Management of carcinoid syndrome: A systematic review and meta-analysis. Endocr.-Relat. Cancer 2019, 26, R145–R156. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.; Penalver, J.L.; Lo, K.B.U.; Rangaswami, J.; Pressman, G.S. Carcinoid Heart Disease: Review of Current Knowledge. Tex. Hear. Inst. J. 2019, 46, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Khattar, R.S. Carcinoid heart disease: Presentation, diagnosis, and management. Heart 2004, 90, 1224–1228. [Google Scholar] [CrossRef]
- A Pellikka, P.; Tajik, A.J.; Khandheria, B.K.; Seward, J.B.; A Callahan, J.; Pitot, H.C.; Kvols, L.K. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation 1993, 87, 1188–1196. [Google Scholar] [CrossRef]
- O’Toole, D.; Ducreux, M.; Bommelaer, G.; Wemeau, J.L.; Bouche, O.; Catus, F. Treatment of carcinoid syndrome: A prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 2000, 88, 770–776. [Google Scholar] [CrossRef]
- Ruszniewski, P.; Ish-Shalom, S.; Wymenga, M.; O’toole, D.; Arnold, R.; Tomassetti, P.; Bax, N.; Caplin, M.; Eriksson, B.; Glaser, B.; et al. Rapid and Sustained Relief from the Symptoms of Carcinoid Syndrome: Results from an Open 6-Month Study of the 28-Day Prolonged-Release Formulation of Lanreotide. Neuroendocrinology 2004, 80, 244–251. [Google Scholar] [CrossRef]
- Best, J.; Nijhout, H.F.; Reed, M. Serotonin synthesis, release and reuptake in terminals: A mathematical model. Theor. Biol. Med Model. 2010, 7, 34. [Google Scholar] [CrossRef]
- Lapuerta, P.; Zambrowicz, B.; Fleming, D.; Wheeler, D.; Sands, A. Telotristat etiprate, a novel inhibitor of serotonin synthesis for the treatment of carcinoid sy ndrome. Clin. Investig. 2015, 5, 447–456. [Google Scholar] [CrossRef]
- Kulke, M.H.; Hörsch, D.; Caplin, M.E.; Anthony, L.B.; Bergsland, E.; Öberg, K.; Welin, S.; Warner, R.R.; Lombard-Bohas, C.; Kunz, P.L.; et al. Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome. J. Clin. Oncol. 2017, 35, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Gross, D.J.; Benavent, M.; Perros, P.; Srirajaskanthan, R.; Warner, R.R.P. Telotristat ethyl in carcinoid syndrome: Safety and efficacy in the TELECAST phase 3 trial. Endocr. Relat. Cancer 2018, 25, 309–322. [Google Scholar] [CrossRef]
- Norlén, O.; Stålberg, P.; Öberg, K.; Eriksson, J.; Hedberg, J.; Hessman, O.; Janson, E.T.; Hellman, P.; Åkerström, G. Long-Term Results of Surgery for Small Intestinal Neuroendocrine Tumors at a Tertiary Referral Center. Mol. Med. 2011, 36, 1419–1431. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Lardière-Deguelte, S.; de Mestier, L.; Appéré, F.; Vullierme, M.-P.; Zappa, M.; Hoeffel, C.; Noaves, M.; Brixi, H.; Hentic, O.; Ruszniewski, P.; et al. Toward a Preoperative Classification of Lymph Node Metastases in Patients with Small Intestinal Neuroendocrine Tumors in the Era of Intestinal-Sparing Surgery. Neuroendocrinology 2015, 103, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Pasquer, A.; Walter, T.; Hervieu, V.; Forestier, J.; Scoazec, J.-Y.; Lombard-Bohas, C.; Poncet, G. Surgical Management of Small Bowel Neuroendocrine Tumors: Specific Requirements and Their Impact on Staging and Prognosis. Ann. Surg. Oncol. 2015, 22, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, G.; Caplin, M.; Davies, P.; Ferone, D.; Garcia-Carbonero, R.; Grozinsky-Glasberg, S.; Hörsch, D.; Janson, E.T.; Kianmanesh, R.; Kos-Kudla, B.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology 2017, 105, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.C.; Luis, S.A.; Shabtaie, S.A.; Pellikka, P.A.; Connolly, H.M.; Miranda, W.; Schaff, H.; Shen, W.-K.; Killu, A.M.; Cha, Y.-M.; et al. Outcomes and periprocedural management of cardiac implantable electronic devices in patients with carcinoid heart disease. Hear. Rhythm. 2021, 18, 2094–2100. [Google Scholar] [CrossRef]
- Evers, B.M.; Ishizuka, J.; Townsend, C.M.; Thompson, J.C. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann. N. Y. Acad. Sci. 1994, 733, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Jeng, Y.-J.; Townsend, C.M.; Nagasawa, S.; Chuo, S.; Kern, K.; Yanaihara, N.; Ferrar, R.S.; Hill, F.L.; Thompson, J.C.; Greeley, G.H. Regulation of Pancreastatin Release from a Human Pancreatic Carcinoid Cell Line in Vitro*. Endocrinology 1991, 128, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Feelders, R.A.; Van den Dungen, R.; Dogan-Oruc, F.; Van Koetsveld, P.M.; Castaño, J.P.; De Herder, W.W.; Hofland, L.J. Effect of the Tryptophan Hydroxylase Inhibitor Telotristat on Growth and Serotonin Secretion in 2D and 3D Cultured Pancreatic Neuroendocrine Tumor Cells. Neuroendocrinology 2020, 110, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martinez, A.D.; Feelders, R.A.; de Herder, W.W.; Castano, J.P.; Galvez Moreno, M.A.; Dogan, F. Effects of Ketoconazole on ACTH-Producing and Non-ACTH-Producing Neuroendocrine Tumor Cells. Horm. Cancer 2019, 10, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Dungen, R.v.D.; Dogan-Oruc, F.; van Koetsveld, P.M.; Culler, M.D.; de Herder, W.W.; Luque, R.M.; A Feelders, R.; Hofland, L.J. Effects of novel somatostatin-dopamine chimeric drugs in 2D and 3D cell culture models of neuroendocrine tumors. Endocr. Relat. Cancer 2019, 26, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Jiménez-Vacas, J.M.; Alors-Pérez, E.; Herrero-Aguayo, V.; Fuentes-Fayos, A.C.; Pedraza-Arévalo, S.; Castaño, J.P.; Luque, R.M. Mouse models of endocrine tumors. J. Endocrinol. 2019, 240, R73–R96. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.N.; Chen, L.A.; Larson, S.D.; Silva, S.R.; Rychahou, P.G.; Boor, P.J. Development and characterization of a novel in vivo model of carcinoid syndrome. Clin. Cancer Res. 2009, 15, 2747–2755. [Google Scholar] [CrossRef]
- Evers, B.M.; Townsend, C.M.; Upp, J.R.; Allen, E.; Hurlbut, S.C.; Kim, S.W. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 1991, 101, 303–311. [Google Scholar] [CrossRef]
- Strosberg, J.; Kvols, L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2010, 16, 2963–2970. [Google Scholar] [CrossRef]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Herrera-Martínez, Y.; Teomiro, C.A.; Idougourram, S.L.; Puertas, M.J.M.; Continente, A.C.; Blanch, R.S.; Castaño, J.P.; Moreno, M.G.; Gahete, M.D.; Luque, R.M.; et al. Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Maasberg, S.; Knappe-Drzikova, B.; Vonderbeck, D.; Jann, H.; Weylandt, K.H.; Grieser, C.; Pascher, A.; Schefold, J.C.; Pavel, M.; Wiedenmann, B.; et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology 2015, 104, 11–25. [Google Scholar] [CrossRef] [PubMed]
- A Qureshi, S.; Burch, N.; Druce, M.; Hattersley, J.G.; Khan, S.; Gopalakrishnan, K.; Darby, C.; Wong, J.L.H.; Davies, L.; Fletcher, S.; et al. Screening for malnutrition in patients with gastro-entero-pancreatic neuroendocrine tumours: A cross-sectional study. BMJ Open 2016, 6, e010765. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Clarke, S.J.; Engel, A.; Diakos, C.I.; Pavlakis, N.; Roach, P.J.; Bailey, D.L.; Bauer, J.; Findlay, M. Computed tomography (CT)-defined sarcopenia and myosteatosis are prevalent in patients with neuroendocrine neoplasms (NENs) treated with peptide receptor radionuclide therapy (PRRT). Eur. J. Clin. Nutr. 2021, 76, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- A Oates, J.; A Pettinger, W.; Doctor, R.B. Evidence for the release of bradykinin in carcinoid syndrome. J. Clin. Investig. 1966, 45, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Oates, J.A.; Melmon, K.; Sjoerdsma, A.; Gillespie, L.; Mason, D.T. Release of a kinin peptide in the carcinoid syndrome. Lancet 1964, 1, 514–517. [Google Scholar] [CrossRef]
- Rorstad, O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J. Surg. Oncol. 2005, 89, 151–160. [Google Scholar] [CrossRef]
- Kulke, M.H.; Shah, M.H.; Benson, A.B., 3rd; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 78–108. [Google Scholar] [CrossRef]
- van der Lely, A.J.; de Herder, W.W. Carcinoid syndrome: Diagnosis and medical management. Arq. Bras. Endocrinol. Metabol. 2005, 49, 850–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zandee, W.T.; Kamp, K.; van Adrichem, R.C.S.; A Feelders, R.; de Herder, W.W. Limited value for urinary 5-HIAA excretion as prognostic marker in gastrointestinal neuroendocrine tumours. Eur. J. Endocrinol. 2016, 175, 361–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindström, M.; Tohmola, N.; Renkonen, R.; Hämäläinen, E.; Schalin-Jäntti, C.; Itkonen, O. Comparison of serum serotonin and serum 5-HIAA LC-MS/MS assays in the diagnosis of serotonin producing neuroendocrine neoplasms: A pilot study. Clin. Chim. Acta 2018, 482, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Toumpanakis, C.; Caplin, M.E.; Davar, J. Usefulness of N-terminal Pro–Brain Natriuretic Peptide as a Biomarker of the Presence of Carcinoid Heart Disease. Am. J. Cardiol. 2008, 102, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Burgess, M.I.; Banks, M.; Pritchard, D.M.; Vora, J.; Valle, J.W.; Wong, C.; Chadwick, C.; George, K.; Keevil, B.; et al. The Association of a Panel of Biomarkers with the Presence and Severity of Carcinoid Heart Disease: A Cross-Sectional Study. PLoS ONE 2013, 8, e73679. [Google Scholar] [CrossRef] [PubMed]
- Davar, J.; Connolly, H.M.; Caplin, M.E.; Pavel, M.; Zacks, J.; Bhattacharyya, S. Diagnosing and Managing Carcinoid Heart Disease in Patients with Neuroendocrine Tumors: An Expert Statement. J. Am. Coll. Cardiol. 2017, 69, 1288–1304. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Stockton, S.S.; A Hassan, S. Carcinoid Heart Disease Management: A Multi-Disciplinary Collaboration. Oncologist 2023, 28, 575–583. [Google Scholar] [CrossRef]
- O’brien, M.; Ernst, M.; Poh, A.R. An intrasplenic injection model of pancreatic cancer metastasis to the liver in mice. STAR Protoc. 2023, 4, 102021. [Google Scholar] [CrossRef]
- Parekh, D.; Ishizuka, J.; Townsend, C.M.; Haber, B.; Beauchamp, R.D.; Karp, G. Characterization of a human pancreatic carcinoid in vitro: Morphology, amine and peptide storage, and secretion. Pancreas 1994, 9, 83–90. [Google Scholar] [CrossRef]
- Puri, S.; García-Núñez, A.; Hebrok, M.; Cano, D.A. Elimination of Von Hippel-Lindau Function Perturbs Pancreas Endocrine Homeostasis in Mice. PLoS ONE 2013, 8, e72213. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Martínez, A.D.; Fuentes-Fayos, A.C.; Sanchez-Sanchez, R.; Montero, A.J.; Sarmento-Cabral, A.; Gálvez-Moreno, M.A.; Gahete, M.D.; Luque, R.M. Does Telotristat Have a Role in Preventing Carcinoid Heart Disease? Int. J. Mol. Sci. 2024, 25, 2036. https://doi.org/10.3390/ijms25042036
Herrera-Martínez AD, Fuentes-Fayos AC, Sanchez-Sanchez R, Montero AJ, Sarmento-Cabral A, Gálvez-Moreno MA, Gahete MD, Luque RM. Does Telotristat Have a Role in Preventing Carcinoid Heart Disease? International Journal of Molecular Sciences. 2024; 25(4):2036. https://doi.org/10.3390/ijms25042036
Chicago/Turabian StyleHerrera-Martínez, Aura D., Antonio C. Fuentes-Fayos, Rafael Sanchez-Sanchez, Antonio J. Montero, André Sarmento-Cabral, María A. Gálvez-Moreno, Manuel D. Gahete, and Raúl M. Luque. 2024. "Does Telotristat Have a Role in Preventing Carcinoid Heart Disease?" International Journal of Molecular Sciences 25, no. 4: 2036. https://doi.org/10.3390/ijms25042036
APA StyleHerrera-Martínez, A. D., Fuentes-Fayos, A. C., Sanchez-Sanchez, R., Montero, A. J., Sarmento-Cabral, A., Gálvez-Moreno, M. A., Gahete, M. D., & Luque, R. M. (2024). Does Telotristat Have a Role in Preventing Carcinoid Heart Disease? International Journal of Molecular Sciences, 25(4), 2036. https://doi.org/10.3390/ijms25042036







