Identification of Germline FOXE1 and Somatic MAPK Pathway Gene Alterations in Patients with Malignant Struma Ovarii, Cleft Palate and Thyroid Cancer
Abstract
1. Introduction
2. Results
2.1. Identification of FOXE1 Promoter Variants in Two Portuguese Families
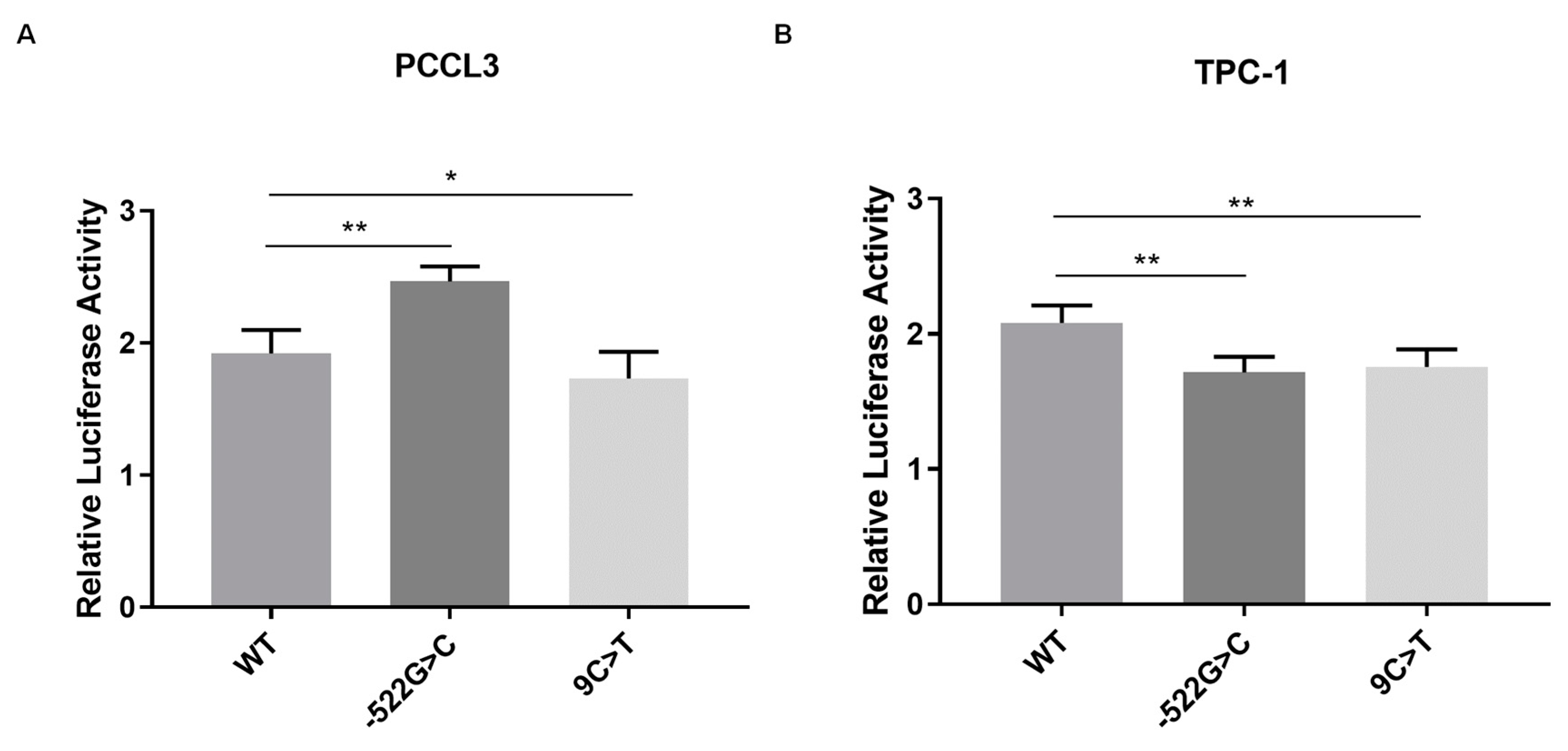
2.2. Analysis of the Effect of FOXE1 Variants on FOXE1 Promoter Activity In Vitro
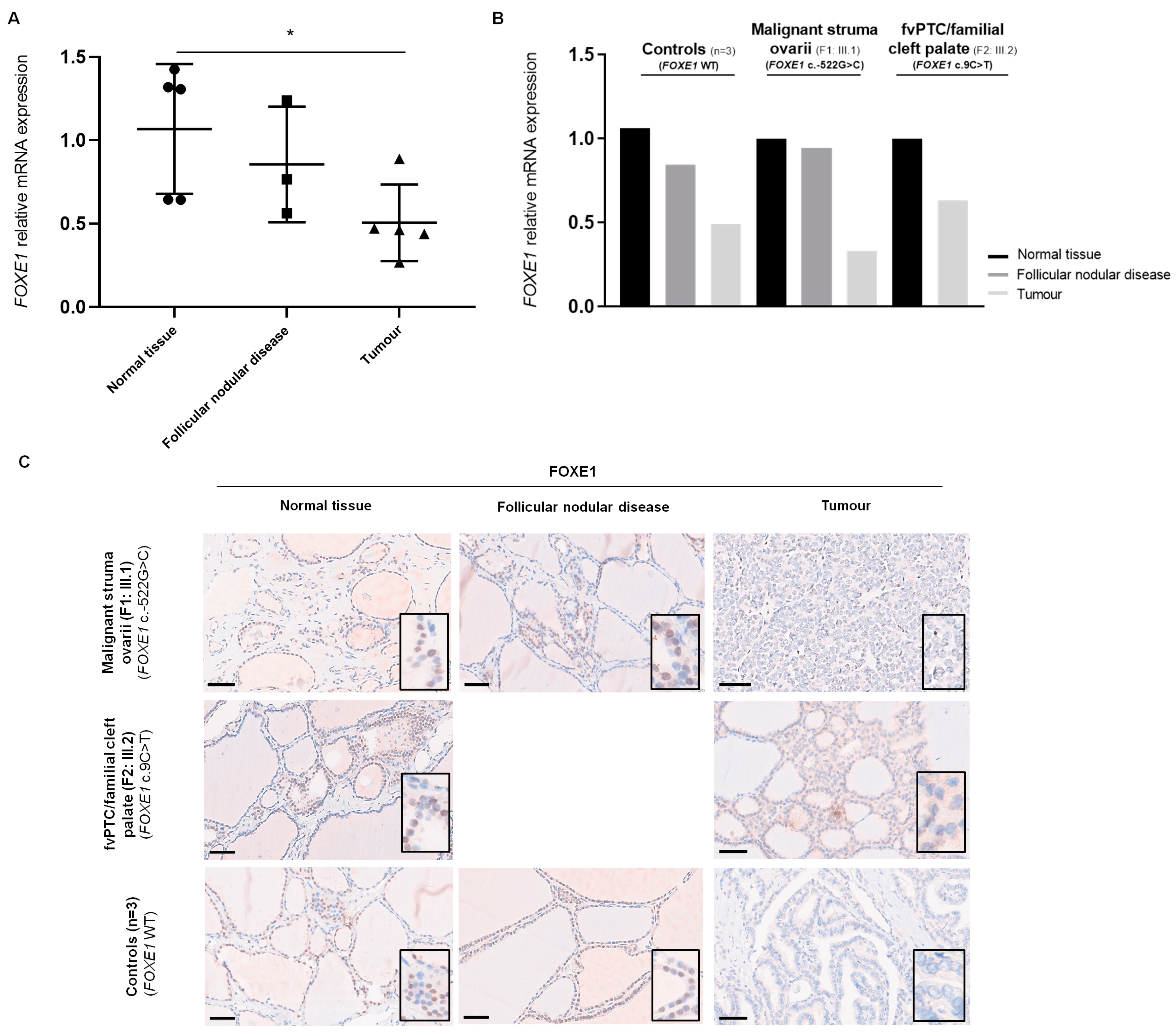
2.3. Analysis of FOXE1 mRNA Expression in Patients’ Thyroid Tissue by RT-qPCR
2.4. Analysis of FOXE1 Protein Expression in Patients’ Thyroid Tissue by Immunohistochemistry
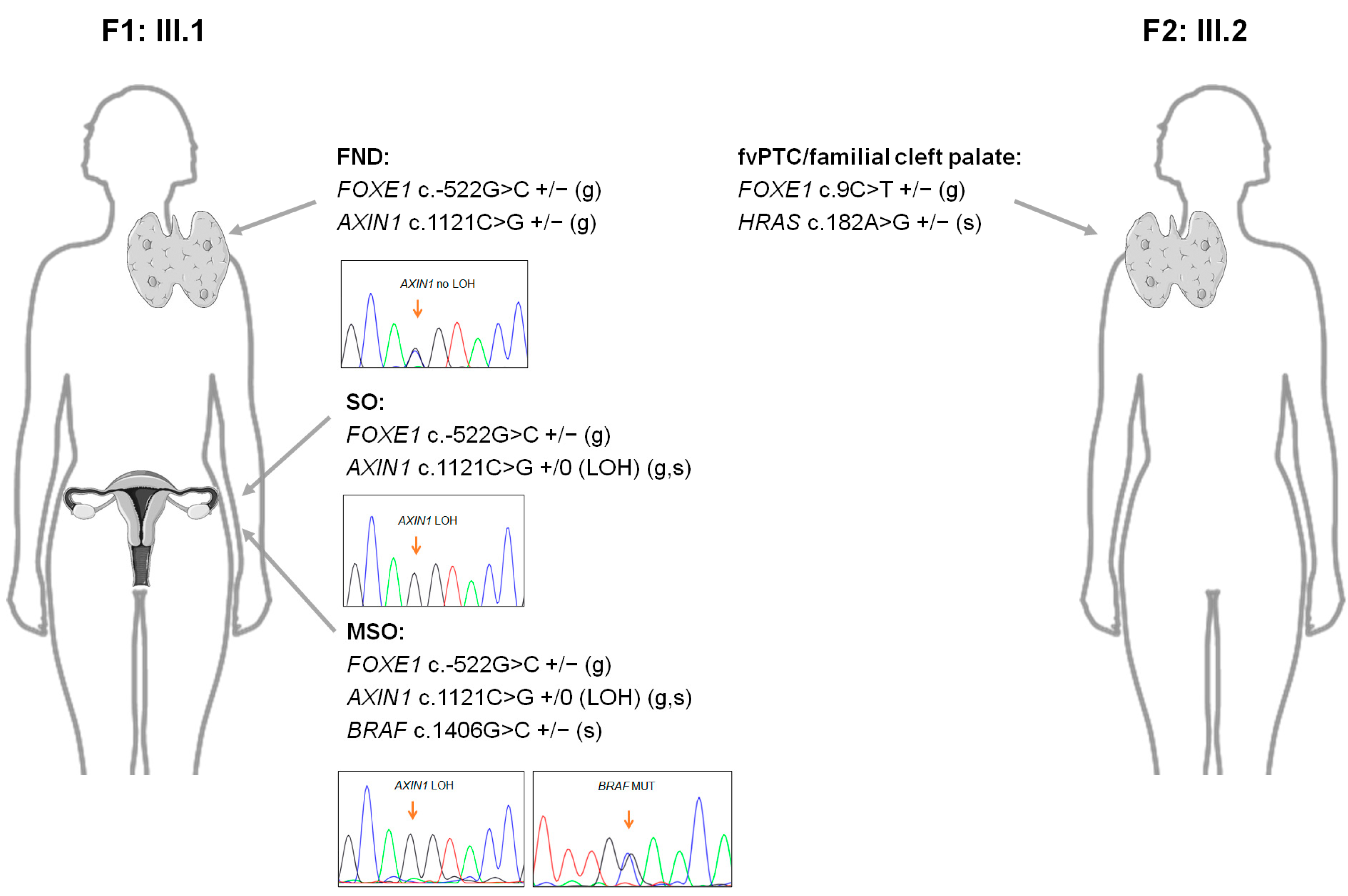
2.5. Next-Generation Sequencing Analyses of the Probands from Families F1 and F2
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Nucleic Acid Extraction
4.3. Sanger Sequencing Analysis
4.4. Next-Generation Sequencing (NGS)
4.5. Quantitative Reverse Transcription PCR (RT-qPCR)
4.6. Immunohistochemistry (IHC)
4.7. Cell Culture
4.8. Constructs, Transient Transfections and Luciferase Reporter Gene Assays
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on fundamental mechanisms of thyroid cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Veschi, V.; Turdo, A.; Modica, C.; Verona, F.; Di Franco, S.; Gaggianesi, M.; Tirrò, E.; Di Bella, S.; Iacono, M.L.; Pantina, V.D.; et al. Recapitulating thyroid cancer histotypes through engineering embryonic stem cells. Nat. Commun. 2023, 14, 1351. [Google Scholar] [CrossRef]
- Donato, S.; Simões, H.; Leite, V. Malignant Struma Ovarii with Concurrent Thyroid Cancer: Outcomes during and after Pregnancy. Eur. Thyroid J. 2021, 10, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakayama, K.; Kanno, K.; Ishibashi, T.; Ishikawa, M.; Sato, S.; Iida, K.; Razia, S.; Kyo, S. Identifying the Carcinogenic Mechanism of Malignant Struma Ovarii Using Whole-Exome Sequencing and DNA Methylation Analysis. Curr. Issues Mol. Biol. 2023, 45, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- De Felice, M.; Di Lauro, R. Thyroid development and its disorders: Genetics and molecular mechanisms. Endocr. Rev. 2004, 25, 722–746. [Google Scholar] [CrossRef] [PubMed]
- De Felice, M.; Ovitt, C.; Biffali, E.; Rodriguez-Mallon, A.; Arra, C.; Anastassiadis, K.; Macchia, P.E.; Mattei, M.G.; Mariano, A.; Scholer, H.; et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat. Genet. 1998, 19, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.P.; Lopez-Marquez, A.; Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 2015, 11, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Parlato, R.; Rosica, A.; Rodriguez-Mallon, A.; Affuso, A.; Postiglione, M.P.; Arra, C.; Mansouri, A.; Kimura, S.; Di Lauro, R.; De Felice, M. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev. Biol. 2004, 276, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Bernal, J.; Fernández, L.P.; Santisteban, P. FOXE1 regulates migration and invasion in thyroid cancer cells and targets ZEB1. Endocr. Relat. Cancer 2020, 27, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Sigurdsson, A.; Bergthorsson, J.T.; He, H.; Blondal, T.; Geller, F.; Jakobsdottir, M.; et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat. Genet. 2009, 41, 460–464. [Google Scholar] [CrossRef]
- Matsuse, M.; Takahashi, M.; Mitsutake, N.; Nishihara, E.; Hirokawa, M.; Kawaguchi, T.; Rogounovitch, T.; Saenko, V.; Bychkov, A.; Suzuki, K.; et al. The FOXE1 and NKX2-1 loci are associated with susceptibility to papillary thyroid carcinoma in the Japanese population. J. Med. Genet. 2011, 48, 645–648. [Google Scholar] [CrossRef]
- Penna-Martinez, M.; Epp, F.; Kahles, H.; Ramos-Lopez, E.; Hinsch, N.; Hansmann, M.L.; Selkinski, I.; Grünwald, F.; Holzer, K.; Bechstein, W.O.; et al. FOXE1 association with differentiated thyroid cancer and its progression. Thyroid 2014, 24, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ruiz-Llorente, S.; Montero-Conde, C.; Inglada-Perez, L.; Schiavi, F.; Leskela, S.; Pita, G.; Milne, R.; Maravall, J.; Ramos, I.; et al. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet. 2009, 5, e1000637. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; Duncan, E.L.; O’Neill, C.; Tacon, L.; Sywak, M.; Sidhu, S.; Delbridge, L.; Learoyd, D.; Robinson, B.G.; Ludgate, M.; et al. Association of FOXE1 polyalanine repeat region with papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, E1814–E1819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomaz, R.A.; Sousa, I.; Silva, J.G.; Santos, C.; Teixeira, M.R.; Leite, V.; Cavaco, B.M. FOXE1 polymorphisms are associated with familial and sporadic nonmedullary thyroid cancer susceptibility. Clin. Endocrinol. 2012, 77, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.S.; da Silva, J.G.; Tomaz, R.A.; Pinto, A.E.; Bugalho, M.J.; Leite, V.; Cavaco, B.M. Identification of a novel germline FOXE1 variant in patients with familial non-medullary thyroid carcinoma (FNMTC). Endocrine 2015, 49, 204–214. [Google Scholar] [CrossRef]
- Clifton-Bligh, R.J.; Wentworth, J.M.; Heinz, P.; Crisp, M.S.; John, R.; Lazarus, J.H.; Ludgate, M.; Chatterjee, V.K. Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat. Genet. 1998, 19, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Castanet, M.; Park, S.M.; Smith, A.; Bost, M.; Leger, J.; Lyonnet, S.; Pelet, A.; Czernichow, P.; Chatterjee, K.; Polak, M. A novel loss-of-function mutation in TTF-2 is associated with congenital hypothyroidism, thyroid agenesis and cleft palate. Hum. Mol. Genet. 2002, 11, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Barış, I.; Arısoy, A.E.; Smith, A.; Agostini, M.; Mitchell, C.S.; Park, S.M.; Halefoglu, A.M.; Zengin, E.; Chatterjee, V.K.; Battaloglu, E. A novel missense mutation in human TTF-2 (FKHL15) gene associated with congenital hypothyroidism but not athyreosis. J. Clin. Endocrinol. Metab. 2006, 91, 4183–4187. [Google Scholar] [CrossRef]
- Castanet, M.; Mallya, U.; Agostini, M.; Schoenmakers, E.; Mitchell, C.; Demuth, S.; Raymond, F.L.; Schwabe, J.; Gurnell, M.; Chatterjee, V.K. Maternal isodisomy for chromosome 9 causing homozygosity for a novel FOXE1 mutation in syndromic congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2010, 95, 4031–4036. [Google Scholar] [CrossRef]
- Carré, A.; Hamza, R.T.; Kariyawasam, D.; Guillot, L.; Teissier, R.; Tron, E.; Castanet, M.; Dupuy, C.; El Kholy, M.; Polak, M. A novel FOXE1 mutation (R73S) in Bamforth–Lazarus syndrome causing increased thyroidal gene expression. Thyroid 2014, 24, 649–654. [Google Scholar] [CrossRef]
- de Filippis, T.; Gelmini, G.; Paraboschi, E.; Vigone, M.C.; Di Frenna, M.; Marelli, F.; Bonomi, M.; Cassio, A.; Larizza, D.; Moro, M.; et al. A frequent oligogenic involvement in congenital hypothyroidism. Hum. Mol. Genet. 2017, 26, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, J.S.; Hughes, I.A.; Lazarus, J.H.; Weaver, C.M.; Harper, P.S. Congenital hypothyroidism, spiky hair, and cleft palate. J. Med. Genet. 1989, 26, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ding, Z.; Yang, Z.; Deng, X.; Kang, J.; Wu, B.; Zheng, Q. Expression and clinical significance of FOXE1 in papillary thyroid carcinoma. Mol. Med. Rep. 2013, 8, 123–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, Z.; Ke, R.; Zhang, Y.; Fan, Y.; Fan, J. FOXE1 inhibits cell proliferation, migration and invasion of papillary thyroid cancer by regulating PDGFA. Mol. Cell. Endocrinol. 2019, 493, 110420. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, D.; Tang, Y.; Chiriboga, L.; Rivera, M.; Ghossein, R. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod. Pathol. 2008, 21, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Credendino, S.C.; De Menna, M.; Cantone, I.; Moccia, C.; Esposito, M.; Di Guida, L.; De Felice, M.; De Vita, G. FOXE1-Dependent Regulation of Macrophage Chemotaxis by Thyroid Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 7666. [Google Scholar] [CrossRef] [PubMed]
- Lidral, A.C.; Liu, H.; Bullard, S.A.; Bonde, G.; Machida, J.; Visel, A.; Uribe, L.M.; Li, X.; Amendt, B.; Cornell, R.A. A single nucleotide polymorphism associated with isolated cleft lip and palate, thyroid cancer and hypothyroidism alters the activity of an oral epithelium and thyroid enhancer near FOXE1. Hum. Mol. Genet. 2015, 24, 3895–3907. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.P.; Cortinhas-Alves, E.A.; de Oliveira, E.H.C.; Santana-da-Silva, L.C. Does the Polymorphism in the Length of the Polyalanine Tract of FOXE1 Gene Influence the Risk of Thyroid Dysgenesis Occurrence? J. Thyroid Res. 2017, 2017, 2793205. [Google Scholar] [CrossRef]
- Grassi, E.S.; Rurale, G.; de Filippis, T.; Gentilini, D.; Carbone, E.; Coscia, F.; Uraghi, S.; Bullock, M.; Clifton-Bligh, R.J.; Gupta, A.K.; et al. The length of FOXE1 polyalanine tract in congenital hypothyroidism: Evidence for a pathogenic role from familial, molecular and cohort studies. Front. Endocrinol. 2023, 14, 1127312. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- Kurihara, T.; Ikeda, S.; Ishizaki, Y.; Fujimori, M.; Tokumoto, N.; Hirata, Y.; Ozaki, S.; Okajima, M.; Sugino, K.; Asahara, T. Immunohistochemical and sequencing analyses of the Wnt signaling components in Japanese anaplastic thyroid cancers. Thyroid 2004, 14, 1020–1029. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Yardy, G.W.; Bicknell, D.C.; Wilding, J.L.; Bartlett, S.; Liu, Y.; Winney, B.; Turner, G.D.; Brewster, S.F.; Bodmer, W.F. Mutations in the AXIN1 gene in advanced prostate cancer. Eur. Urol. 2009, 56, 486–494. [Google Scholar] [CrossRef]
- Tan, A.; Stewart, C.J.R.; Garrett, K.L.; Rye, M.; Cohen, P.A. Novel BRAF and KRAS mutations in papillary thyroid carcinoma arising in struma ovarii. Endocr. Pathol. 2015, 26, 296–301. [Google Scholar] [CrossRef]
- Poli, R.; Scatolini, M.; Grosso, E.; Maletta, F.; Gallo, M.; Liscia, D.; Nelva, A.; Cesario, F.; Forte, G.; Metovic, J.; et al. Malignant struma ovarii: Next-generation sequencing of six cases revealed Nras, Braf, and Jak3 mutations. Endocrine 2021, 71, 216–224. [Google Scholar] [CrossRef]
- Neyrand, S.; Trecourt, A.; Lopez, J.; Just, P.A.; Descotes, F.; Borson-Chazot, F.; Ray-Coquard, I.; Decaussin-Petrucci, M.; Devouassoux-Shisheboran, M. Role of gene sequencing in classifying struma ovarii: BRAF p. G469A mutation and TERT promoter alterations favour malignant struma ovarii. Histopathology 2023, 84, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Mosrati, M.A.; Willander, K.; Falk, I.J.; Hermanson, M.; Höglund, M.; Stockelberg, D.; Wei, Y.; Lotfi, K.; Söderkvist, P. Association between TERT promoter polymorphisms and acute myeloid leukemia risk and prognosis. Oncotarget 2015, 6, 25109–25120. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.R.; Mayall, E.S.; Wyllie, F.S.; Williams, E.D.; Goyns, M.; Stringer, B.; Wynford-Thomas, D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 1989, 4, 159–164. [Google Scholar] [PubMed]
- Ma, D.; Guseva, N.V.; Dahmoush, L.; Robinson, R.A. Struma ovarii with malignant transformation and germline KIT mutation: A case report with review of the literature. Int. J. Gynecol. Pathol. 2016, 35, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Persaud, T.V.N. The Developing Human: Clinically Oriented Embryology, 5th ed.; WB Saunders: Philadelphia, PA, USA, 1993. [Google Scholar]
- Fundakowski, C.; Felger, E.; Maghami, E. Thyroglossal Duct Cysts and Ectopic Thyroid Tissue. In Surgery of the Thyroid and Parathyroid Glands, 3rd ed.; Randolph, G.W., Ed.; Elsevier: Philadelphia, PA, USA, 2021; pp. 50–52. [Google Scholar]
- Moreno, L.M.; Mansilla, M.A.; Bullard, S.A.; Cooper, M.E.; Busch, T.D.; Machida, J.; Johnson, M.K.; Brauer, D.; Krahn, K.; Daack-Hirsch, S.; et al. FOXE1 association with both isolated cleft lip with or without cleft palate, and isolated cleft palate. Hum. Mol. Genet. 2009, 18, 4879–4896. [Google Scholar] [CrossRef] [PubMed]
- Credendino, S.C.; Moccia, C.; Amendola, E.; D’Avino, G.; Di Guida, L.; Clery, E.; Greco, A.; Bellevicine, C.; Brunetti, A.; De Felice, M.; et al. FOXE1 gene dosage affects thyroid cancer histology and differentiation in vivo. Int. J. Mol. Sci. 2020, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.; Saenko, V.; Nakashima, M.; Mitsutake, N.; Rogounovitch, T.; Nikitski, A.; Yamashita, S. Patterns of FOXE1 expression in papillary thyroid carcinoma by immunohistochemistry. Thyroid 2013, 23, 817–828. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, W.; Liyanarachchi, S.; Srinivas, M.; Wang, Y.; Akagi, K.; Wang, Y.; Wu, D.; Wang, Q.; Jin, V.; et al. Multiple functional variants in long-range enhancer elements contribute to the risk of SNP rs965513 in thyroid cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 6128–6133. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, H.; Li, W.; Phay, J.; Shen, R.; Yu, L.; Hancioglu, B.; de la Chapelle, A. MYH9 binds to lncRNA gene PTCSC2 and regulates FOXE1 in the 9q22 thyroid cancer risk locus. Proc. Natl. Acad. Sci. USA 2017, 114, 474–479. [Google Scholar] [CrossRef]
- Zhang, P.; Zuo, H.; Nakamura, Y.; Nakamura, M.; Wakasa, T.; Kakudo, K. Immunohistochemical analysis of thyroid-specific transcription factors in thyroid tumors. Pathol. Int. 2006, 56, 240–245. [Google Scholar] [CrossRef]
- Sequeira, M.J.; Morgan, J.M.; Fuhrer, D.; Wheeler, M.H.; Jasani, B.; Ludgate, M. Thyroid transcription factor-2 gene expression in benign and malignant thyroid lesions. Thyroid 2001, 11, 995–1001. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Magne, F.; Chanoine, J.P.; Deal, C.; Van Vliet, G.; Deladoëy, J. Role for tissue-dependent methylation differences in the expression of FOXE1 in nontumoral thyroid glands. J. Clin. Endocrinol. Metab. 2014, 99, E1120–E1129. [Google Scholar] [CrossRef][Green Version]
- Nikitski, A.; Saenko, V.; Shimamura, M.; Nakashima, M.; Matsuse, M.; Suzuki, K.; Rogounovitch, T.; Bogdanova, T.; Shibusawa, N.; Yamada, M.; et al. Targeted Foxe1 overexpression in mouse thyroid causes the development of multinodular goiter but does not promote carcinogenesis. Endocrinology 2016, 157, 2182–2195. [Google Scholar] [CrossRef]
- Cavaco, B.M.; Batista, P.F.; Martins, C.; Banito, A.; do Rosário, F.; Limbert, E.; Sobrinho, L.G.; Leite, V. Familial non-medullary thyroid carcinoma (FNMTC): Analysis of fPTC/PRN, NMTC1, MNG1 and TCO susceptibility loci and identification of somatic BRAF and RAS mutations. Endocr. Relat. Cancer 2008, 15, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Moses, W.; Weng, J.; Kebebew, E. Prevalence, clinicopathologic features, and somatic genetic mutation profile in familial versus sporadic nonmedullary thyroid cancer. Thyroid 2011, 21, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Gomes, I.; Pojo, M.; Pires, C.; Moura, M.M.; Cabrera, R.; Santos, C.; van IJcken, W.F.J.; Teixeira, M.R.; Ramalho, J.S.; et al. Identification of SPRY4 as a novel candidate susceptibility gene for familial nonmedullary thyroid cancer. Thyroid 2021, 31, 1366–1375. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, T.; Sun, J.; Wang, F.; Cheng, C.; He, S.; Chen, L.; Xie, D.; Fu, L.; Guan, X.; et al. Germ-line mutations in WDR77 predispose to familial papillary thyroid cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2026327118. [Google Scholar] [CrossRef]
- Bann, D.V.; Jin, Q.; Sheldon, K.E.; Houser, K.R.; Nguyen, L.; Warrick, J.I.; Baker, M.J.; Broach, J.R.; Gerhard, G.S.; Goldenberg, D. Genetic variants implicate dual oxidase-2 in familial and sporadic nonmedullary thyroid cancer. Cancer Res. 2019, 79, 5490–5499. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Perona, A.; Santisteban, P. Role of the wnt pathway in thyroid cancer. Front. Endocrinol. 2012, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Shimizu, S.I.; Chong, J.M.; Hishima, T.; Funata, N.; Kashiwagi, H.; Nagai, H.; Miyaki, M.; Fukayama, M. Nuclear localization of immunoreactive β-catenin is specific to familial adenomatous polyposis in papillary thyroid carcinoma. Jpn. J. Clin. Oncol. 2000, 91, 1100–1102. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Simões-Pereira, J.; Saramago, A.; Rodrigues, R.; Pojo, M.; Pires, C.; Horta, M.; López-Presa, D.; Rito, M.; Cabrera, R.; Ferreira, T.C.; et al. Clinical and molecular characterisation of metastatic papillary thyroid cancer according to radioiodine therapy outcomes. Endocrine 2023. [Google Scholar] [CrossRef]

| Gene | Status | DNA | Protein | dbSNP ID | MAF (%) a | Variant’s Predicted Impact b | |
|---|---|---|---|---|---|---|---|
| F1: III.1 | FOXE1 | Germline | c.-522G>C | - | rs890127391 | 0.006 | VUS |
| AXIN1 | Germline | c.1121C>G | p.Thr374Arg | n/a | n/a | VUS | |
| TERTp | Germline | c.-245T>C | - | rs2853669 | 30.6 | Polymorphism | |
| BRAF | Somatic | c.1406G>C | p.Gly469Ala | rs121913355 | 0.0 | Deleterious (10/14) c | |
| RAF1 | Somatic | Gain | |||||
| CTNNB1 | Somatic | Gain | |||||
| PIK3CA | Somatic | Gain | |||||
| DCUN1D1 | Somatic | Gain | |||||
| F2: III.2 | FOXE1 | Germline | c.9C>T | p.Ala3= | rs911627696 | 0.006 | VUS |
| HRAS | Somatic | c.182A>G | p.Gln61Arg | rs121913233 | 0.0 | Deleterious (10/14) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, C.; Saramago, A.; Moura, M.M.; Li, J.; Donato, S.; Marques, I.J.; Belo, H.; Machado, A.C.; Cabrera, R.; Grünewald, T.G.P.; et al. Identification of Germline FOXE1 and Somatic MAPK Pathway Gene Alterations in Patients with Malignant Struma Ovarii, Cleft Palate and Thyroid Cancer. Int. J. Mol. Sci. 2024, 25, 1966. https://doi.org/10.3390/ijms25041966
Pires C, Saramago A, Moura MM, Li J, Donato S, Marques IJ, Belo H, Machado AC, Cabrera R, Grünewald TGP, et al. Identification of Germline FOXE1 and Somatic MAPK Pathway Gene Alterations in Patients with Malignant Struma Ovarii, Cleft Palate and Thyroid Cancer. International Journal of Molecular Sciences. 2024; 25(4):1966. https://doi.org/10.3390/ijms25041966
Chicago/Turabian StylePires, Carolina, Ana Saramago, Margarida M. Moura, Jing Li, Sara Donato, Inês J. Marques, Hélio Belo, Ana C. Machado, Rafael Cabrera, Thomas G. P. Grünewald, and et al. 2024. "Identification of Germline FOXE1 and Somatic MAPK Pathway Gene Alterations in Patients with Malignant Struma Ovarii, Cleft Palate and Thyroid Cancer" International Journal of Molecular Sciences 25, no. 4: 1966. https://doi.org/10.3390/ijms25041966
APA StylePires, C., Saramago, A., Moura, M. M., Li, J., Donato, S., Marques, I. J., Belo, H., Machado, A. C., Cabrera, R., Grünewald, T. G. P., Leite, V., & Cavaco, B. M. (2024). Identification of Germline FOXE1 and Somatic MAPK Pathway Gene Alterations in Patients with Malignant Struma Ovarii, Cleft Palate and Thyroid Cancer. International Journal of Molecular Sciences, 25(4), 1966. https://doi.org/10.3390/ijms25041966






