Exploring the Roles of Vitamins C and D and Etifoxine in Combination with Citalopram in Depression/Anxiety Model: A Focus on ICAM-1, SIRT1 and Nitric Oxide
Abstract
1. Introduction
2. Results
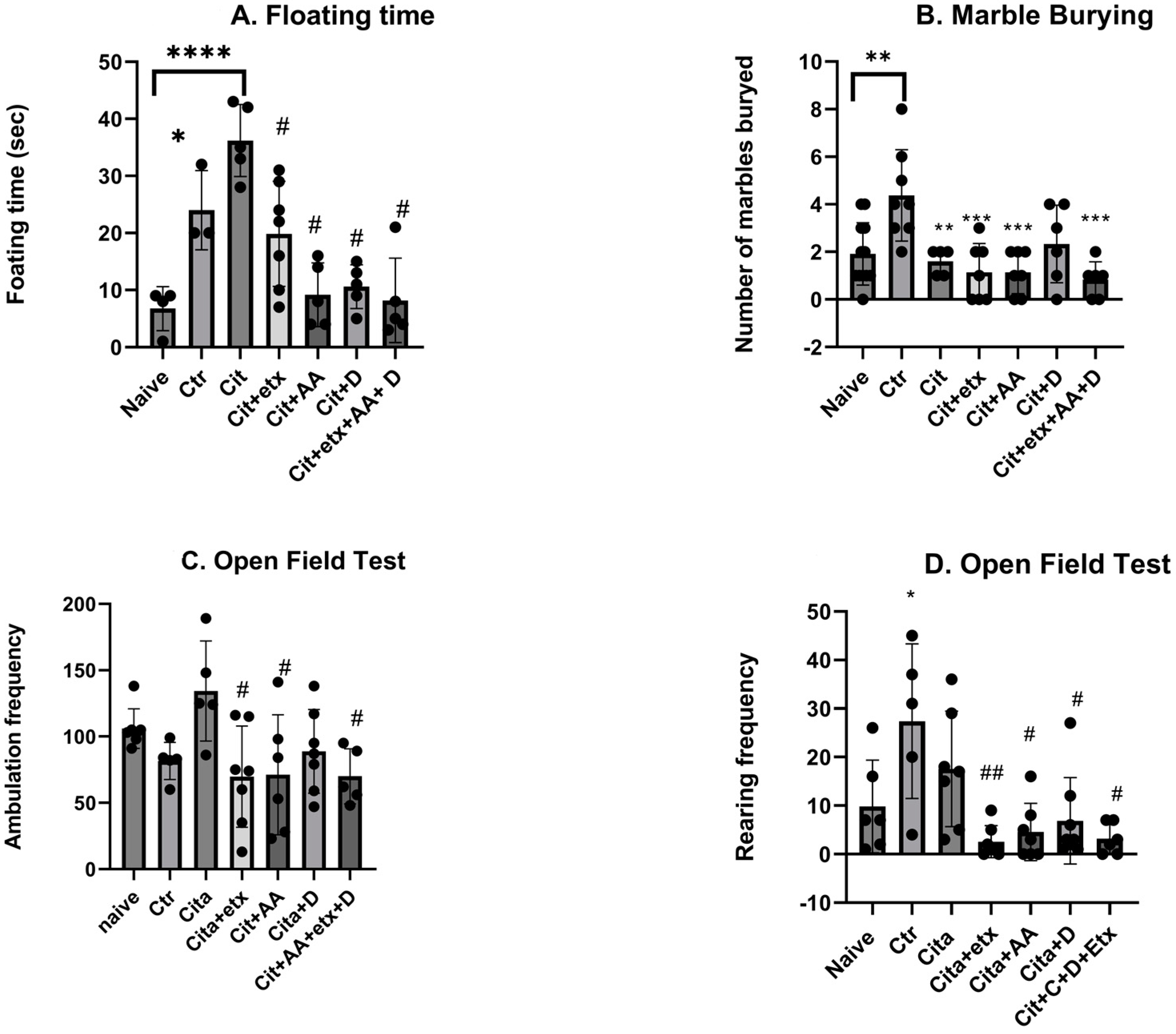
2.1. Behavioural Tests
2.2. ICAM-1 and SIRT1
2.3. Nitric Oxide
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Study Design and Treatments
4.3. Acute Restraint Model
4.4. Behavioural Paradigms
4.4.1. Forced Swim Test
4.4.2. Marble Burying Test
4.4.3. Open Field Test
4.5. Tissue Collection
4.5.1. Western Blots
4.5.2. Nitric Oxide Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fazel, M.; Wheeler, J.; Danesh, J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: A systematic review. Lancet 2005, 365, 1309–1314. [Google Scholar] [CrossRef]
- Bonadiman, C.S.C.; Malta, D.C.; Passos, V.M.d.A.; Naghavi, M.; Melo, A.P.S. Depressive disorders in Brazil: Results from the Global Burden of Disease Study 2017. Popul. Health Metrics 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef]
- Müller, N. Immunology of Major Depression. Neuroimmunomodulation 2014, 21, 123–130. [Google Scholar] [CrossRef]
- FLotrich, E.; Pittsburgh, S. Inflammatory cytokine-associated depression. Brain Res. 2016, 1617, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Irwin, M.R. HHS Public Access. Brain Behav. Immun. 2021, 83, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Zhang, Y.; Li, J.; Li, Z.; Zhang, Y.; Ye, X.; Tang, Q.; Sun, W. Comparative Efficacy and Acceptability of Anti-inflammatory Agents on Major Depressive Disorder: A Network Meta-Analysis. Front. Pharmacol. 2021, 12, 691200. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, O.; Mayyas, F.; Elhajji, F.D. Chlorpheniramine and escitalopram: Similar antidepressant and nitric oxide lowering roles in a mouse model of anxiety. Biomed. Rep. 2017, 6, 675–680. [Google Scholar] [CrossRef]
- Szarka, A.; Rigo, J., Jr.; Lázár, L.; Bekő, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef]
- Lee, S.J.; Benveniste, E.N. Adhesion molecule expression and regulation on cells of the central nervous system. J. Neuroimmunol. 1999, 98, 77–88. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Borawska, M.H. Soluble intercellular adhesion molecule-1 (sICAM-1): An overview. Eur. Cytokine Netw. 2004, 15, 91–98. [Google Scholar]
- Baghai, T.C.; Varallo-Bedarida, G.; Born, C.; Häfner, S.; Schüle, C.; Eser, D.; Zill, P.; Manook, A.; Weigl, J.; Jooyandeh, S.; et al. Classical Risk Factors and Inflammatory Biomarkers: One of the Missing Biological Links between Cardiovascular Disease and Major Depressive Disorder. Int. J. Mol. Sci. 2018, 19, 1740. [Google Scholar] [CrossRef]
- Watroba, M.; Szukiewicz, D. Sirtuins promote brain homeostasis, preventing Alzheimer’s disease through targeting neuroinflammation. Front. Physiol. 2022, 13, 962769. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Yoshimura, R.; Kitajima, T.; Okochi, T.; Okumura, T.; Tsunoka, T.; Yamanouchi, Y.; Kinoshita, Y.; Kawashima, K.; Fukuo, Y.; et al. SIRT1 gene is associated with major depressive disorder in the Japanese population. J. Affect. Disord. 2010, 126, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-J.; Zhang, C. Down-Regulation of SIRT1 Gene Expression in Major Depressive Disorder. Am. J. Psychiatry 2016, 173, 1046. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Akinfiresoye, L.; Kalejaiye, O.; Tizabi, Y. Antidepressant effects of resveratrol in an animal model of depression. Behav. Brain Res. 2014, 268, 1–7. [Google Scholar] [CrossRef]
- Ali, S.H.; Madhana, R.M.; Athira, K.V.; Kasala, E.R.; Bodduluru, L.N.; Pitta, S.; Mahareddy, J.R.; Lahkar, M. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids 2015, 101, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Guix, F.X.; Uribesalgo, I.; Coma, M.; Muñoz, F.J. The physiology and pathophysiology of nitric oxide in the brain. Prog. Neurobiol. 2005, 76, 126–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Paik, J.-W.; Lee, S.-W.; Yoon, D.; Han, C.; Lee, B.-H. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Lee, S.-W.; Yoon, D.; Lee, H.-J.; Yang, J.-C.; Shim, S.-H.; Kim, D.-H.; Ryu, S.-H.; Han, C.; Kim, Y.-K. Increased Plasma Nitric Oxide Metabolites in Suicide Attempters. Neuropsychobiology 2006, 53, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kevil, C.G.; Orr, A.W.; Langston, W.; Mickett, K.; Murphy-Ullrich, J.; Patel, R.P.; Kucik, D.F.; Bullard, D.C. Intercellular Adhesion Molecule-1 (ICAM-1) Regulates Endothelial Cell Motility through a Nitric Oxide-dependent Pathway. J. Biol. Chem. 2004, 279, 19230–19238. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sarna, S.K. Nitric oxide modifies chromatin to suppress ICAM-1 expression during colonic inflammation. Am. J. Physiol. Liver Physiol. 2012, 303, G103–G110. [Google Scholar] [CrossRef] [PubMed]
- Rogacka, D.; Audzeyenka, I.; Rachubik, P.; Szrejder, M.; Typiak, M.; Angielski, S.; Piwkowska, A. Involvement of nitric oxide synthase/nitric oxide pathway in the regulation of SIRT1–AMPK crosstalk in podocytes: Impact on glucose uptake. Arch. Biochem. Biophys. 2021, 709, 108985. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, G.; Pae, C.-U.; Masand, P.S. Beyond serotonin: Newer antidepressants in the future. Expert Rev. Neurother. 2017, 17, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.E.; Ros, S.; Agüera, L.; de la Gándara, J.; de Pedro, J.M. Combined antidepressants: Clinical experience. Acta Psychiatr. Scand. 2005, 112, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Shaikh, A.S.; Han, W.; Chen, D.; Guo, Y.; Jiang, P. Vitamin D and depression: Mechanisms, determination and application. Asia Pac. J. Clin. Nutr 2019, 28, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Binfaré, R.W.; Rosa, A.O.; Lobato, K.R.; Santos, A.R.; Rodrigues, A.L.S. Ascorbic acid administration produces an antidepressant-like effect: Evidence for the involvement of monoaminergic neurotransmission. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Amr, M.; El-Mogy, A.; Shams, T.; Vieira, K.; Lakhan, S.E. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. Nutr. J. 2013, 12, 31. [Google Scholar] [CrossRef]
- Vicente, B. Etifoxine is non-inferior than clonazepam for reduction of anxiety symptoms in the treatment of anxiety disorders: A randomized, double blind, non-inferiority trial. Psychopharmacology 2020, 237, 3357–3367. [Google Scholar] [CrossRef]
- Stein, D.J. Etifoxine versus alprazolam for the treatment of adjustment disorder with anxiety: A randomized controlled trial. Adv. Ther. 2015, 32, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Machawal, L.; Kumar, A. Possible involvement of nitric oxide mechanism in the neuroprotective effect of rutin against immobilization stress induced anxiety like behaviour, oxidative damage in mice. Pharmacol. Rep. 2014, 66, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Nitzke, A.M.; Doukas, D.G.; Seiglie, M.P.; Dulawa, S.C. Antidepressant response to chronic citalopram treatment in eight inbred mouse strains. Psychopharmacology 2011, 213, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Cervo, L.; Canetta, A.; Calcagno, E.; Burbassi, S.; Sacchetti, G.; Caccia, S.; Fracasso, C.; Albani, D.; Forloni, G.; Invernizzi, R.W. Genotype-Dependent Activity of Tryptophan Hydroxylase-2 Determines the Response to Citalopram in a Mouse Model of Depression. J. Neurosci. 2005, 25, 8165–8172. [Google Scholar] [CrossRef] [PubMed]
- Kala, M.; Nivsarkar, M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen. Comp. Endocrinol. 2016, 225, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Fava, M.; Thase, M.E. Treatment of SSRI-Resistant Depression: Across-Class Switches. Biol. Psychiatry 2008, 63, 699–704. [Google Scholar] [CrossRef]
- Undurraga, J.; Baldessarini, R.J. Randomized, Placebo-Controlled Trials of Antidepressants for Acute Major Depression: Thirty-Year Meta-Analytic Review. Neuropsychopharmacology 2012, 37, 851–864. [Google Scholar] [CrossRef]
- Kiraly, S.J.; Kiraly, M.A.; Hawe, R.D.; Makhani, N. Vitamin D as a Neuroactive Substance: Review. Sci. World J. 2006, 6, 125–139. [Google Scholar] [CrossRef]
- Coplan, J.D.; Aaronson, C.J.; Panthangi, V.; Kim, Y. Treating comorbid anxiety and depression: Psychosocial and pharmacological approaches. World J. Psychiatry 2015, 5, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, A.; Ghanizadeh, A.; Kazemeini, F. Vitamin C as an adjuvant for treating major depressive disorder and suicidal behavior, a randomized placebo-controlled clinical trial. Trials 2015, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.J.; Joca, S.R.; Del Bel, E.; Guimarães, F.S. The antidepressant-like effect of doxycycline is associated with decreased nitric oxide metabolite levels in the prefrontal cortex. Behav. Brain Res. 2024, 458, 114764. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Torrisi, S.A.; Musso, N.; Geraci, F.; Tropea, M.R.; Privitera, A.; Tascedda, F.; Puzzo, D.; et al. Antioxidant Activity of Fluoxetine and Vortioxetine in a Non-Transgenic Animal Model of Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 809541. [Google Scholar] [CrossRef]
- Suzuki, E.; Yagi, G.; Nakaki, T.; Kanba, S.; Asai, M. Elevated plasma nitrate levels in depressive states. J. Affect. Disord. 2001, 63, 221–224. [Google Scholar] [CrossRef]
- Harkin, A.J.; Bruce, K.H.; Craft, B.; Paul, I.A. Nitric oxide synthase inhibitors have antidepressant-like properties in mice 1. Acute treatments are active in the forced swim test. Eur. J. Pharmacol. 1999, 372, 207–213. [Google Scholar] [CrossRef]
- Jesse, C.R.; Bortolatto, C.; Savegnago, L.; da Rocha, J.B.T.; Nogueira, C.W. Involvement of l-arginine–nitric oxide–cyclic guanosine monophosphate pathway in the antidepressant-like effect of tramadol in the rat forced swimming test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1838–1843. [Google Scholar] [CrossRef]
- Dhir, A.; Kulkarni, S. Involvement of l-arginine–nitric oxide–cyclic guanosine monophosphate pathway in the antidepressant-like effect of venlafaxine in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Wegener, G.; Volke, V. Nitric Oxide Synthase Inhibitors as Antidepressants. Pharmaceuticals 2010, 3, 273. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, L. Vitamin D3/vitamin D receptor signaling mitigates symptoms of post-stroke depression in mice by upregulating hippocampal BDNF expression. Neurosci. Res. 2021, 170, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, O.; Ibrahim, A.; Qnais, E.; Alqudah, A.; Altaber, S.; Aljabali, A.A.A.; Tambuwala, M.M. Vitamins C and D Exhibit Similar Antidepressant Effects to Escitalopram Mediated by NOx and FKBPL in a Stress-Induced Mice Model. Nutrients 2023, 15, 2692. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Kondo, Y.; Isaka, A.; Ishigami, A.; Suzuki, E. Vitamin C impacts anxiety-like behavior and stress-induced anorexia relative to social environment in SMP30/GNL knockout mice. Nutr. Res. 2016, 36, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Kesmati, M.; Zadehdarvish, F.; Jelodar, Z.; Torabi, M. Vitamin C potentiate sedative effect of magnesium oxide nanoparticles on anxiety and nociception in the postpartum depression model. Nanomed. J. 2017, 4, 17–24. [Google Scholar]
- Verleye, M.; Dumas, S.; Heulard, I.; Krafft, N.; Gillardin, J.-M. Differential effects of etifoxine on anxiety-like behaviour and convulsions in BALB/cByJ and C57BL/6J mice: Any relation to overexpression of central GABAA receptor beta2 subunits? Eur. Neuropsychopharmacol. 2011, 21, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Blavet, N.; Deniel, M.; Jalfre, M. Immobility induced by forced swimming in rats: Effects of agents which modify central catecholamine and serotonin activity. Eur. J. Pharmacol. 1979, 57, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, L.B.; Kolb, Y.; Prinssen, E.P. A combined marble burying–locomotor activity test in mice: A practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol. 2006, 547, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.; Armstrong, R.; Delp; Graham, R.; Dunn, A. Open-field behavior is not related to treadmill performance in exercising rats. Physiol. Behav. 1988, 43, 541–546. [Google Scholar] [CrossRef]
- Mirzaii-Dizgah, M.-H.; Mirzaii-Dizgah, M.-R.; Mirzaii-Dizgah, I. Serum and saliva total tau protein as a marker for relapsing-remitting multiple sclerosis. Med. Hypotheses 2020, 135, 109476. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gammoh, O.; Ibrahim, A.; Yehya, A.; Alqudah, A.; Qnais, E.; Altaber, S.; Alrob, O.A.; Aljabali, A.A.A.; Tambuwala, M.M. Exploring the Roles of Vitamins C and D and Etifoxine in Combination with Citalopram in Depression/Anxiety Model: A Focus on ICAM-1, SIRT1 and Nitric Oxide. Int. J. Mol. Sci. 2024, 25, 1960. https://doi.org/10.3390/ijms25041960
Gammoh O, Ibrahim A, Yehya A, Alqudah A, Qnais E, Altaber S, Alrob OA, Aljabali AAA, Tambuwala MM. Exploring the Roles of Vitamins C and D and Etifoxine in Combination with Citalopram in Depression/Anxiety Model: A Focus on ICAM-1, SIRT1 and Nitric Oxide. International Journal of Molecular Sciences. 2024; 25(4):1960. https://doi.org/10.3390/ijms25041960
Chicago/Turabian StyleGammoh, Omar, Aseel Ibrahim, Ala Yehya, Abdelrahim Alqudah, Esam Qnais, Sara Altaber, Osama Abo Alrob, Alaa A. A. Aljabali, and Murtaza M. Tambuwala. 2024. "Exploring the Roles of Vitamins C and D and Etifoxine in Combination with Citalopram in Depression/Anxiety Model: A Focus on ICAM-1, SIRT1 and Nitric Oxide" International Journal of Molecular Sciences 25, no. 4: 1960. https://doi.org/10.3390/ijms25041960
APA StyleGammoh, O., Ibrahim, A., Yehya, A., Alqudah, A., Qnais, E., Altaber, S., Alrob, O. A., Aljabali, A. A. A., & Tambuwala, M. M. (2024). Exploring the Roles of Vitamins C and D and Etifoxine in Combination with Citalopram in Depression/Anxiety Model: A Focus on ICAM-1, SIRT1 and Nitric Oxide. International Journal of Molecular Sciences, 25(4), 1960. https://doi.org/10.3390/ijms25041960









