Multifunctional Biomass-Based Ionic Liquids/CuCl-Catalyzed CO2-Promoted Hydration of Propargylic Alcohols: A Green Synthesis of α-Hydroxy Ketones
Abstract
1. Introduction
2. Results
3. Discussion
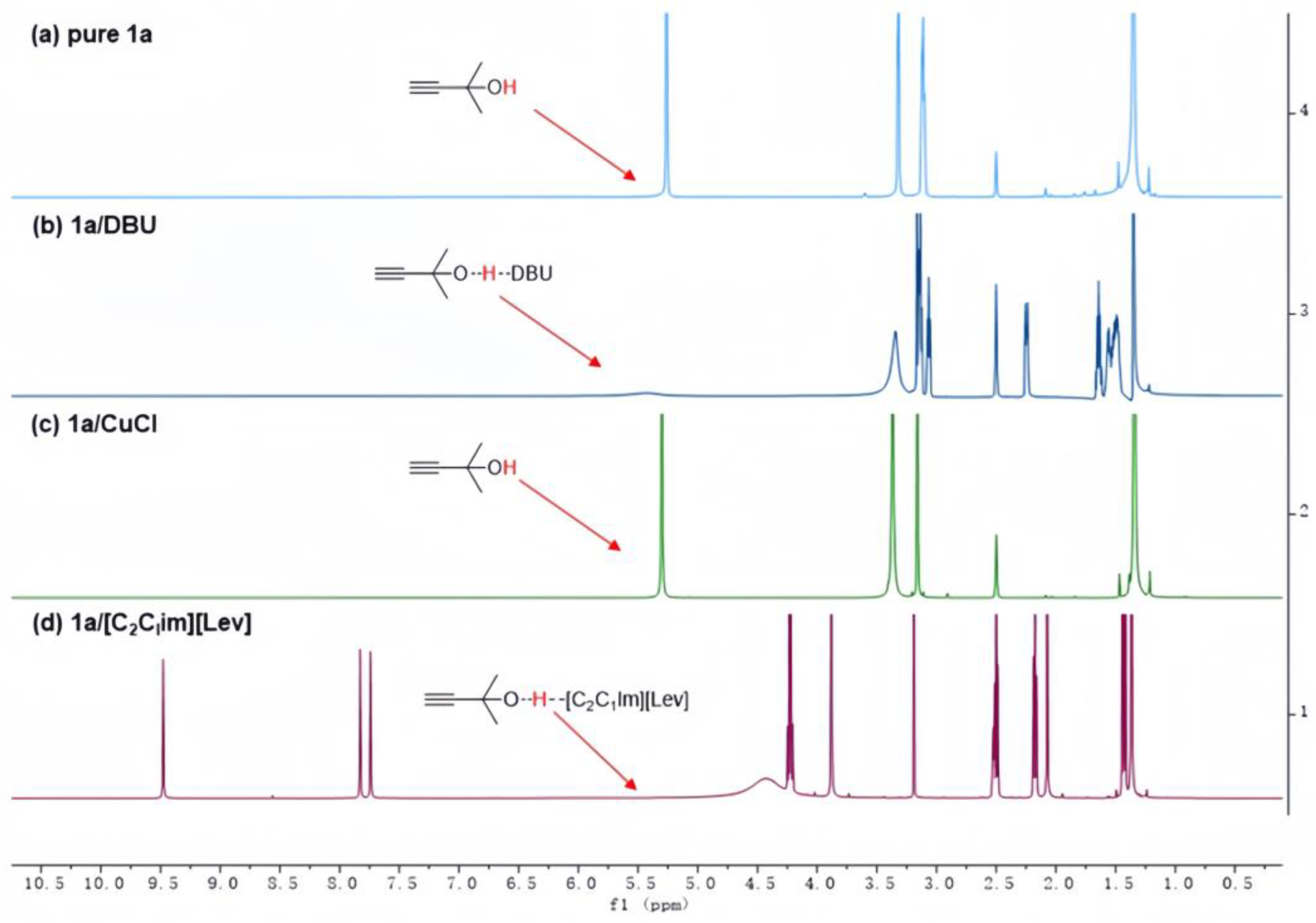
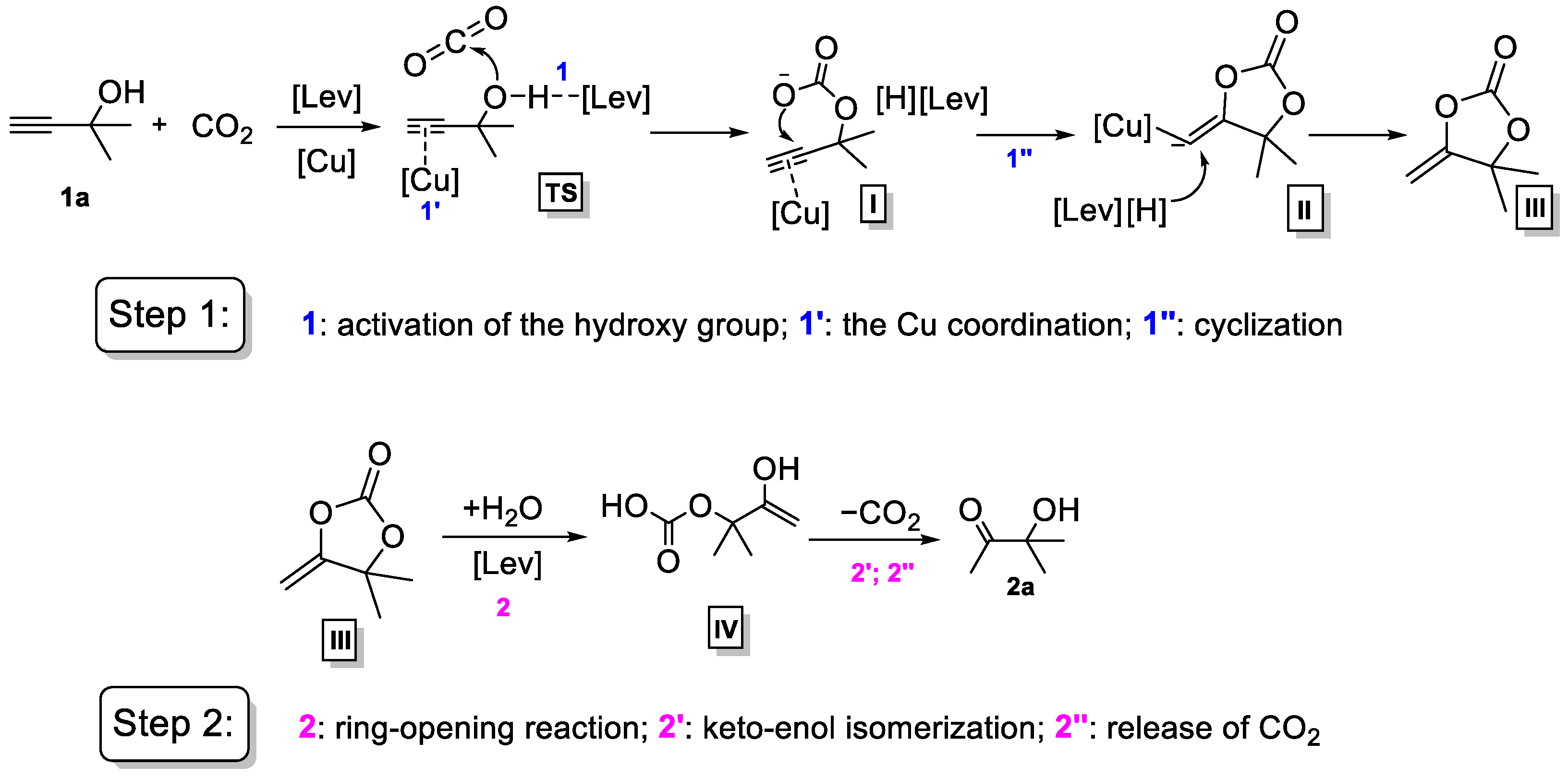
3.1. Identification of Tandem Mechanism
3.2. Activation of the Propargylic Alcohols
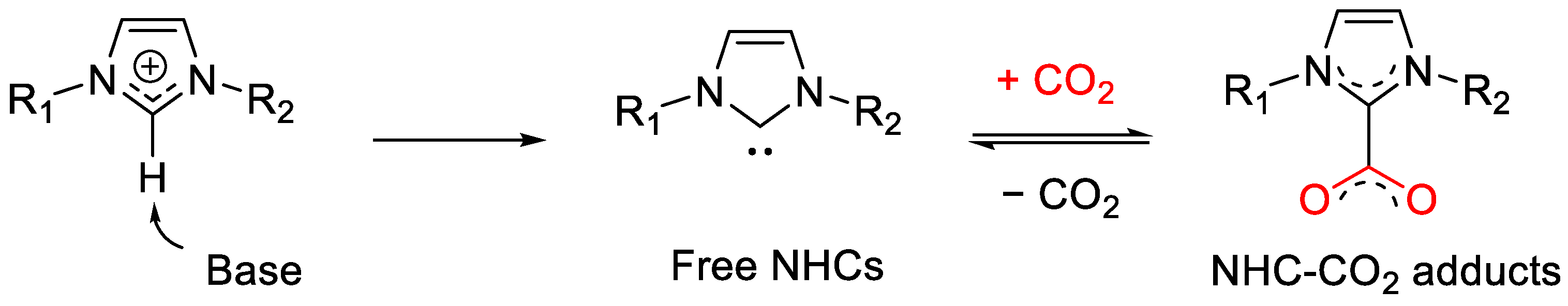
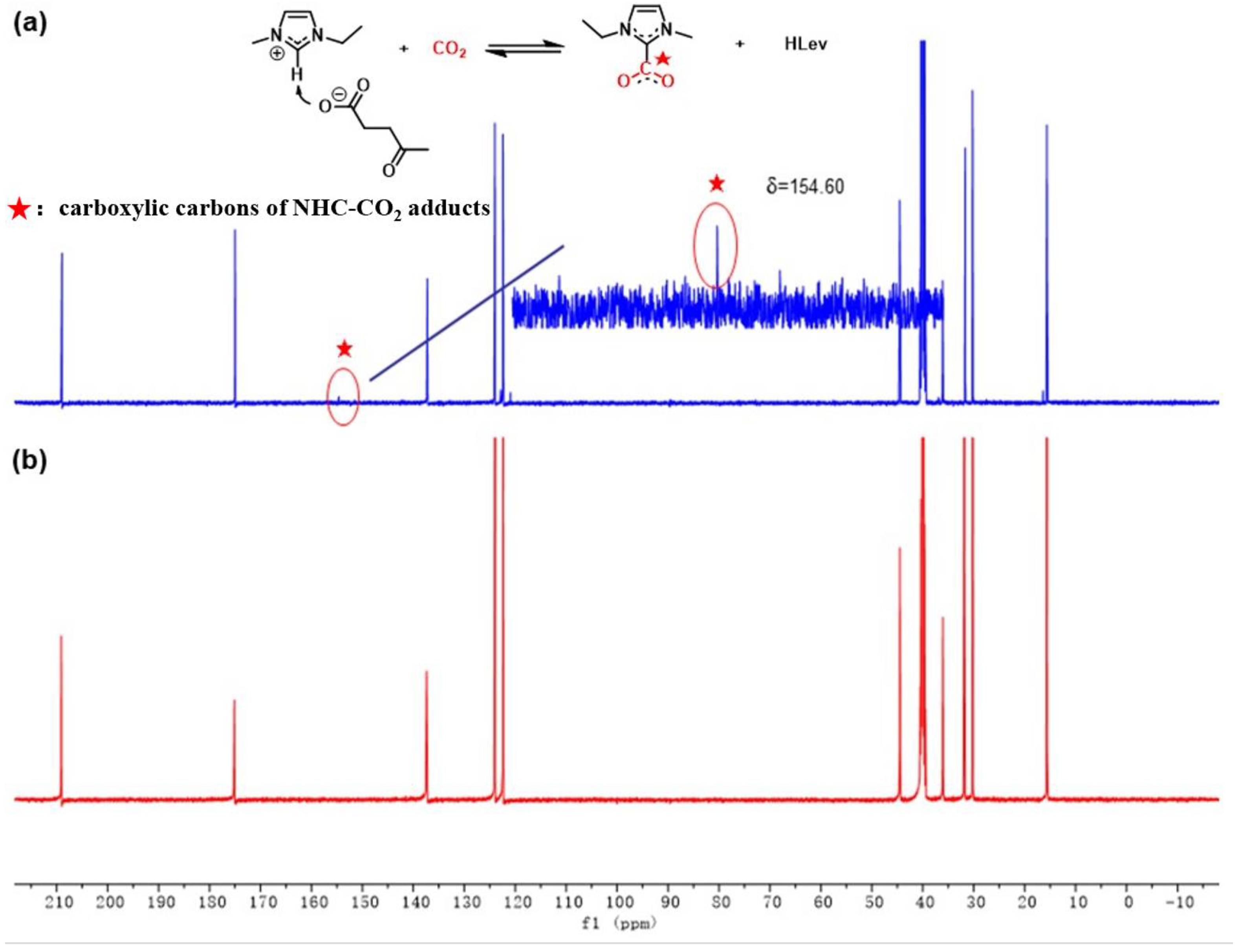
3.3. Generation of NHC-CO2 Adducts
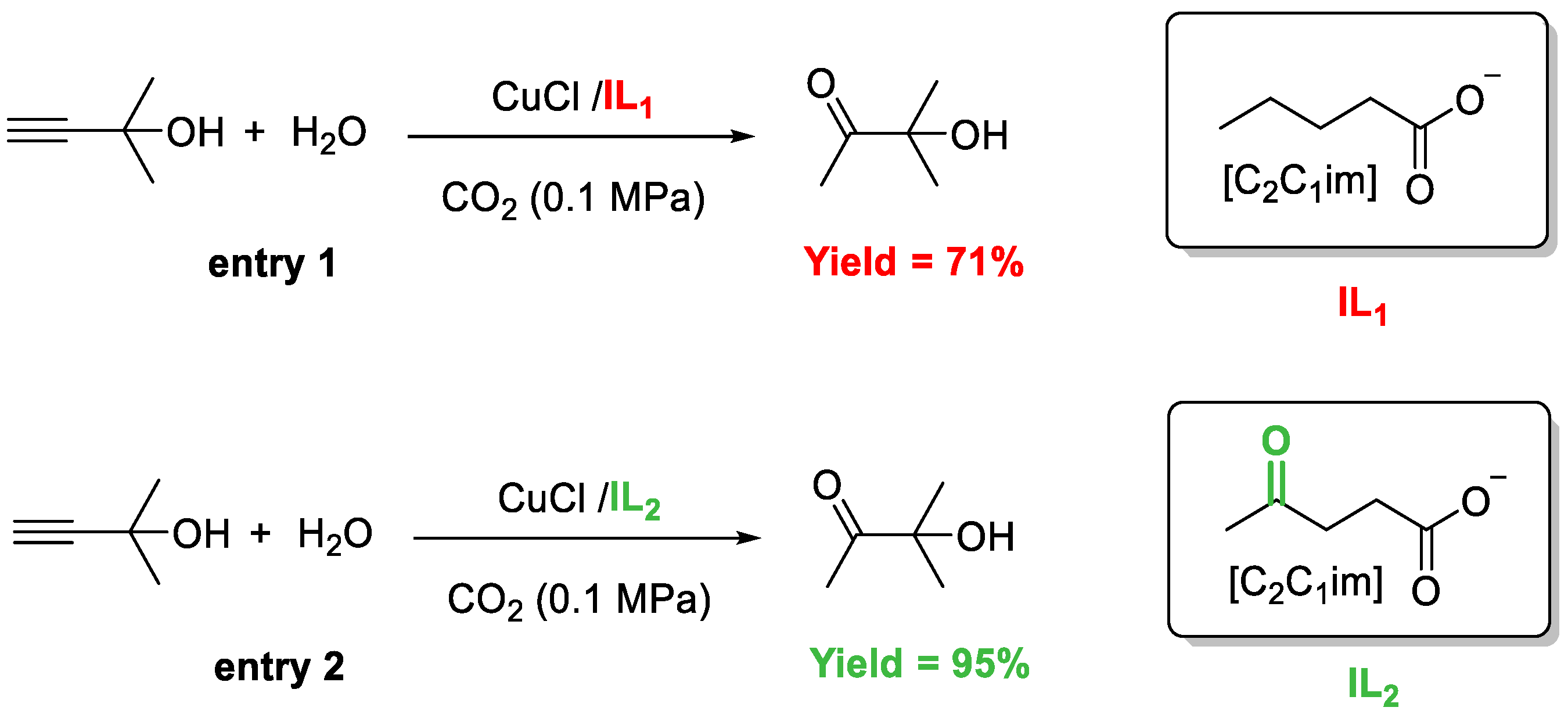
3.4. Interaction between [C2C1im][Lev] and Cu Species
3.5. Proposed Mechanism for the Catalytic Process
4. Materials and Methods
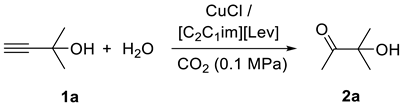
4.1. The CO2-Promoted Hydration of Propargylic Alcohols
4.2. Procedures for Recycling the Catalytic System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oerlemans, J. Glaciers as indicators of a carbon dioxide warming. Nature 1986, 320, 607–609. [Google Scholar] [CrossRef]
- McNutt, M. Time’s up, CO2. Science 2019, 365, 411. [Google Scholar] [CrossRef] [PubMed]
- Mac Dowell, N.; Reiner, D.M.; Haszeldine, R.S. Comparing approaches for carbon dioxide removal. Joule 2022, 6, 2233–2239. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Fischer, E.M.; Gruber, N. Marine heatwaves under global warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; He, L.-N.; Gao, J.; Liu, A.-H.; Yu, B. Carbon dioxide utilization with C–N bond formation: Carbon dioxide capture and subsequent conversion. Energy Environ. Sci. 2012, 5, 6602–6639. [Google Scholar] [CrossRef]
- Huang, K.; Sun, C.-L.; Shi, Z.-J. Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 2435–2452. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Kleij, A.W.; North, M.; Urakawa, A. CO2 Catalysis. ChemSusChem 2017, 10, 1036–1038. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Change 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Hou, S.-L.; Dong, J.; Zhao, B. Formation of C-X Bonds in CO2 Chemical Fixation Catalyzed by Metal−Organic Frameworks. Adv. Mater. 2020, 32, 1806163. [Google Scholar] [CrossRef]
- Dabral, S.; Schaub, T. The Use of Carbon Dioxide (CO2) as a Building Block in Organic Synthesis from an Industrial Perspective. Adv. Synth. Catal. 2019, 361, 223–246. [Google Scholar] [CrossRef]
- Riemer, D.; Schilling, W.; Goetz, A.; Zhang, Y.; Gehrke, S.; Tkach, I.; Hollóczki, O.; Das, S. CO2-Catalyzed Efficient Dehydrogenation of Amines with Detailed Mechanistic and Kinetic Studies. ACS Catal. 2018, 8, 11679–11687. [Google Scholar] [CrossRef]
- Riemer, D.; Mandaviya, B.; Schilling, W.; Götz, A.C.; Kühl, T.; Finger, M.; Das, S. CO2-Catalyzed Oxidation of Benzylic and Allylic Alcohols with DMSO. ACS Catal. 2018, 8, 3030–3034. [Google Scholar] [CrossRef]
- Hirapara, P.; Riemer, D.; Hazra, N.; Gajera, J.; Finger, M.; Das, S. CO2-assisted synthesis of non-symmetric α-diketones directly from aldehydes via C–C bond formation. Green Chem. 2017, 19, 5356–5360. [Google Scholar] [CrossRef]
- Riemer, D.; Hirapara, P.; Das, S. Chemoselective Synthesis of Carbamates using CO2 as Carbon Source. ChemSusChem 2016, 9, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, F.; Fang, T.; Li, C.; Li, W.; Song, Q. Passerini-type reaction of boronic acids enables α-hydroxyketones synthesis. Nat. Commun. 2021, 12, 441. [Google Scholar] [CrossRef]
- Kutscheroff, M. Ueber die Einwirkung der Kohlenwasserstoffe der Acetylenreihe auf Quecksilberoxyd und dessen Salze. Ber. Dtsch. Chem. Ges. 1884, 17, 13–29. [Google Scholar] [CrossRef]
- Kutscheroff, M. Ueber eine neue Methode direkter Addition von Wasser (Hydratation) an die Kohlenwasserstoffe der Acetylenreihe. Ber. Dtsch. Chem. Ges. 1881, 14, 1540–1542. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, M.; Ciccarelli, S.; Lee, J.; Catano, B. AuIII-Catalyzed Formation of α-Halomethyl Ketones from Terminal Alkynes. Eur. J. Org. Chem. 2017, 2017, 781–785. [Google Scholar] [CrossRef]
- Weerasiri, K.C.; Chen, D.; Wozniak, D.I.; Dobereiner, G.E. Internal Alkyne Regio- and Chemoselectivity using a Zwitterionic N-Heterocyclic Carbene Gold Catalyst in a Silver-Free Alkyne Hydration Reaction. Adv. Synth. Catal. 2016, 358, 4106–4113. [Google Scholar] [CrossRef]
- Gatto, M.; Belanzoni, P.; Belpassi, L.; Biasiolo, L.; Del Zotto, A.; Tarantelli, F.; Zuccaccia, D. Solvent-, Silver-, and Acid-Free NHC-Au-X Catalyzed Hydration of Alkynes. The Pivotal Role of the Counterion. ACS Catal. 2016, 6, 7363–7376. [Google Scholar] [CrossRef]
- Ebule, R.E.; Malhotra, D.; Hammond, G.B.; Xu, B. Ligand Effects in the Gold Catalyzed Hydration of Alkynes. Adv. Synth. Catal. 2016, 358, 1478–1481. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Shao, J.; Yang, G.; Wu, Y.; Zhang, Z. Hydration of alkynes at room temperature catalyzed by gold(i) isocyanide compounds. Green Chem. 2015, 17, 532–537. [Google Scholar] [CrossRef]
- Liang, S.; Jasinski, J.; Hammond, G.B.; Xu, B. Supported Gold Nanoparticle-Catalyzed Hydration of Alkynes under Basic Conditions. Org. Lett. 2015, 17, 162–165. [Google Scholar] [CrossRef]
- Ibrahim, H.; de Frémont, P.; Braunstein, P.; Théry, V.; Nauton, L.; Cisnetti, F.; Gautier, A. Water-Soluble Gold–N-Heterocyclic Carbene Complexes for the Catalytic Homogeneous Acid- and Silver-Free Hydration of Hydrophilic Alkynes. Adv. Synth. Catal. 2015, 357, 3893–3900. [Google Scholar] [CrossRef]
- Chen, T.; Cai, C. Catalytic hydration of alkynes to ketones by a salen–gold(III) complex. Catal. Commun. 2015, 65, 102–104. [Google Scholar] [CrossRef]
- Xie, L.; Wu, Y.; Yi, W.; Zhu, L.; Xiang, J.; He, W. Gold-Catalyzed Hydration of Haloalkynes to α-Halomethyl Ketones. J. Org. Chem. 2013, 78, 9190–9195. [Google Scholar] [CrossRef] [PubMed]
- Oonishi, Y.; Gómez-Suárez, A.; Martin, A.R.; Nolan, S.P. Hydrophenoxylation of Alkynes by Cooperative Gold Catalysis. Angew. Chem. Int. Ed. 2013, 52, 9767–9771. [Google Scholar] [CrossRef] [PubMed]
- Leyva, A.; Corma, A. Isolable Gold(I) Complexes Having One Low-Coordinating Ligand as Catalysts for the Selective Hydration of Substituted Alkynes at Room Temperature without Acidic Promoters. J. Org. Chem. 2009, 74, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Santhi, J.; Baire, B. Carbonyl Directed Regioselective Hydration of Alkynes under Ag-Catalysis. ChemistrySelect 2017, 2, 4338–4342. [Google Scholar] [CrossRef]
- Dong, Q.; Li, N.; Qiu, R.; Wang, J.; Guo, C.; Xu, X. Silver-containing microemulsion as a high-efficient and recyclable catalytic system for hydration of alkynes. J. Organomet. Chem. 2015, 799–800, 122–127. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Ye, D.-N.; Ye, M.; Zhou, Z.-G.; Li, S.-H.; Liu, L.-X. AgF/TFA-promoted highly efficient synthesis of α-haloketones from haloalkynes. Tetrahedron Lett. 2014, 55, 1373–1375. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Ye, D.-N.; Qian, Y.-P.; Ye, M.; Liu, L.-X. Highly efficient AgBF4-catalyzed synthesis of methyl ketones from terminal alkynes. Tetrahedron 2013, 69, 6116–6120. [Google Scholar] [CrossRef]
- Venkateswara Rao, K.T.; Sai Prasad, P.S.; Lingaiah, N. Solvent-free hydration of alkynes over a heterogeneous silver exchanged silicotungstic acid catalyst. Green Chem. 2012, 14, 1507–1514. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, D. AgOTf catalyzed hydration of terminal alkynes. Appl. Organomet. Chem. 2012, 26, 722–726. [Google Scholar] [CrossRef]
- Grotjahn, D.B.; Lev, D.A. A General Bifunctional Catalyst for the Anti-Markovnikov Hydration of Terminal Alkynes to Aldehydes Gives Enzyme-Like Rate and Selectivity Enhancements. J. Am. Chem. Soc. 2004, 126, 12232–12233. [Google Scholar] [CrossRef]
- Banerjee, S.; Ambegave, S.B.; Mule, R.D.; Senthilkumar, B.; Patil, N.T. Gold-Catalyzed Alkynylative Meyer–Schuster Rearrangement. Org. Lett. 2020, 22, 4792–4796. [Google Scholar] [CrossRef]
- Yoshida, S.; Fukui, K.; Kikuchi, S.; Yamada, T. Silver-Catalyzed Enantioselective Carbon Dioxide Incorporation into Bispropargylic Alcohols. J. Am. Chem. Soc. 2010, 132, 4072–4073. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Qi, C.; Hu, X.; Guan, Y.; Jiang, H. Efficient synthesis of tertiary α-hydroxy ketones through CO2-promoted regioselective hydration of propargylic alcohols. Green Chem. 2014, 16, 3729–3733. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Z.; Yu, B.; Zhang, H.; Xu, H.; Hao, L.; Han, B.; Liu, Z. Task-specific ionic liquid and CO2-cocatalysed efficient hydration of propargylic alcohols to α-hydroxy ketones. Chem. Sci. 2015, 6, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Zhang, X.; Huang, Y.-F.; Chen, K.-H.; He, L.-N. Synthesis of α-hydroxy ketones by copper(I)-catalyzed hydration of propargylic alcohols: CO2 as a cocatalyst under atmospheric pressure. Chin. J. Catal. 2019, 40, 1345–1351. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Du, M.; Bu, C.; Chen, C.; Chaemcheun, S.; Hu, J.; Zhang, Y.; Yuan, Y.; Verpoort, F. CO2-Promoted Hydration of Propargylic Alcohols: Green Synthesis of α-Hydroxy Ketones by an Efficient and Recyclable AgOAc/Ionic Liquid System. ACS Sustain. Chem. Eng. 2020, 8, 8148–8155. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Xu, Y.; Yan, X.; Zhang, S.; Duan, K.; Chen, C.; Yuan, Y.; Verpoort, F. CO2-induced dissolution of ZnO into ionic liquids and its catalytic application for the hydration of propargylic alcohols. Appl. Catal. B 2022, 310, 121270. [Google Scholar] [CrossRef]
- Guo, X.-X.; Cai, Z.-T.; Muhammad, Y.; Zhang, F.-L.; Wei, R.-P.; Gao, L.-J.; Xiao, G.-M. Silver-anchored porous aromatic framework for efficient conversion of propargylic alcohols with CO2 at ambient pressure. Chin. Chem. Lett. 2023, 34, 107740. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Muhammad, Y.; Yang, Z.; Wei, R.; Gao, L.; Xiao, G. Amino-functionalized organic polymer loaded with highly dispersed CuI for efficient catalytic conversion of CO2 with PA. Microporous Mesoporous Mater. 2023, 352, 112507. [Google Scholar] [CrossRef]
- Simon, N.M.; Zanatta, M.; dos Santos, F.P.; Corvo, M.C.; Cabrita, E.J.; Dupont, J. Carbon Dioxide Capture by Aqueous Ionic Liquid Solutions. ChemSusChem 2017, 10, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Y.; Dong, W.-X.; Deng, Y.; Chen, L.-F.; Zhao, C.-F.; Zhang, C.-L.; Zhou, J.; Qu, Y.-F.; Li, Y.-S.; Li, D.-J.; et al. Biomass-based biomimetic-oriented Janus nanoarchitecture for efficient heavy-metal enrichment and interfacial solar water sanitation. Interdiscip. Mater. 2022, 1, 537–547. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Conversion of Biomass and Its Derivatives to Levulinic Acid and Levulinate Esters via Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 4749–4766. [Google Scholar] [CrossRef]
- Bernardo, J.R.; Oliveira, M.C.; Fernandes, A.C. HReO4 as highly efficient and selective catalyst for the conversion of carbohydrates into value added chemicals. Mol. Catal. 2019, 465, 87–94. [Google Scholar] [CrossRef]
- Werpy, T.A.; Holladay, J.E.; White, J.F. Top Value Added Chemicals From Biomass: I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2004.
- Hu, Y.; Song, J.; Xie, C.; Wu, H.; Jiang, T.; Yang, G.; Han, B. Transformation of CO2 into α-Alkylidene Csights into the levulinate-based ionic liquid class: By CuI and Ionic Liquid with Biomass-Derived Levulinate Anion. ACS Sustain. Chem. Eng. 2019, 7, 5614–5619. [Google Scholar] [CrossRef]
- Grignard, B.; Ngassamtounzoua, C.; Gennen, S.; Gilbert, B.; Méreau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Boosting the Catalytic Performance of Organic Salts for the Fast and Selective Synthesis of α-Alkylidene Cyclic Carbonates from Carbon Dioxide and Propargylic Alcohols. ChemCatChem 2018, 10, 2584–2592. [Google Scholar] [CrossRef]
- Kayaki, Y.; Yamamoto, M.; Ikariya, T. Stereoselective Formation of α-Alkylidene Cyclic Carbonates via Carboxylative Cyclization of Propargyl Alcohols in Supercritical Carbon Dioxide. J. Org. Chem. 2007, 72, 647–649. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to ‘green’ chemistry—Which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, F.; Zhao, Y.; Wang, Y.; Ke, Z.; Li, R.; Zeng, W.; Han, B.; Liu, Z. A CO2-mediated base catalysis approach for the hydration of triple bonds in ionic liquids. Green Chem. 2021, 23, 9870–9875. [Google Scholar] [CrossRef]
- Kayaki, Y.; Yamamoto, M.; Ikariya, T. N-Heterocyclic Carbenes as Efficient Organocatalysts for CO2 Fixation Reactions. Angew. Chem. Int. Ed. 2009, 48, 4194–4197. [Google Scholar] [CrossRef]
- Gurau, G.; Rodríguez, H.; Kelley, S.P.; Janiczek, P.; Kalb, R.S.; Rogers, R.D. Demonstration of Chemisorption of Carbon Dioxide in 1,3-Dialkylimidazolium Acetate Ionic Liquids. Angew. Chem. Int. Ed. 2011, 50, 12024–12026. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, L.; Qiu, J.; Li, Z.; Wang, H.; Cui, G.; Zhang, S.; Wang, J. Remarkable synergistic effect between copper(I) and ionic liquids for promoting chemical fixation of CO2. J. CO2 Util. 2017, 22, 374–381. [Google Scholar] [CrossRef]
- Shi, G.; Zhai, R.; Li, H.; Wang, C. Highly efficient synthesis of alkylidene cyclic carbonates from low concentration CO2 using hydroxyl and azolate dual functionalized ionic liquids. Green Chem. 2021, 23, 592–596. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, X.; He, H.; Qi, C.; Xiong, W.; Ren, Y.; Jiang, H. Copper-Promoted Coupling of Carbon Dioxide and Propargylic Alcohols: Expansion of Substrate Scope and Trapping of Vinyl Copper Intermediate. Adv. Synth. Catal. 2015, 357, 2556–2565. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Deng, L.; Yue, W.; Zhang, L.; Guo, Y.; Xie, H.; Zheng, Q.; Zou, G.; Chen, P. Biobased Protic Ionic Liquids as Sustainable Solvents for Wool Keratin/Cellulose Simultaneous Dissolution: Solution Properties and Composited Membrane Preparation. ACS Sustain. Chem. Eng. 2022, 10, 2158–2168. [Google Scholar] [CrossRef]



 | |||
|---|---|---|---|
| Entry | Metal Salt | IL | Yield (%) b |
| 1 | - c | - d | 0 |
| 2 | CuCl | - d | 0 |
| 3 | - c | [C2C1im][Lev] | 0 |
| 4 e | CuCl | [C2C1im][Lev] | 0 |
| 5 | CuCl | [N4444][Lev] | 94 |
| 6 | CuCl | [N4444]2[ITa] | 80 |
| 7 | CuCl | [N4444]2[Sa] | 88 |
| 8 | CuCl | [N4444][La] | 74 |
| 9 | CuCl | [P4444][Lev] | 87 |
| 10 | CuCl | [DBUH][Lev] | 75 |
| 11 | CuCl | [DBNH][Lev] | 47 |
| 12 | CuCl | [C4C1im][Lev] | 85 |
| 13 | CuCl | [C2C1im][Lev] | 95 |
| 14 | CuBr | [C2C1im][Lev] | 89 |
| 15 | CuI | [C2C1im][Lev] | 66 |
| 16 | Cu2O | [C2C1im][Lev] | 86 |
| 17 | Cu2S | [C2C1im][Lev] | 72 |
| 18 | CuCl2 | [C2C1im][Lev] | 67 |
| 19 | Cu(OAc)2 | [C2C1im][Lev] | 80 |
| 20 | CuSO4 | [C2C1im][Lev] | 73 |
 | |||||
|---|---|---|---|---|---|
| Entry | CuCl (mol %) c | IL (equiv.) c | Temperature (°C) | Time (h) | Yield (%) b |
| 1 | 1 | 0.5 | 80 | 12 | 41 |
| 2 | 1 | 0.75 | 80 | 12 | 62 |
| 3 | 1 | 1 | 80 | 12 | 95 |
| 4 | 0.75 | 1 | 80 | 12 | 76 |
| 5 | 0.5 | 1 | 80 | 12 | 51 |
| 6 | 0.25 | 1 | 80 | 12 | 42 |
| 7 | 1 | 1 | 40 | 12 | 5 |
| 8 | 1 | 1 | 60 | 12 | 78 |
| 9 | 1 | 1 | 100 | 12 | 93 |
| 10 | 1 | 1 | 80 | 3 | 40 |
| 11 | 1 | 1 | 80 | 6 | 60 |
| 12 | 1 | 1 | 80 | 9 | 80 |
 | |||||
|---|---|---|---|---|---|
| Substrate | Product | Time (h) | Yield (%) b | ||
| 1a | 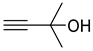 | 2a | 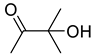 | 12 | 95 (90 c) |
| 1b | 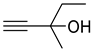 | 2b | 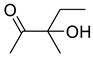 | 12 | 89 |
| 1c | 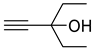 | 2c | 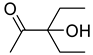 | 12 | 87 |
| 1d | 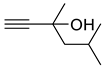 | 2d |  | 12 | 85 |
| 1e |  | 2e | 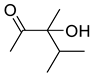 | 12 | 81 |
| 1f | 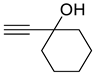 | 2f | 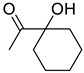 | 12 24 | 69 79 |
| 1g |  | 2g |  | 12 36 | 33 73 |
| 1h |  | 2h | 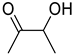 | 12 | 0 |
| 1i |  | 2i | 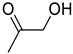 | 12 | 0 |
| Ref. | Catalyst | Solvent | AE (%) | E-Factor (kg/kg) | CE (%) | RME (%) | MI (kg/kg) | MP (%) |
|---|---|---|---|---|---|---|---|---|
| 2015 [45] | [Bu4P][Im] | / | 100 | 11.2 | 92 | 78.2 | 12.2 | 8.2 |
| 2019 [46] | Cu2O/DBU cyclohexyldiphenylphosphine | CH3CN | 100 | 10.0 | 97 | 97.1 | 11.0 | 9.1 |
| 2021 [61] | [P4444][2-OP] | / | 100 | 4.9 | 83 | 83.0 | 5.9 | 16.9 |
| 2023 [49] | Silver-anchored porous aromatic framework Ag@PAF-DAB | CH3CN | 100 | 17.7 | 99 | 86.3 | 18.7 | 5.4 |
| this work | CuCl/[C2C1im][Lev] | / | 100 | 3.0 | 95 | 80.8 | 4.0 | 24.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Zhang, S.; Duan, K.; Xu, Y.; Guo, K.; Chen, C.; Chaemchuen, S.; Cao, D.; Verpoort, F. Multifunctional Biomass-Based Ionic Liquids/CuCl-Catalyzed CO2-Promoted Hydration of Propargylic Alcohols: A Green Synthesis of α-Hydroxy Ketones. Int. J. Mol. Sci. 2024, 25, 1937. https://doi.org/10.3390/ijms25031937
Yuan Y, Zhang S, Duan K, Xu Y, Guo K, Chen C, Chaemchuen S, Cao D, Verpoort F. Multifunctional Biomass-Based Ionic Liquids/CuCl-Catalyzed CO2-Promoted Hydration of Propargylic Alcohols: A Green Synthesis of α-Hydroxy Ketones. International Journal of Molecular Sciences. 2024; 25(3):1937. https://doi.org/10.3390/ijms25031937
Chicago/Turabian StyleYuan, Ye, Siqi Zhang, Kang Duan, Yong Xu, Kaixuan Guo, Cheng Chen, Somboon Chaemchuen, Dongfeng Cao, and Francis Verpoort. 2024. "Multifunctional Biomass-Based Ionic Liquids/CuCl-Catalyzed CO2-Promoted Hydration of Propargylic Alcohols: A Green Synthesis of α-Hydroxy Ketones" International Journal of Molecular Sciences 25, no. 3: 1937. https://doi.org/10.3390/ijms25031937
APA StyleYuan, Y., Zhang, S., Duan, K., Xu, Y., Guo, K., Chen, C., Chaemchuen, S., Cao, D., & Verpoort, F. (2024). Multifunctional Biomass-Based Ionic Liquids/CuCl-Catalyzed CO2-Promoted Hydration of Propargylic Alcohols: A Green Synthesis of α-Hydroxy Ketones. International Journal of Molecular Sciences, 25(3), 1937. https://doi.org/10.3390/ijms25031937











