Construction of a g-C3N4/Bi(OH)3 Heterojunction for the Enhancement of Visible Light Photocatalytic Antibacterial Activity
Abstract
1. Introduction
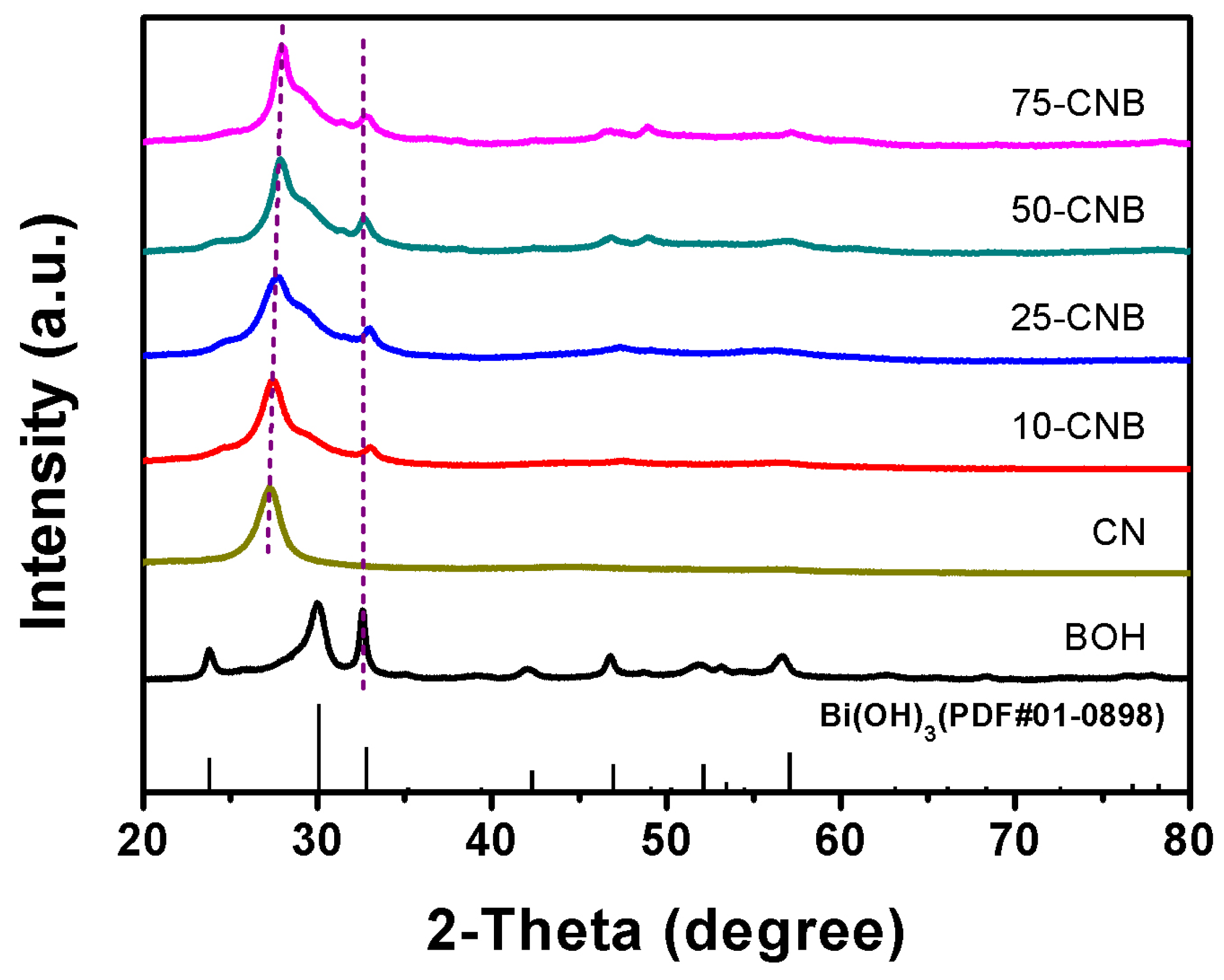
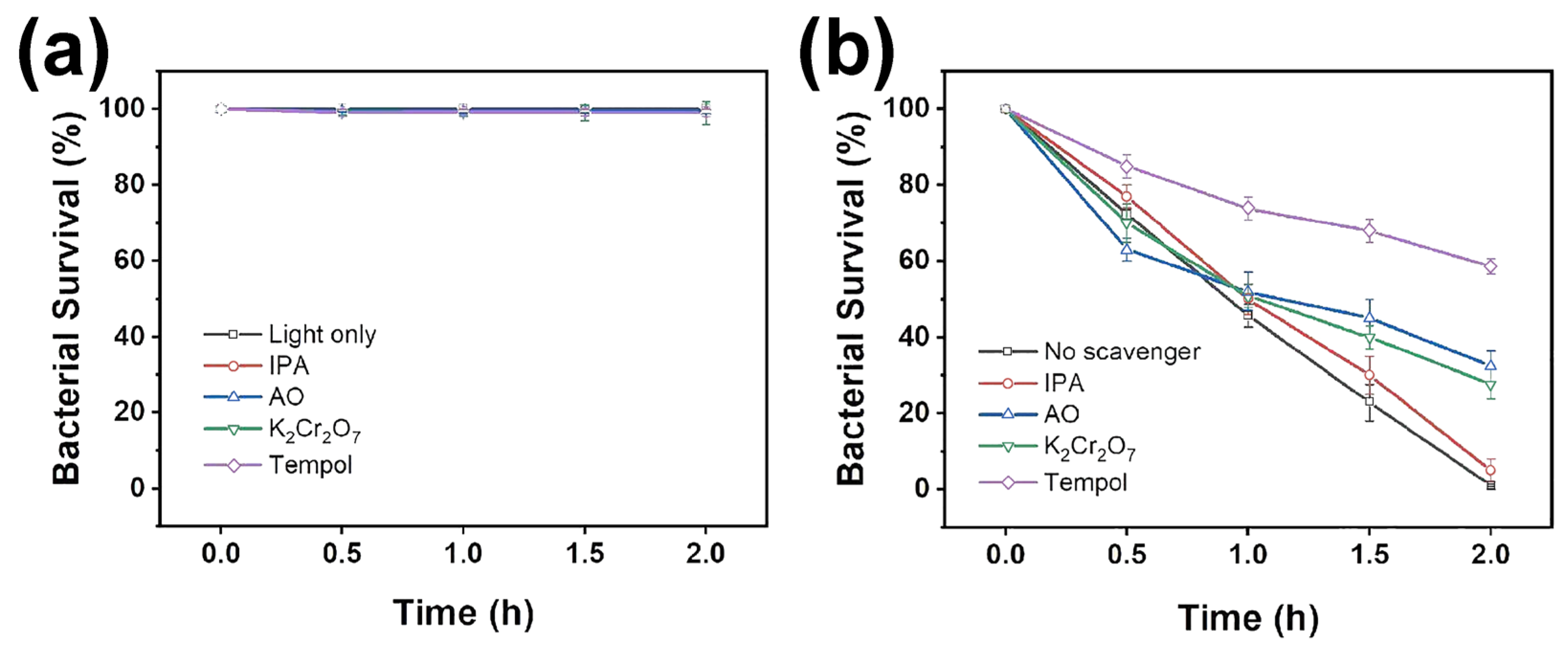
2. Results and Discussion
3. Material and Methods
3.1. Sample Preparation
3.2. Characterization
3.3. Photocatalytic Antibacterial Activity
3.4. Live/Dead Cell Staining of Bacteria
3.5. Analysis of Total Protein
3.6. Radical Capture Experiment
3.7. Photoelectrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Ye, Y.; Sun, J.; Li, Z.; Ping, J.; Sun, X. Recent Advances in g-C3N4-Based Photocatalysts for Pollutant Degradation and Bacterial Disinfection: Design Strategies, Mechanisms, and Applications. Small 2022, 18, 2105089. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Kiruthika, A.R. Photocatalytic disinfection of micro-organisms: Mechanisms and applications. Environ. Technol. Innov. 2021, 24, 101909. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.; Song, M.; Li, X.; Li, Q.; Shang, J.K. Directing photocatalytic pathway to exceedingly high antibacterial activity in water by functionalizing holey ultrathin nanosheets of graphitic carbon nitride. Water Res. 2021, 198, 117125. [Google Scholar] [CrossRef] [PubMed]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Mohite, B.M. Role of Nanotechnology in Photocatalysis Application. Recent Pat. Nanotechnol. 2023, 17, 5–7. [Google Scholar] [CrossRef]
- Sohrabi, H.; Arbabzadeh, O.; Falaki, M.; Vatanpour, V.; Majidi, M.R.; Kudaibergenov, N.; Joo, S.W.; Khataee, A. Advances in fabrication, physio-chemical properties, and sensing applications of non-metal boron nitride and boron carbon nitride-based nanomaterials. Surf. Interfaces 2023, 41, 103152. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Kim, H. Photocatalytic degradation of tetracycline antibiotics using hydrothermally synthesized two-dimensional molybdenum disulfide/titanium dioxide composites. J. Colloid Interface Sci. 2022, 606, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W.; Fujishima, A.; Terashima, C. Enhanced Photocatalytic Degradation Activity Using the V2O5/RGO Composite. Nanomaterials 2023, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, B.; Liu, X.; Li, Z.; Zhu, S.; Liang, Y.; Cui, Z.; Wu, S. Recent Progress in Photocatalytic Antibacterial. ACS Appl. Bio Mater. 2021, 4, 3909–3936. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Z.; Jiang, H.; Wang, X. Progress on photocatalytic semiconductor hybrids for bacterial inactivation. Mater. Horiz. 2021, 8, 2964–3008. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, R.; Zhang, W.; Shi, S.; Zhu, W.; Liu, M.; Yang, C.; Xie, X.; Wang, J. Graphitic carbon nitride (g-C3N4)-based nanostructured materials for photodynamic inactivation: Synthesis, efficacy and mechanism. Chem. Eng. J. 2021, 404, 126528. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, L.; He, D.; Xiao, B.; Ran, X.; Li, R.; Xu, H.; Feng, J. Ag@Bi5O7I nanoparticles deposited on Bi(OH)3 nanosheets for boosting photocatalytic antibacterial activity under visible light irradiation. J. Environ. Chem. Eng. 2023, 11, 109996. [Google Scholar] [CrossRef]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Yan, K.; Mu, C.; Meng, L.; Fei, Z.; Dyson, P.J. Recent advances in graphite carbon nitride-based nanocomposites: Structure, antibacterial properties and synergies. Nanoscale Adv. 2021, 3, 3708–3729. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, X.; Zheng, Y.; Chu, P.K.; Zhang, Y.; Wu, S. Graphitic carbon nitride-based materials for photocatalytic antibacterial application. Mat. Sci. Eng. R 2021, 145, 100610. [Google Scholar] [CrossRef]
- Huo, X.; Yi, H.; Fu, Y.; An, Z.; Qin, L.; Liu, X.; Li, B.; Liu, S.; Li, L.; Zhang, M.; et al. Porous graphitic carbon nitride nanomaterials for water treatment. Environ. Sci. Nano 2021, 8, 1835–1862. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Yang, L.; Yun, S.; Que, M.; Zheng, H.; Zhao, Y.; Yang, T.; Liu, Z. 2D/2D g-C3N4/TiO2 with exposed (001) facets Z-Scheme composites accelerating separation of interfacial charge and visible photocatalytic degradation of Rhodamine B. J. Phys. Chem. Solids 2022, 160, 110339. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Pan, K.; Wang, S.; Sun, H.; Xie, Y.; Xu, Y.; Wu, J.; Zhou, W. Engineering surface N-vacancy defects of ultrathin mesoporous carbon nitride nanosheets as efficient visible-light-driven photocatalysts. Sol. RRL 2020, 5, 2170011. [Google Scholar] [CrossRef]
- Tian, S.; Wang, B.; Gong, W.; He, Z.; Xu, Q.; Chen, W.; Zhang, Q.; Zhu, Y.; Yang, J.; Fu, Q.; et al. Dual-atom Pt heterogeneous catalyst with excellent catalytic performances for the selective hydrogenation and epoxidation. Nat. Commun. 2021, 12, 3181. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Bi, Z.; Chen, X.; Hu, X.; Pan, W. Recent advances and perspectives of g-C3N4–based materials for photocatalytic dyes degradation. Chemosphere 2022, 295, 133834. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0D/2D S-Scheme Heterojunction over Polymeric Carbon Nitride for Visible-Light Photocatalytic Inactivation of Bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Patnaik, S.; Mansingh, S.; Behera, A.; Mohanty, A.; Acharya, C.; Parida, K.M. Enhanced photocatalytic activities of polypyrrole sensitized zinc ferrite/graphitic carbon nitride n-n heterojunction towards ciprofloxacin degradation, hydrogen evolution and antibacterial studies. J. Colloid Interface Sci. 2020, 561, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.A.; Abubshait, H.A.; Ramay, S.M.; Mahmood, A.; Ghaithan, H.M. Designing of highly active g-C3N4/Co@ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Environ. Chem. Eng. 2021, 9, 105534. [Google Scholar] [CrossRef]
- Orooji, Y.; Ghanbari, M.; Amiri, O.; Salavati-Niasari, M. Facile fabrication of silver iodide/graphitic carbon nitride nanocomposites by notable photo-catalytic performance through sunlight and antimicrobial activity. J. Hazard. Mater. 2020, 389, 122079. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yue, L.; Lian, F.; Wang, C.; Cheng, B.; Lv, J.; Wang, Z.; Xing, B. CuO nanoparticles doping recovered the photocatalytic antialgal activity of graphitic carbon nitride. J. Hazard. Mater. 2021, 403, 123621. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ren, G.; Gao, X.; Li, Z.; Wang, L.; Meng, X. A novel bismuth hydroxide Bi(OH)3 semiconductor with highly-efficient photocatalytic activity. Chem. Commun. 2022, 58, 8198–8201. [Google Scholar]
- Feng, J.; Cao, M.; Wang, L.; Ran, X.; Xiao, B.; Zhu, J.; Liu, Z.; Xi, X.; Feng, G.; Li, R. Ultra-thin DyFeO3/g-C3N4 p-n heterojunctions for highly efficient photo-Fenton removal of oxytetracycline and antibacterial activity. J. Alloys Compd. 2023, 939, 168789. [Google Scholar] [CrossRef]
- Chu, Z.; Li, J.; Lan, Y.; Chen, C.; Yang, J.; Ning, D.; Xia, X.; Mao, X. KCl–LiCl molten salt synthesis of LaOCl/CeO2-g-C3N4 with excellent photocatalytic-adsorbed removal performance for organic dye pollutant. Ceram. Int. 2022, 48, 15439–15450. [Google Scholar] [CrossRef]
- Rani, B.; Nayak, A.K.; Sahu, N.K. Degradation of mixed cationic dye pollutant by metal free melem derivatives and graphitic carbon nitride. Chemosphere 2022, 298, 134249. [Google Scholar] [CrossRef]
- Liu, H.; Yu, D.; Sun, T.; Du, H.; Jiang, W.; Muhammad, Y.; Huang, L. Fabrication of surface alkalinized g-C3N4 and TiO2 composite for the synergistic adsorption- photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2019, 473, 855–863. [Google Scholar] [CrossRef]
- Zhai, W.; He, J.; Hu, S.; Liang, Y.; Chen, F.; Wang, Y.; He, G.; He, Q. Enhanced photocatalytic degradation of tetracycline over magnetic La0.7Sr0.3MnO3/g-C3N4 p–n heterojunction arising from the synergistic effects of oxygen vacancy defects and high-potential photogenerated electrons. J. Alloys Compd. 2022, 918, 165699. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Liu, C.; Wu, Y.; Xia, M.; Wang, F. Porous P, Fe-doped g-C3N4 nanostructure with enhanced photo-Fenton activity for removal of tetracycline hydrochloride: Mechanism insight, DFT calculation and degradation pathways. Chemosphere 2022, 291, 133039. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zheng, H.; Sun, X.; Zhu, M.; Zhou, Y.; Wang, D.; Zhang, D.; Zhang, L. New and highly efficient ultra-thin g-C3N4/FeOCl nanocomposites as photo-Fenton catalysts for pollutants degradation and antibacterial effect under visible light. Chemosphere 2022, 290, 133324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, M.Q.; Tang, Z.R.; Xu, Y.J. Toward Improving the Graphene-Semiconductor Composite Photoactivity via the Addition of Metal Ions as Generic Interfacial Mediator. ACS Nano 2014, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, C.; Mei, J.; Yang, S.; Wong, P.K. Faster electron injection and higher interface reactivity in g-C3N4/Fe2O3 nanohybrid for efficient photo-Fenton-like activity toward antibiotics degradation. Environ. Res. 2021, 195, 110842. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Si, Q.; Jia, W.; Zheng, S.; Wang, H.; Zhao, Q.; Luo, H.; Jiang, J.; Ren, N. Atomically dispersed cobalt on carbon nitride for peroxymonosulfate activation: Switchable catalysis enabled by light irradiation. Chem. Eng. J. 2022, 446, 137277. [Google Scholar] [CrossRef]
- Shi, H.; Fan, J.; Zhao, Y.; Hu, X.; Zhang, X.; Tang, Z. Visible light driven CuBi2O4/Bi2MoO6 p-n heterojunction with enhanced photocatalytic inactivation of E. coli and mechanism insight. J. Hazard. Mater. 2020, 381, 121006. [Google Scholar]
- Chen, J.; Shan, M.; Shi, X.; Zhang, S.; Li, J.; Luan, J.; Duan, L.; Hou, H. BiSnSbO6–TiO2 composites enhance LED light-driven photocatalytic antibacterial activity. Ceram. Int. 2022, 48, 19036–19046. [Google Scholar] [CrossRef]
- Fang, Y.; Pei, S.; Zhuo, L.; Cheng, P.; Yuan, H.; Zhang, L. Phosphorus and sulfur codoped carbon nitride nanosheets with enhanced photocatalytic antibacterial activity and promotion of wound healing. Appl. Surf. Sci. 2022, 586, 152761. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Zhou, Q. Superior Antibacterial Activity of Fe3O4-TiO2 Nanosheets under Solar Light. ACS Appl. Mater. Interfaces 2015, 7, 21875–21883. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Zhang, X.; Chen, S.; Li, J.; Sun, Z.; Ma, Z.; Yang, W.; Zhang, B.; Yang, K.; Guo, S. Highly efficient visible-light photocatalytic degradation and antibacterial activity by GaN:ZnO solid solution nanoparticles. J. Mater. Sci. Technol. 2021, 94, 67–76. [Google Scholar] [CrossRef]
- Huo, P.; Liu, C.; Wu, D.; Guan, J.; Li, J.; Wang, H.; Tang, Q.; Li, X.; Yan, Y.; Yuan, S. Fabricated Ag/Ag2S/reduced graphene oxide composite photocatalysts for enhancing visible light photocatalytic and antibacterial activity. J. Ind. Eng. Chem. 2018, 57, 125–133. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Li, Q.-Q.; Ma, T.; Gao, J.-Q.; Xue, J.-B.; Gao, S. Graphitic carbon nitride loaded with bismuth nanoparticles displays antibacterial photocatalytic activity. Rare Met. 2022, 41, 1570–1582. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.; Jia, S.; Xie, H.; Kang, Z.; Chen, W.; Cui, M.; Wang, B.; Wang, B.; Chen, X.; et al. Intrinsic carbon defects induced nickel phosphate/carbon photocatalyst for high performance bacterial disinfection. Chem. Eng. J. 2022, 438, 135624. [Google Scholar] [CrossRef]
- Yang, G.; Wang, L.; Zhang, C.; Li, P.; Du, H.; Mao, Y.; Qiu, M.; Li, Q.; Hao, D.; Wang, Q. Novel graphene quantum dots modified NH2-MIL-125 photocatalytic composites for effective antibacterial property and mechanism insight. Sep. Purif. Technol. 2023, 312, 123433. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wang, L.; Xiao, B.; Ran, X.; Wang, C.; Zhu, J.; Liu, Z.; Li, C.; Cui, X.; Li, R.; et al. Construction of a g-C3N4/Bi(OH)3 Heterojunction for the Enhancement of Visible Light Photocatalytic Antibacterial Activity. Int. J. Mol. Sci. 2024, 25, 1872. https://doi.org/10.3390/ijms25031872
Feng J, Wang L, Xiao B, Ran X, Wang C, Zhu J, Liu Z, Li C, Cui X, Li R, et al. Construction of a g-C3N4/Bi(OH)3 Heterojunction for the Enhancement of Visible Light Photocatalytic Antibacterial Activity. International Journal of Molecular Sciences. 2024; 25(3):1872. https://doi.org/10.3390/ijms25031872
Chicago/Turabian StyleFeng, Jian, Li Wang, Bo Xiao, Xia Ran, Caiying Wang, Jinming Zhu, Zuoji Liu, Chaozhong Li, Xinai Cui, Rong Li, and et al. 2024. "Construction of a g-C3N4/Bi(OH)3 Heterojunction for the Enhancement of Visible Light Photocatalytic Antibacterial Activity" International Journal of Molecular Sciences 25, no. 3: 1872. https://doi.org/10.3390/ijms25031872
APA StyleFeng, J., Wang, L., Xiao, B., Ran, X., Wang, C., Zhu, J., Liu, Z., Li, C., Cui, X., Li, R., Feng, G., & Dai, Z. (2024). Construction of a g-C3N4/Bi(OH)3 Heterojunction for the Enhancement of Visible Light Photocatalytic Antibacterial Activity. International Journal of Molecular Sciences, 25(3), 1872. https://doi.org/10.3390/ijms25031872





