Annulus Fibrosus Injury Induces Acute Neuroinflammation and Chronic Glial Response in Dorsal Root Ganglion and Spinal Cord—An In Vivo Rat Discogenic Pain Model
Abstract
1. Introduction
2. Results
2.1. AF Puncture Injury Did Not Affect Rat General Health
2.2. AF Puncture Injury Induced IVD Degeneration
2.3. AF Puncture Injury Induced Macrophage-Associated Inflammatory Responses in the Spine
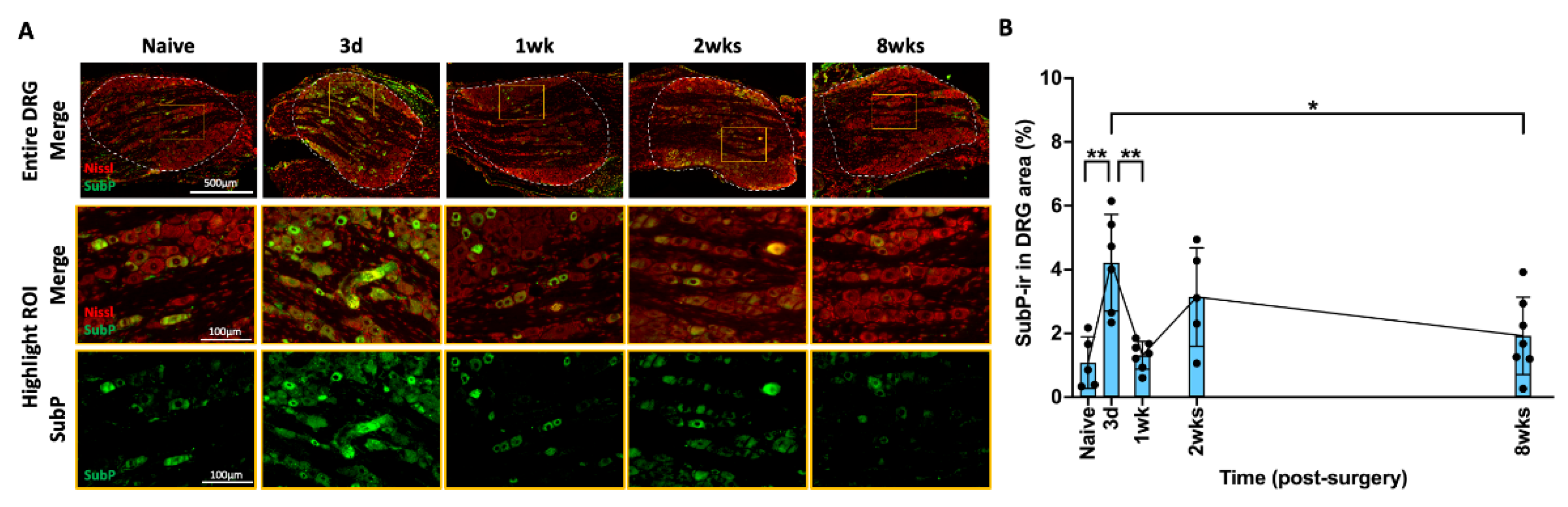
2.4. AF Injury Induced Temporary Increase in SubP in DRG
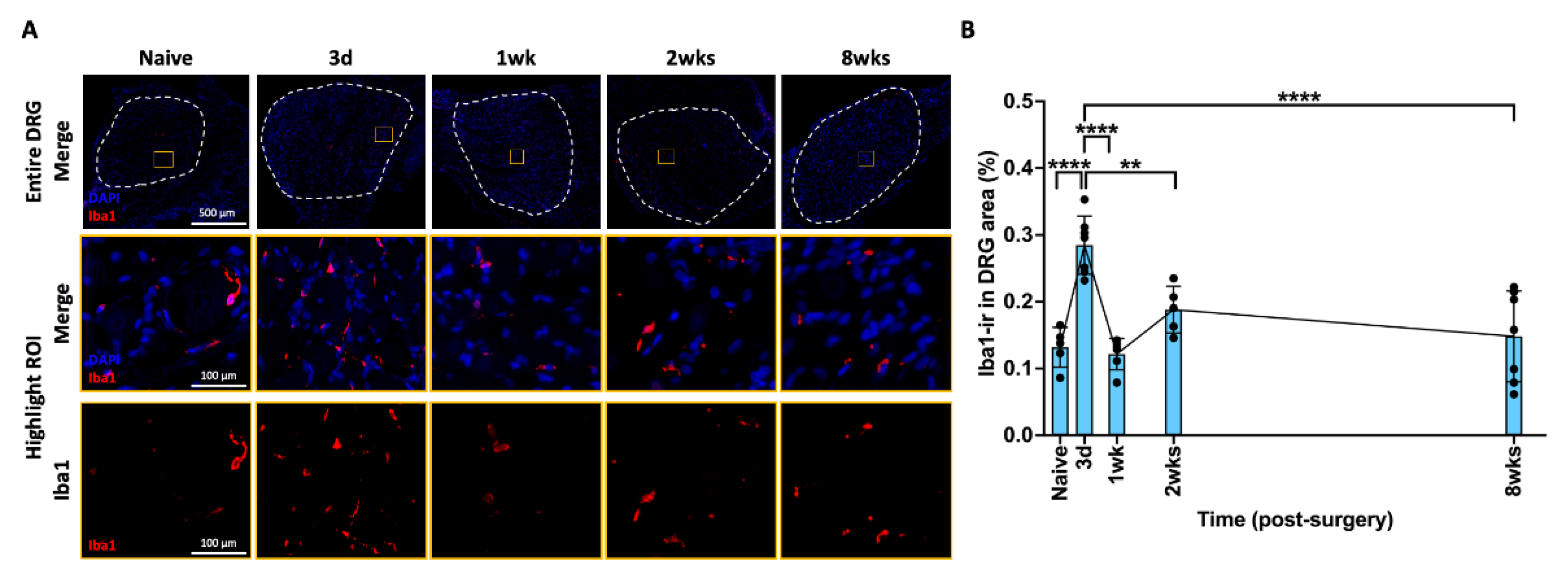
2.5. AF Injury Induced Temporary Neuroinflammatory Response in DRG
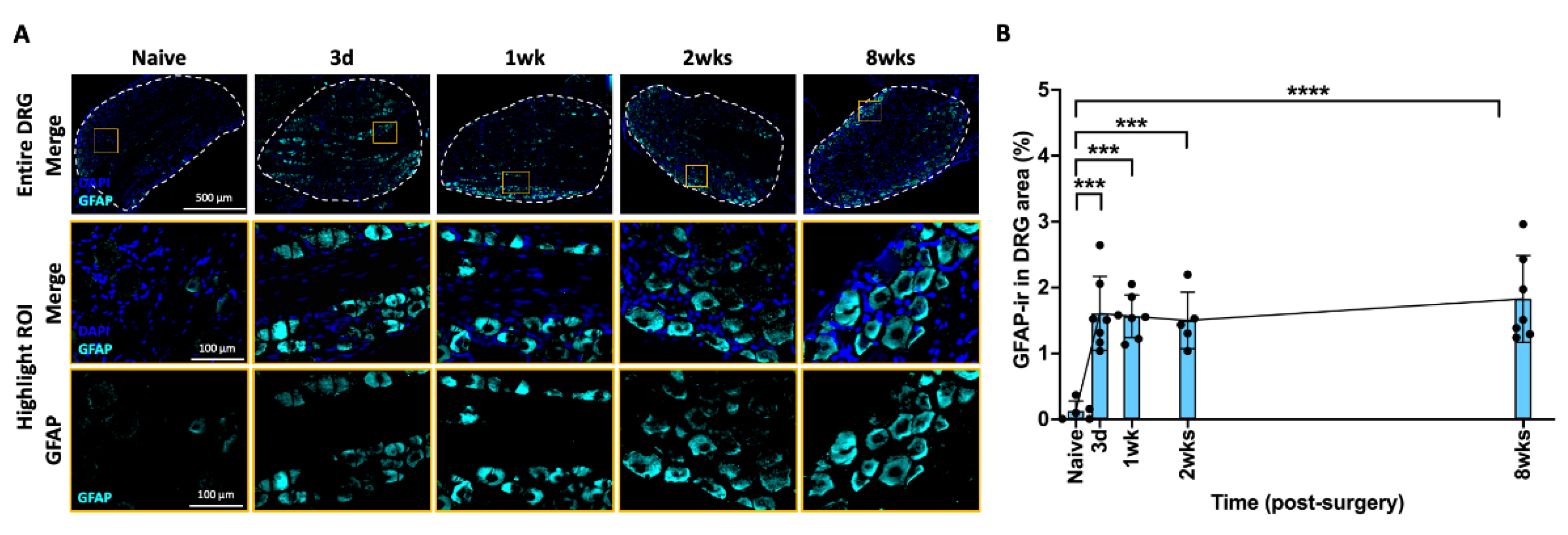
2.6. AF Injury Induced Sustained Increase in Satellite Glial Cell in DRG
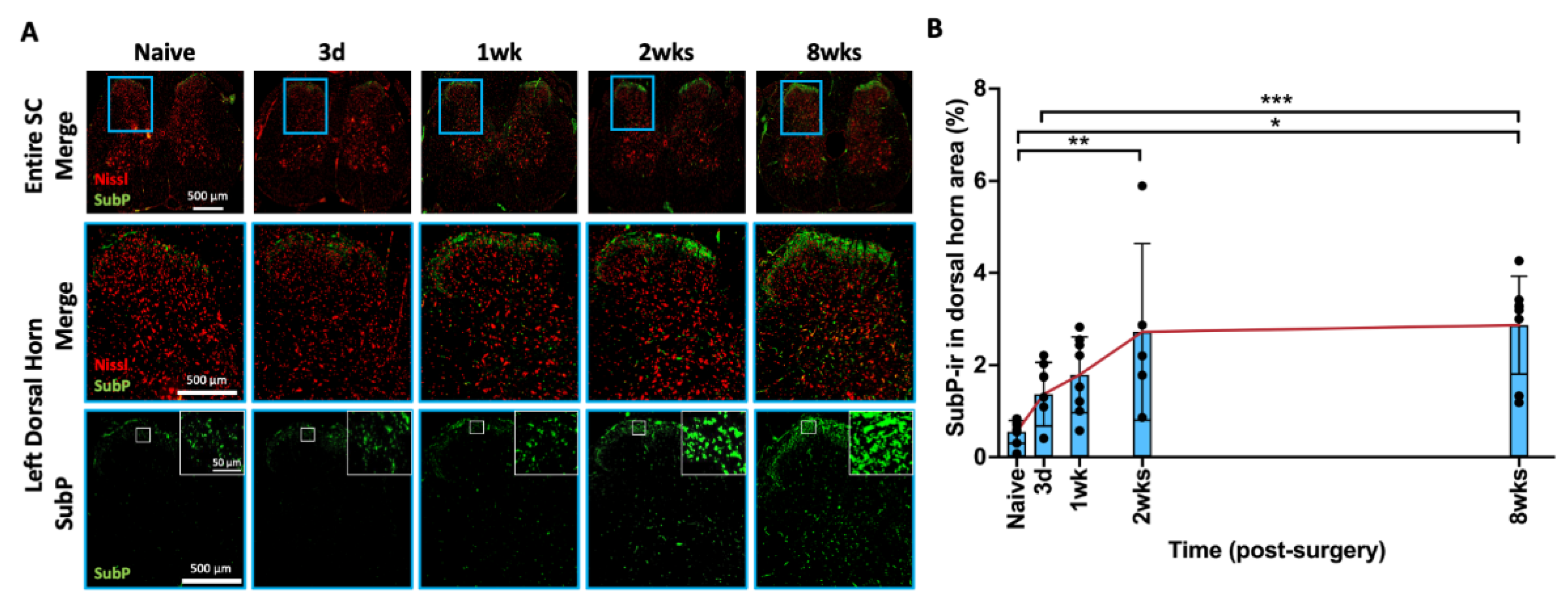
2.7. AF Injury Induced Persistent Spinal Cord Sensitization
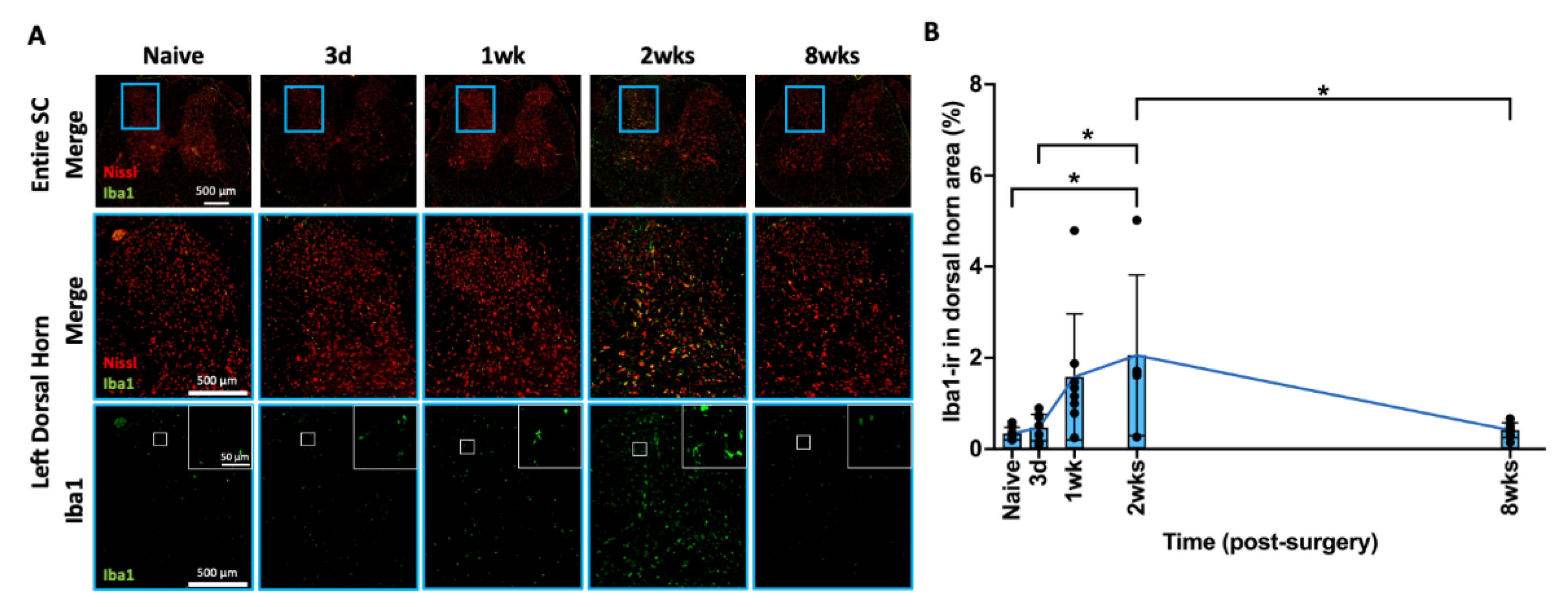
2.8. AF Injury Induced Temporary Microglia-Mediated Neuroinflammatory Response
2.9. AF Injury Induced Sustained and Progressive Astroglial Response
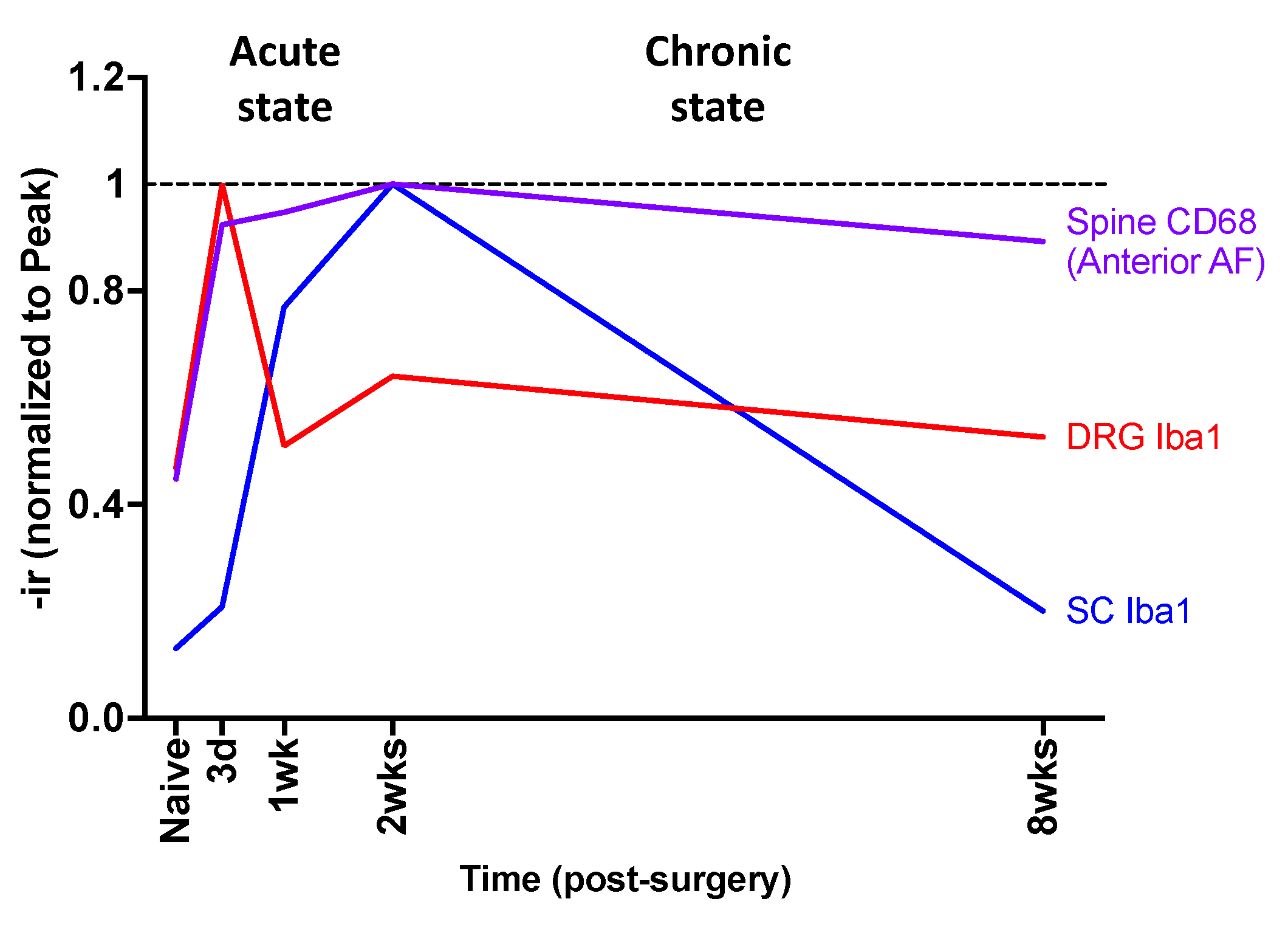
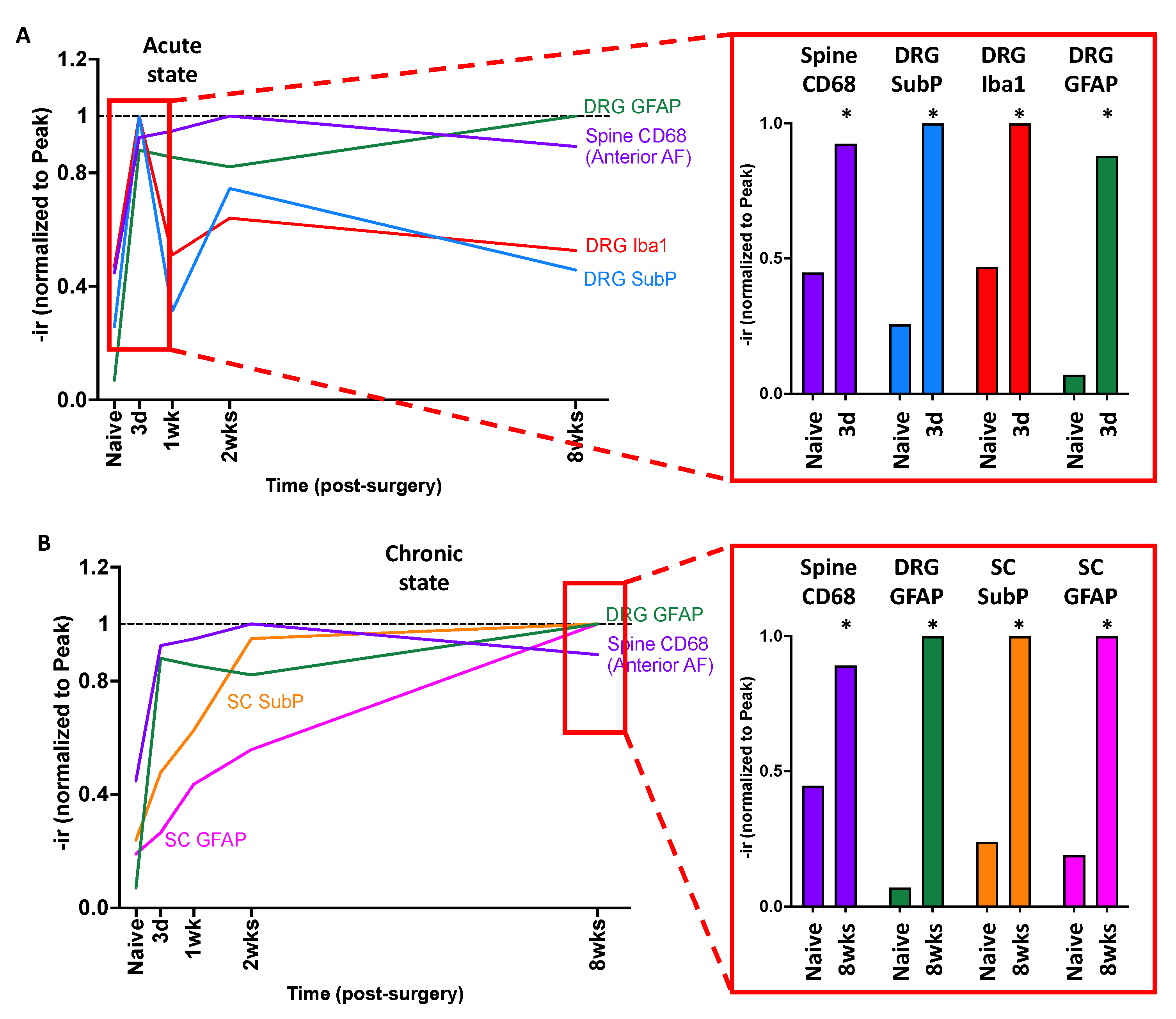
2.10. Crosstalk between Spine, DRG, and SC
3. Discussion
4. Materials and Methods
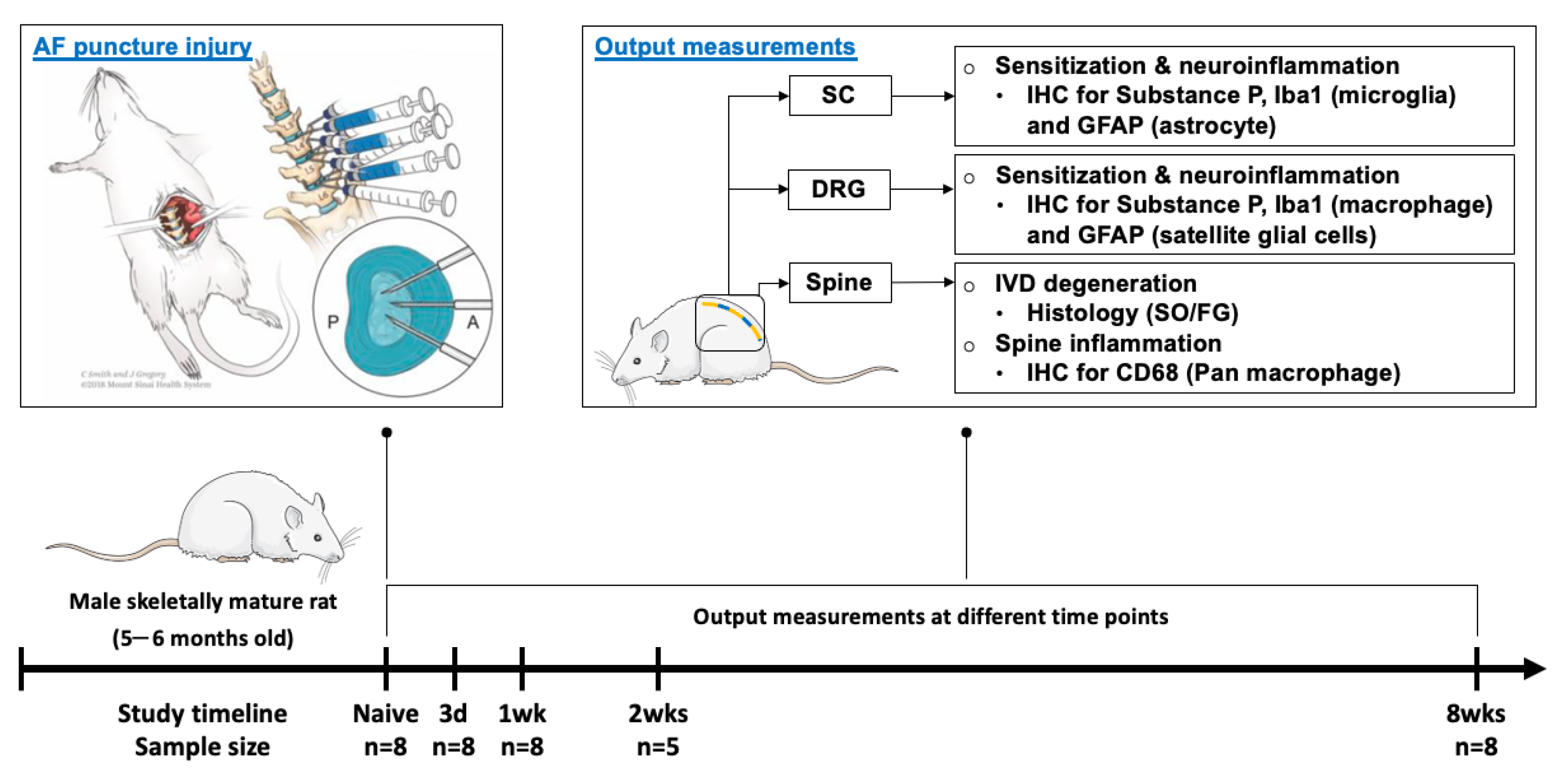
4.1. Study Design
4.2. Surgical Procedure and AF Puncture Injury
4.3. Tissue Collection
4.4. Spine Morphology and IVD Degeneration Grading
4.5. Immunohistochemical Analysis for Spine Macrophage
4.6. Immunohistochemical Analysis for DRG Sensitization and Neuroinflammation
4.7. Immunohistochemical Analysis for Spinal Cord Sensitization and Neuroinflammation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, M.A.; Dolan, P. Intervertebral disc degeneration: Evidence for two distinct phenotypes. J. Anat. 2012, 221, 497–506. [Google Scholar] [CrossRef]
- Battié, M.C.; Videman, T.; Levälahti, E.; Gill, K.; Kaprio, J. Genetic and environmental effects on disc degeneration by phenotype and spinal level: A multivariate twin study. Spine 2008, 33, 2801–2808. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, H.; Liu, A.; Li, C.; Yang, K.; Zheng, S.; Wang, L.; Wang, J.C.; Buser, Z. Analysis of the relationship between the facet fluid sign and lumbar spine motion of degenerative spondylolytic segment using Kinematic MRI. Eur. J. Radiol. 2017, 94, 6–12. [Google Scholar] [CrossRef]
- Fujii, K.; Yamazaki, M.; Kang, J.D.; Risbud, M.V.; Cho, S.K.; Qureshi, S.A.; Hecht, A.C.; Iatridis, J.C. Discogenic Back Pain: Literature Review of Definition, Diagnosis, and Treatment. JBMR Plus 2019, 3, e10180. [Google Scholar] [CrossRef]
- Samartzis, D.K.J.L.; Williams, F.M.K. Spine Phenotypes, 1st ed.; Academic Press: Cambridge, MA, USA, 2022; p. 416. [Google Scholar]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Mirza, S.K.; Deyo, R.A.; Heagerty, P.J.; Turner, J.A.; Martin, B.I.; Comstock, B.A. One-year outcomes of surgical versus nonsurgical treatments for discogenic back pain: A community-based prospective cohort study. Spine J. 2013, 13, 1421–1433. [Google Scholar] [CrossRef]
- Arts, M.P.; Kols, N.I.; Onderwater, S.M.; Peul, W.C. Clinical outcome of instrumented fusion for the treatment of failed back surgery syndrome: A case series of 100 patients. Acta Neurochir. 2012, 154, 1213–1217. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.H.; Song, K.-S.; Hong, J.-Y. Neuropathic Pain after Spinal Surgery. Asian Spine J. 2017, 11, 642–652. [Google Scholar] [CrossRef]
- Duggal, N.; Mendiondo, I.; Pares, H.R.; Jhawar, B.S.; Das, K.; Kenny, K.J.; Dickman, C.A. Anterior lumbar interbody fusion for treatment of failed back surgery syndrome: An outcome analysis. Neurosurgery 2004, 54, 636–643. [Google Scholar] [CrossRef]
- Fritsch, E.W.; Heisel, J.; Rupp, S. The failed back surgery syndrome: Reasons, intraoperative findings, and long-term results: A report of 182 operative treatments. Spine 1996, 21, 626–633. [Google Scholar] [CrossRef]
- Markwalder, T.-M.; Battaglia, M. Failed back surgery syndrome part II: Surgical techniques, implant choice, and operative results in 171 patients with instability of the lumbar spine. Acta Neurochir. 1993, 123, 129–134. [Google Scholar] [CrossRef]
- Skaf, G.; Bouclaous, C.; Alaraj, A.; Chamoun, R. Clinical outcome of surgical treatment of failed back surgery syndrome. Surg. Neurol. 2005, 64, 483–488. [Google Scholar] [CrossRef]
- Anderson, J.T.; Haas, A.R.; Percy, R.; Woods, S.T.; Ahn, U.M.; Ahn, N.U. Single-level lumbar fusion for degenerative disc disease is associated with worse outcomes compared with fusion for spondylolisthesis in a workers’ compensation setting. Spine 2015, 40, 323–331. [Google Scholar] [CrossRef]
- Endler, P.; Ekman, P.; Berglund, I.; Möller, H.; Gerdhem, P. Long-term outcome of fusion for degenerative disc disease in the lumbar spine. Bone Jt. J. 2019, 101-B, 1526–1533. [Google Scholar] [CrossRef]
- Schizas, C.; Kulik, G.; Kosmopoulos, V. Disc degeneration: Current surgical options. Eur. Cells Mater. 2010, 20, 306–315. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Tomaszewski, K.A.; Saganiak, K.; Gładysz, T.; Walocha, J.A. The biology behind the human intervertebral disc and its endplates. Folia Morphol. 2015, 74, 157–168. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Hodges, P.W.; Barbe, M.F.; Loggia, M.L.; Nijs, J.; Stone, L.S. Diverse Role of Biological Plasticity in Low Back Pain and Its Impact on Sensorimotor Control of the Spine. J. Orthop. Sports Phys. Ther. 2019, 49, 389–401. [Google Scholar] [CrossRef]
- Ji, R.-R.; Kohno, T.; Moore, K.A.; Woolf, C.J. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003, 26, 696–705. [Google Scholar] [CrossRef]
- Crosby, N.D.; Gilliland, T.M.; Winkelstein, B.A. Early afferent activity from the facet joint after painful trauma to its capsule potentiates neuronal excitability and glutamate signaling in the spinal cord. Pain 2014, 155, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.T.; Evangelista, B.G.; Venega, R.A.; Seminowicz, D.A.; Chacur, M. Anti-NGF treatment can reduce chronic neuropathic pain by changing peripheral mediators and brain activity in rats. Behav. Pharmacol. 2019, 30, 79–88. [Google Scholar] [CrossRef]
- Ita, M.E.; Crosby, N.D.; Bulka, B.A.M.; Winkelstein, B.A. Painful Cervical Facet Joint Injury Is Accompanied by Changes in the Number of Excitatory and Inhibitory Synapses in the Superficial Dorsal Horn That Differentially Relate to Local Tissue Injury Severity. Spine 2017, 42, E695–E701. [Google Scholar] [CrossRef]
- Lee, S.; Shi, X.Q.; Fan, A.; West, B.; Zhang, J. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Mol. Pain 2018, 14, 1744806918764979. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.P.; Dong, L.; Golder, F.J.; Winkelstein, B.A. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain 2010, 151, 414–421. [Google Scholar] [CrossRef]
- McGinnis, A.; Ji, R.-R. The Similar and Distinct Roles of Satellite Glial Cells and Spinal Astrocytes in Neuropathic Pain. Cells 2023, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Du, J.; Tan, H.Y.; Nesic, O.; Hargett, G.L.; Bopp, A.C.; Yamani, A.; Lin, Q.; Willis, W.D.; Hulsebosch, C.E. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009, 147, 265–276. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Baniasadi, M.; Manaheji, H.; Maghsoudi, N.; Danyali, S.; Zakeri, Z.; Maghsoudi, A.; Zaringhalam, J. Microglial-induced apoptosis is potentially responsible for hyperalgesia variations during CFA-induced inflammation. Inflammopharmacology 2020, 28, 475–485. [Google Scholar] [CrossRef]
- Colburn, R.; DeLeo, J.; Rickman, A.; Yeager, M.; Kwon, P.; Hickey, W. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J. Neuroimmunol. 1997, 79, 163–175. [Google Scholar] [CrossRef]
- Kartha, S.; Weisshaar, C.L.; Philips, B.H.; Winkelstein, B.A. Pre-treatment with Meloxicam Prevents the Spinal Inflammation and Oxidative Stress in DRG Neurons that Accompany Painful Cervical Radiculopathy. Neuroscience 2018, 388, 393–404. [Google Scholar] [CrossRef]
- Zhang, J.; De Koninck, Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 2006, 97, 772–783. [Google Scholar] [CrossRef]
- Abbadie, C.; Brown, J.; Mantyh, P.; Basbaum, A. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience 1996, 70, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, J.-H. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci. Res. 2007, 58, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesthesia 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Moss, A.; Beggs, S.; Vega-Avelaira, D.; Costigan, M.; Hathway, G.J.; Salter, M.W.; Fitzgerald, M. Spinal microglia and neuropathic pain in young rats. Pain 2007, 128, 215–224. [Google Scholar] [CrossRef]
- Myers, R.R.; Campana, W.M.; Shubayev, V.I. The role of neuroinflammation in neuropathic pain: Mechanisms and therapeutic targets. Drug Discov. Today 2006, 11, 8–20. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Mei, X.; Huang, J.; Wei, Y.; Wang, Y.; Wu, S.; Li, Y. Crosstalk between spinal astrocytes and neurons in nerve injury-induced neuropathic pain. PLoS ONE 2009, 4, e6973. [Google Scholar] [CrossRef]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Chiba, T.; Kurokawa, M.; Aoki, Y.; Takahashi, K.; Yamagata, M. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine 2003, 28, 871–880. [Google Scholar] [CrossRef]
- Lai, A.; Moon, A.; Purmessur, D.; Skovrlj, B.; Laudier, D.M.; Winkelstein, B.A.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 2016, 16, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Moon, A.; Purmessur, D.; Skovrlj, B.; Winkelstein, B.A.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Assessment of functional and behavioral changes sensitive to painful disc degeneration. J. Orthop. Res. 2015, 33, 755–764. [Google Scholar] [CrossRef]
- Evashwick-Rogler, T.W.; Lai, A.; Watanabe, H.; Salandra, J.M.; Winkelstein, B.A.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Inhibiting tumor necrosis factor-alpha at time of induced intervertebral disc injury limits long-term pain and degeneration in a rat model. JOR Spine 2018, 1, e1014. [Google Scholar] [CrossRef]
- Mosley, G.E.B.; Hoy, R.C.B.; Nasser, P.; Kaseta, T.B.; Lai, A.; Evashwick-Rogler, T.W.; Lee, M.B.; Iatridis, J.C. Sex Differences in Rat Intervertebral Disc Structure and Function Following Annular Puncture Injury. Spine 2019, 44, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Lamas, S.; Gonçalves, R.M.; Barbosa, M.A. Joint analysis of IVD herniation and degeneration by rat caudal needle puncture model. J. Orthop. Res. 2017, 35, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhu, K.; Li, F.-C.; Xiao, Y.-X.; Feng, J.; Shi, Z.-L.; Lin, M.; Wang, J.; Chen, Q.-X. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine 2008, 33, 1925–1934. [Google Scholar] [CrossRef]
- Hsieh, A.H.; Hwang, D.; Ryan, D.A.; Freeman, A.K.; Kim, H. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine 2009, 34, 998–1005. [Google Scholar] [CrossRef]
- Masuda, K.; Aota, Y.; Muehleman, C.; Imai, Y.; Okuma, M.; Thonar, E.J.; Andersson, G.B.; An, H.S. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: Correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 2005, 30, 5–14. [Google Scholar] [CrossRef]
- Millecamps, M.; Stone, L.S. Delayed onset of persistent discogenic axial and radiating pain after a single-level lumbar intervertebral disc injury in mice. Pain 2018, 159, 1843–1855. [Google Scholar] [CrossRef]
- Yang, F.; Leung, V.Y.; Luk, K.D.; Chan, D.; MC Cheung, K. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J. Pathol. 2009, 218, 113–121. [Google Scholar] [CrossRef]
- Zhang, K.-B.; Zheng, Z.-M.; Liu, H.; Liu, X.-G. The effects of punctured nucleus pulposus on lumbar radicular pain in rats: A behavioral and immunohistochemical study. J. Neurosurg. Spine 2009, 11, 492–500. [Google Scholar] [CrossRef]
- Orita, S.; Ohtori, S.; Nagata, M.; Horii, M.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Suzuki, M.; Eguchi, Y.; Kamoda, H.; et al. Inhibiting nerve growth factor or its receptors downregulates calcitonin gene-related peptide expression in rat lumbar dorsal root ganglia innervating injured intervertebral discs. J. Orthop. Res. 2010, 28, 1614–1620. [Google Scholar] [CrossRef]
- Sainoh, T.; Sakuma, Y.; Miyagi, M.; Orita, S.; Yamauchi, K.; Inoue, G.; Kamoda, H.; Ishikawa, T.; Suzuki, M.; Kubota, G.; et al. Efficacy of Anti–Nerve Growth Factor Therapy for Discogenic Neck Pain in Rats. Spine 2014, 39, E757–E762. [Google Scholar] [CrossRef]
- Horii, M.; Orita, S.; Nagata, M.; Takaso, M.; Yamauchi, K.; Yamashita, M.; Inoue, G.; Eguchi, Y.; Ochiai, N.; Kishida, S.; et al. Direct application of the tumor necrosis factor-α inhibitor, etanercept, into a punctured intervertebral disc decreases calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. Spine 2011, 36, E80–E85. [Google Scholar] [CrossRef] [PubMed]
- Inage, K.; Orita, S.; Yamauchi, K.; Suzuki, T.; Suzuki, M.; Sakuma, Y.; Kubota, G.; Oikawa, Y.; Sainoh, T.; Sato, J.; et al. Dose Optimization for Single Intradiscal Administration of the Tumor Necrosis Factor-α Inhibitor, Etanercept, in Rat Disc Injury Models. Asian Spine J. 2016, 10, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Orita, S.; Eguchi, Y.; Kamoda, H.; Arai, G.; Ishikawa, T.; Miyagi, M.; Inoue, G.; Suzuki, M.; Toyone, T.; Aoki, Y.; et al. Brain-Derived neurotrophic factor inhibition at the punctured intervertebral disc downregulates the production of calcitonin gene-related peptide in dorsal root ganglia in rats. Spine 2011, 36, 1737–1743. [Google Scholar] [CrossRef]
- Miyagi, M.; Uchida, K.; Takano, S.; Fujimaki, H.; Aikawa, J.; Sekiguchi, H.; Nagura, N.; Ohtori, S.; Inoue, G.; Takaso, M. Macrophage-derived inflammatory cytokines regulate growth factors and pain-related molecules in mice with intervertebral disc injury. J. Orthop. Res. 2018, 36, 2274–2279. [Google Scholar] [CrossRef]
- Nojima, D.; Inage, K.; Sakuma, Y.; Sato, J.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kuniyoshi, K.; Aoki, Y.; et al. Efficacy of Anti-NaV1.7 Antibody on the Sensory Nervous System in a Rat Model of Lumbar Intervertebral Disc Injury. Yonsei Med. J. 2016, 57, 748–753. [Google Scholar] [CrossRef]
- Mosley, G.E.; Wang, M.; Nasser, P.; Lai, A.; Charen, D.A.; Zhang, B.; Iatridis, J.C. Males and females exhibit distinct relationships between intervertebral disc degeneration and pain in a rat model. Sci. Rep. 2020, 10, 15120. [Google Scholar] [CrossRef] [PubMed]
- Mosley, G.E.; Evashwick-Rogler, T.W.; Lai, A.; Iatridis, J.C. Looking beyond the intervertebral disc: The need for behavioral assays in models of discogenic pain. Ann. N. Y. Acad. Sci. 2017, 1409, 51–66. [Google Scholar] [CrossRef]
- Wawrose, R.A.; Couch, B.K.; Dombrowski, M.; Chen, S.R.; Oyekan, A.; Dong, Q.; Wang, D.; Zhou, C.; Chen, J.; Modali, K.; et al. Percutaneous lumbar annular puncture: A rat model to study intervertebral disc degeneration and pain-related behavior. JOR Spine 2022, 5, e1202. [Google Scholar] [CrossRef]
- Lai, A.; Gansau, J.; Gullbrand, S.E.; Crowley, J.; Cunha, C.; Dudli, S.; Engiles, J.B.; Fusellier, M.; Goncalves, R.M.; Nakashima, D.; et al. Development of a standardized histopathology scoring system for intervertebral disc degeneration in rat models: An initiative of the ORS spine section. JOR Spine 2021, 4, e1150. [Google Scholar] [CrossRef]
- Ohtori, S.; Nakamura, S.; Koshi, T.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Orita, S.; Eguchi, Y.; Suzuki, M.; Ochiai, N.; et al. Effectiveness of L2 spinal nerve infiltration for selective discogenic low back pain patients. J. Orthop. Sci. 2010, 15, 731–736. [Google Scholar] [CrossRef]
- Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Lillyman, D.J.; Lee, F.S.; Barnett, E.C.; Miller, T.J.; Alvaro, M.L.; Drvol, H.C.; Wachs, R.A. Axial hypersensitivity is associated with aberrant nerve sprouting in a novel model of disc degeneration in female Sprague Dawley rats. JOR Spine 2022, 5, e1212. [Google Scholar] [CrossRef]
- Miyagi, M.; Ishikawa, T.; Orita, S.; Eguchi, Y.; Kamoda, H.; Arai, G.; Suzuki, M.; Inoue, G.; Aoki, Y.; Toyone, T.; et al. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators. Spine 2011, 36, 2260–2266. [Google Scholar] [CrossRef]
- Yang, G.; Chen, L.; Gao, Z.; Wang, Y. Implication of microglia activation and CSF1/CSF1R pathway in lumbar disc degeneration-related back pain. Mol. Pain 2018, 14, 1744806918811238. [Google Scholar] [CrossRef]
- Merighi, A.; Carmignoto, G.; Gobbo, S.; Lossi, L.; Salio, C.; Vergnano, A.M.; Zonta, M. Neurotrophins in spinal cord nociceptive pathways. Prog. Brain Res. 2004, 146, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Isami, K.; Imai, S.; Sukeishi, A.; Nagayasu, K.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. The impact of mouse strain-specific spatial and temporal immune responses on the progression of neuropathic pain. Brain Behav. Immun. 2018, 74, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, Y.-Q.; Xu, B.-Y.; Li, J.-Y.; Zhang, L.-Q.; Li, D.-Y.; Zhang, S.; Wu, J.-Y.; Gao, S.-J.; Ye, D.-W.; et al. STING/NF-κB/IL-6-Mediated Inflammation in Microglia Contributes to Spared Nerve Injury (SNI)-Induced Pain Initiation. J. Neuroimmune Pharmacol. 2022, 17, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sandoval, A.; Chai, N.; Nutile-McMenemy, N.; DeLeo, J.A. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008, 1219, 116–126. [Google Scholar] [CrossRef]
- Zhu, D.; Fan, T.; Chen, Y.; Huo, X.; Li, Y.; Liu, D.; Cai, Y.; Cheung, C.W.; Tang, J.; Cui, J.; et al. CXCR4/CX43 Regulate Diabetic Neuropathic Pain via Intercellular Interactions between Activated Neurons and Dysfunctional Astrocytes during Late Phase of Diabetes in Rats and the Effects of Antioxidant N-Acetyl-L-Cysteine. Oxidative Med. Cell. Longev. 2022, 2022, 8547563. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Nutu, M.; Størkson, R. Changes in Spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective tnf-alpha inhibition. Spine 2003, 28, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Onda, A.; Murata, Y.; Rydevik, B.; Larsson, K.; Kikuchi, S.; Olmarker, K. Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine 2004, 29, 1857–1861. [Google Scholar] [CrossRef]
- Sainoh, T.; Orita, S.; Miyagi, M.; Inoue, G.; Kamoda, H.; Ishikawa, T.; Yamauchi, K.; Suzuki, M.; Sakuma, Y.; Kubota, G.; et al. Single Intradiscal Administration of the Tumor Necrosis Factor-Alpha Inhibitor, Etanercept, for Patients with Discogenic Low Back Pain. Pain Med. 2016, 17, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Tobinick, E.; Davoodifar, S. Efficacy of etanercept delivered by perispinal administration for chronic back and/or neck disc-related pain: A study of clinical observations in 143 patients. Curr. Med. Res. Opin. 2004, 20, 1075–1085. [Google Scholar] [CrossRef]
- Cohen, S.P.; Wenzell, D.; Hurley, R.W.; Kurihara, C.; Buckenmaier, C.C., 3rd; Griffith, S.; Larkin, T.M.; Dahl, E.; Morlando, B.J. A Double-blind, placebo-controlled, dose–response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology 2007, 107, 99–105. [Google Scholar] [CrossRef]
- Parisien, M.; Lima, L.V.; Dagostino, C.; El-Hachem, N.; Drury, G.L.; Grant, A.V.; Huising, J.; Verma, V.; Meloto, C.B.; Silva, J.R.; et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci. Transl. Med. 2022, 14, eabj9954. [Google Scholar] [CrossRef]
- Zhang, H.; Mei, X.; Zhang, P.; Ma, C.; White, F.A.; Donnelly, D.F.; Lamotte, R.H. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia 2009, 57, 1588–1599. [Google Scholar] [CrossRef]
- Zhuang, Z.-Y.; Gerner, P.; Woolf, C.J.; Ji, R.-R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Palmisani, S.; Smith, T.E.; Carganillo, R.; Houghton, R.; Pang, D.; Burgoyne, W.; Lam, K.; Lucas, J. Long-Term Improvements in Chronic Axial Low Back Pain Patients Without Previous Spinal Surgery: A Cohort Analysis of 10-kHz High-Frequency Spinal Cord Stimulation over 36 Months. Pain Med. 2018, 19, 1219–1226. [Google Scholar] [CrossRef]
- Kallewaard, J.W.; Edelbroek, C.; Terheggen, M.; Raza, A.; Geurts, J.W. A Prospective Study of Dorsal Root Ganglion Stimulation for Non-Operated Discogenic Low Back Pain. Neuromodulation Technol. Neural Interface 2020, 23, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Remacle, T.Y.; Bonhomme, V.L.; Renwart, H.-J.P.; Remacle, J.M. Effect of Multicolumn Lead Spinal Cord Stimulation on Low Back Pain in Failed Back Surgery Patients: A Three-Year Follow-Up. Neuromodulation Technol. Neural Interface 2017, 20, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.B.; Groenen, P.S.; Patel, K.V.; Vissers, K.C.; van Helmond, N. T12 Dorsal Root Ganglion Stimulation to Treat Chronic Low Back Pain: A Case Series. Neuromodulation Technol. Neural Interface 2020, 23, 203–212. [Google Scholar] [CrossRef]
- Lai, A.; Ho, L.; Evashwick-Rogler, T.W.; Watanabe, H.; Salandra, J.; Winkelstein, B.A.; Laudier, D.; Hecht, A.C.; Pasinetti, G.M.; Iatridis, J.C. Dietary polyphenols as a safe and novel intervention for modulating pain associated with intervertebral disc degeneration in an in-vivo rat model. PLoS ONE 2019, 14, e0223435. [Google Scholar] [CrossRef]
- LaCroix-Fralish, M.L.; Rutkowski, M.D.; Weinstein, J.N.; Mogil, J.S.; DeLeo, J.A. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine 2005, 30, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S.; Bailey, A.L. Sex and gender differences in pain and analgesia. Prog. Brain Res. 2010, 186, 141–157. [Google Scholar] [CrossRef]
- Watson, R.W. Techniques for studying the brain. In The Brain; Watson, R.W., Ed.; Science Direct; Academic Press: Cambridge, MA, USA, 2010; pp. 153–165. [Google Scholar] [CrossRef]
- Sperry, Z.J.; Graham, R.D.; Peck-Dimit, N.; Lempka, S.F.; Bruns, T.M. Spatial models of cell distribution in human lumbar dorsal root ganglia. J. Comp. Neurol. 2020, 528, 1644–1659. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, A.; Iliff, D.; Zaheer, K.; Gansau, J.; Laudier, D.M.; Zachariou, V.; Iatridis, J.C. Annulus Fibrosus Injury Induces Acute Neuroinflammation and Chronic Glial Response in Dorsal Root Ganglion and Spinal Cord—An In Vivo Rat Discogenic Pain Model. Int. J. Mol. Sci. 2024, 25, 1762. https://doi.org/10.3390/ijms25031762
Lai A, Iliff D, Zaheer K, Gansau J, Laudier DM, Zachariou V, Iatridis JC. Annulus Fibrosus Injury Induces Acute Neuroinflammation and Chronic Glial Response in Dorsal Root Ganglion and Spinal Cord—An In Vivo Rat Discogenic Pain Model. International Journal of Molecular Sciences. 2024; 25(3):1762. https://doi.org/10.3390/ijms25031762
Chicago/Turabian StyleLai, Alon, Denise Iliff, Kashaf Zaheer, Jennifer Gansau, Damien M. Laudier, Venetia Zachariou, and James C. Iatridis. 2024. "Annulus Fibrosus Injury Induces Acute Neuroinflammation and Chronic Glial Response in Dorsal Root Ganglion and Spinal Cord—An In Vivo Rat Discogenic Pain Model" International Journal of Molecular Sciences 25, no. 3: 1762. https://doi.org/10.3390/ijms25031762
APA StyleLai, A., Iliff, D., Zaheer, K., Gansau, J., Laudier, D. M., Zachariou, V., & Iatridis, J. C. (2024). Annulus Fibrosus Injury Induces Acute Neuroinflammation and Chronic Glial Response in Dorsal Root Ganglion and Spinal Cord—An In Vivo Rat Discogenic Pain Model. International Journal of Molecular Sciences, 25(3), 1762. https://doi.org/10.3390/ijms25031762





