Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions
Abstract
1. Introduction
2. Microbiota and Intestinal Mucosa
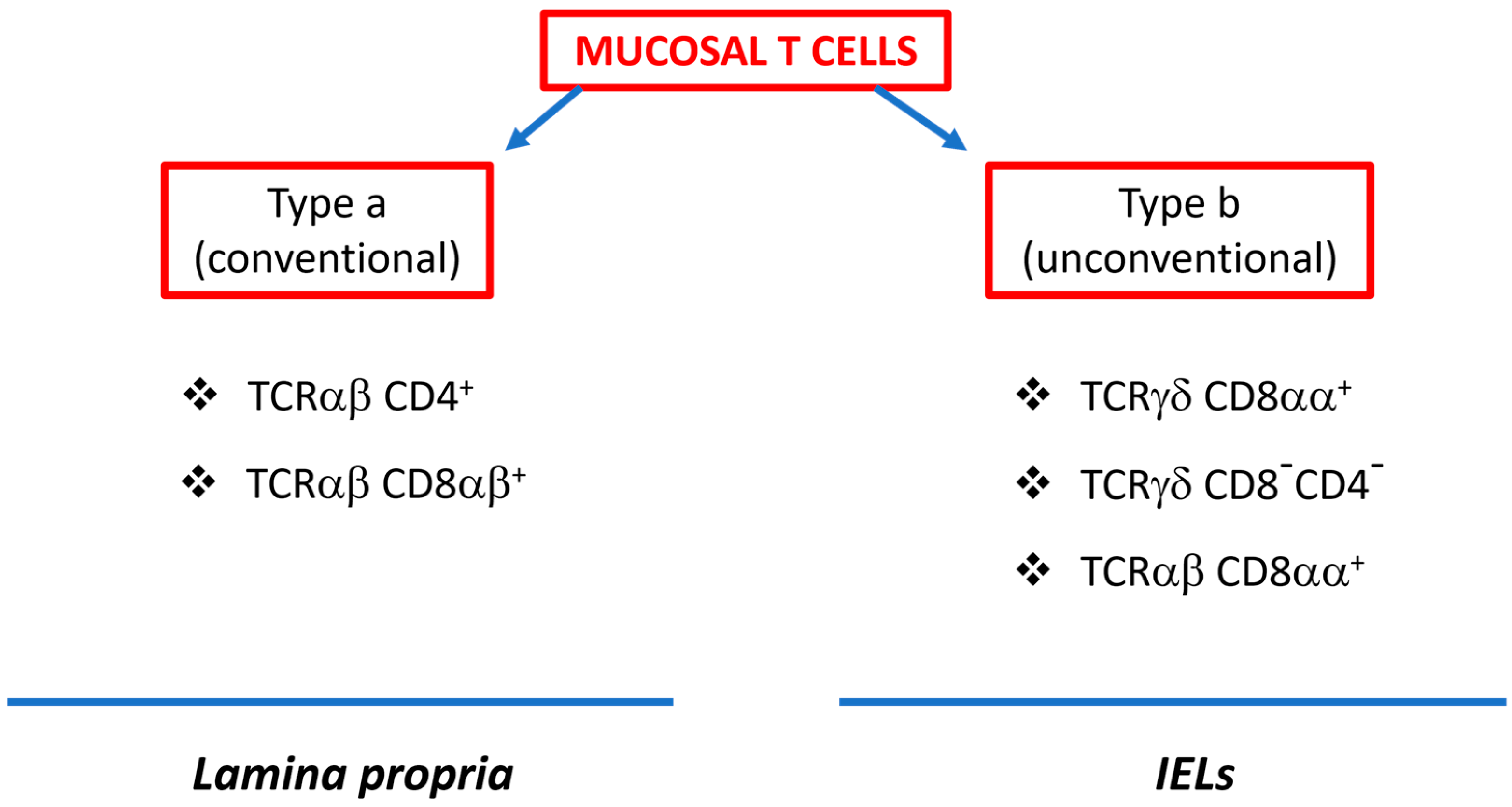
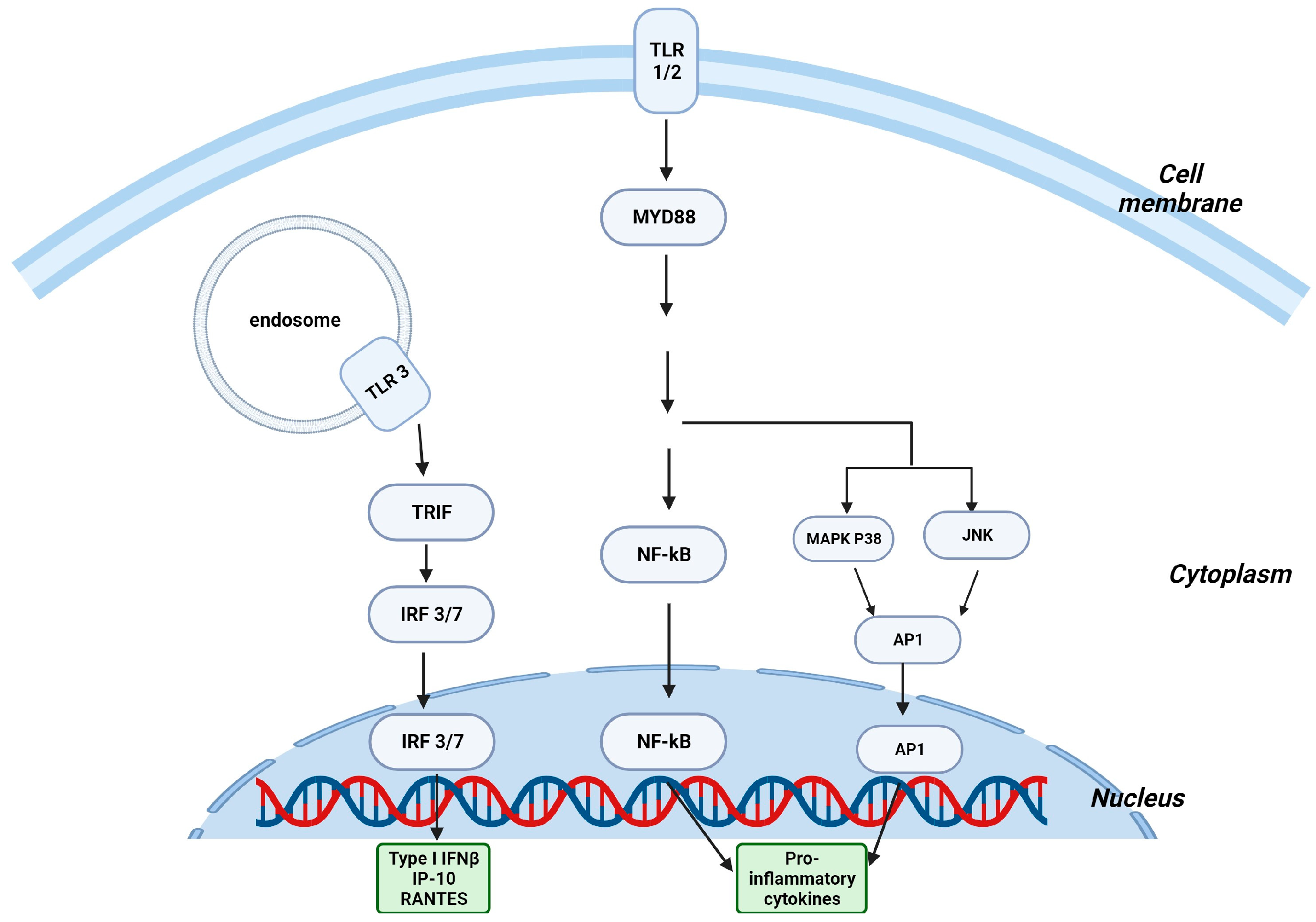
3. Intestinal γδ T Cells
| Ligand | Receptor on γδ T Cells | Effector Factors | Function | References |
|---|---|---|---|---|
| IL-23 | IL-23R | IL-22, IL-17 |
| [60,61,66,67] |
| Xenobiotics Natural products Microbiota metabolites Endogenous molecules | AhR (Aryl hydrocarbon receptor) | |||
| CD30L | CD30 | IL-17 |
| [62,78] |
| MICA/B (stress marker) | NKG2D (natural killer group2, member A) | IFNγ, TNFα KGF-1 |
| [52,53,54,68,79] |
| MAMPs | TLR1 TLR2 Dectin-1 | IFNγ IL-17 Reg III |
| [58,61] |
| IL-12 IL-18 | IL-12R IL-18R | IFNγ ↑CD40L | Enhancement of phagocytic activity | [64,65] |
| E-cadherin (on IECs) | αEβ7 integrin | Granzyme A/B Perforin | Lysis of infected and transformed intestinal cells | [72,80] |
| Microorganisms-derived proteins Lipopeptides Self-proteins Btnl | TCR | IFNγ IL-17 TNFα | Development of effector subsets | [39,56,57,58] |
| MIC (stress marker) | NKG2A | IL-10, TGFβ |
| [75] |
| IL-15 | IL-15R | Maintenance, localization, proliferation, and maturation of γδT cells | [81] | |
| Unknown | GPR18 (orphan G-coupled receptor) | Promotes entry, residence, and maturation of γδT cells in intestinal epithelium (homing) | [49] | |
| CCL25 (epithelial cells) | CCR9 | Promotes entry and residence of γδT cells in the intestinal epithelium (homing) | [43,44,45] |
4. Intestinal T Cells and Microbiota Interactions
5. Dysbiosis and Inflammation
6. Intestinal Neuro-Immune Regulation
| Disease | Gut Microbiota | Microbiota Metabolites | Effect | References |
|---|---|---|---|---|
| Parkinson’s | ↑ Enterobacteriaceae | Postural instability | ||
| ↓ Prevotellaceae ↓ Lactobacillaceae ↓ Lachnospiraceae | ↓ SCFA ↓ Ghrelin | ↑ α-synuclein ↑ Inflammation ↑ Gut permeability ↑ Bacterial dissemination Neurodegeneration | [174,175,176,177,178,179,180] | |
| Chronic Psychosocial Stress | ↓ Bacteroide sps. ↑ Clostridium sps. | ↑ IL-6 ↑ CCL2 ↑ Gut permeability | [159] | |
| Multiple Sclerosis (MS) | ↓ Propionate-generating bacteria | ↓ Propionate | ↑ IL-1β, IL-6, IL-17 ↑ IL-17 ↓ IL-10 ↓ Treg | [161,162] |
| Type 1 Diabetes | ↑ Clostridiales order ↑ Lachnospiraceae | ↑ BCAA | ↑ Gut permeability | [165,166,167] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | acetycholine |
| AChE | acetylcholinesterase |
| AhR | aryl hydrocarbon receptor |
| AKR1B8 | aldo-keto reductase 1B8 |
| AMPs | antimicrobial peptides |
| AP | activator protein 1 |
| BCAAs | branched-chain amino acids |
| BTNL | butyrophilin-like molecules |
| CCL | C-C chemokine ligand |
| CCL5 | C-C chemokine ligand 5 also known as RANTES |
| CCR | C-C chemokine receptor |
| CD30 | also known as TNF receptor superfamily member 8 |
| CLR | C-type lectin receptors |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| cTreg | colonic regulatory T cells |
| CXCL10 | C-X-C motif chemokine ligand 10 or Interferon gamma-induced protein |
| CX3CR1 | CX3C motif chemokine receptor 1 or G-protein coupled receptor 13 |
| DAP10 | DNAX-activating protein of 10KDa |
| DCs | dendritic cells |
| ENS | enteric nervous system |
| EPRC | endothelial protein C receptor |
| GABA | γ-Aminobutyric acid |
| GALT | gut-associated lymphoid tissue |
| GCSF | granulocytes-colony stimulating factor |
| GI | gastro-intestinal |
| GF | germ-free |
| GMPs | granulocyte–monocyte progenitors |
| GPR | G-protein coupled receptor |
| HDAC | histone deacetylase |
| HMBPP | 4-hydroxy-3-methyl-but-2-enyl pyrophosphate |
| 5-HT | 5-hydroxytryptamine |
| IBD | inflammatory bowel disease |
| IEB | intestinal epithelial barrier |
| IEC | intestinal epithelial cells |
| IELs | intra-epithelial lymphocytes |
| IFNβ | interferon beta |
| IFNγ | interferon gamma |
| IL | interleukin |
| ILCs | innate lymphoid cells |
| IL23R | interleukin 23 receptor |
| IP-10 | interferon-inducible protein 10 |
| IRF | interferon regulatory factors |
| JNK | c-Jun N-terminal kinases |
| KGF | keratinocyte growth factor |
| LPLs | lamina propria lymphocytes |
| LPS | lipopolysaccharide |
| MAMPs | microbial-associated molecular patterns |
| MAPK | mitogen-activated protein kinase |
| MHC | major histocompatibility complex |
| MIC | MHC class-I- related chain |
| MNPs | mononuclear phagocytes |
| MyD88 | myeloid differentiation primary response 88 |
| miRNA | microRNA |
| NF-κB: | nuclear factor kappa B |
| NKG2D | natural killer group 2D receptor |
| NLR | Nod-like receptor |
| PD | Parkinson’s disease |
| PRR | patterns recognition receptor |
| RANTES | regulated upon activation, normal T cell expressed and secreted (CCL5) |
| RegIII | regenerating islet-derived protein 3 |
| RORγ | RAR-related orphan receptor gamma |
| SCFA | short-chain fatty acids |
| SFB | segmented filamentous bacteria |
| TCR | T cell receptor |
| TGFβ | transforming growth factor beta |
| TNFα | tumor necrosis factor alpha |
| TLR | toll-like receptor |
| TRIF TIR | (Toll/interleukin-1 receptor) domain-containing adaptor protein inducing interferon beta |
| Treg | regulatory T cell |
| Th17 | T helper 17-secreating cells |
References
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; He, J.; Xiong, X. The Role of the Gut Microbiota in the Development of Ischemic Stroke. Front. Immunol. 2022, 13, 845243. [Google Scholar] [CrossRef]
- El Aidy, S.; Hooiveld, G.; Tremaroli, V.; Bäckhed, F.; Kleerebezem, M. The gut microbiota and mucosal homeostasis: Colonized at birth or at adulthood, does it matter? Gut Microbes 2013, 4, 118–124. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef]
- Hou, Q.; Huang, J.; Ayansola, H.; Masatoshi, H.; Zhang, B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front. Immunol. 2021, 11, 623691. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of gut homeostasis by the mucosal immune system. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 423–435. [Google Scholar] [CrossRef]
- Hayday, A.; Theodoridis, E.; Ramsburg, E.; Shires, J. Intraepithelial lymphocytes: Exploring the Third Way in immunology. Nat. Immunol. 2001, 2, 997–1003. [Google Scholar] [CrossRef]
- Gaud, G.; Lesourne, R.; Love, P.E. Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 2018, 18, 485–497. [Google Scholar] [CrossRef]
- Muro, R.; Takayanagi, H.; Nitta, T. T cell receptor signaling for γδ T cell development. Inflamm. Regen. 2019, 39, 6. [Google Scholar] [CrossRef]
- Girardi, M. Immunosurveillance and immunoregulation by γδ T cells. J. Invest. Dermatol. 2006, 126, 25–31. [Google Scholar] [CrossRef]
- Tlaskalová-Hogenová, H.; Šterzl, J.; Štěpánková, R.; Dlabač, V.; Větvička, V.; Rossmann, P.; Mandel, L.; Rejnek, J. Development of Immunological Capacity under Germfree and Conventional Conditions. Ann. N. Y. Acad. Sci. 1983, 409, 96–113. [Google Scholar] [CrossRef]
- Umesaki, Y.; Setoyama, H.; Matsumoto, S.; Okada, Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology 1993, 79, 32–37. [Google Scholar]
- Williams, A.M.; Probert, C.S.J.; Stepankova, R.; Tlaskalova-Hogenova, H.; Phillips, A.; Bland, P.W. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology 2006, 119, 470–478. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal Host-Bacterial Relationships in the Gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef]
- Togo, A.; Dufour, J.-C.; Lagier, J.-C.; Dubourg, G.; Raoult, D.; Million, M. Repertoire of human breast and milk microbiota: A systematic review. Futur. Microbiol. 2019, 14, 623–641. [Google Scholar] [CrossRef]
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The Relationship Between Breast Milk Components and the Infant Gut Microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Zhou, A.; Yuan, Y.; Yang, M.; Huang, Y.; Li, X.; Li, S.; Yang, S.; Tang, B. Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions. Front. Cell. Infect. Microbiol. 2022, 12, 832672. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.A.E.; Hassan, H.; Mottawea, W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 715506. [Google Scholar] [CrossRef]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441. [Google Scholar] [CrossRef]
- Hrncir, T.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Tlaskalova-Hogenova, H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: Studies in germ-free mice. BMC Immunol. 2008, 9, 65. [Google Scholar] [CrossRef]
- Frosali, S.; Pagliari, D.; Gambassi, G.; Landolfi, R.; Pandolfi, F.; Cianci, R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. J. Immunol. Res. 2015, 2015, 489821. [Google Scholar] [CrossRef]
- You, X.-Y.; Zhang, H.-Y.; Han, X.; Wang, F.; Zhuang, P.-W.; Zhang, Y.-J. Intestinal Mucosal Barrier Is Regulated by Intestinal Tract Neuro-Immune Interplay. Front. Pharmacol. 2021, 12, 659716. [Google Scholar] [CrossRef]
- Kobe, B.; Deisenhofer, J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 1995, 374, 183–186. [Google Scholar] [CrossRef] [PubMed]
- DiPalma, M.P.; Blattman, J.N. The impact of microbiome dysbiosis on T cell function within the tumor microenvironment (TME). Front. Cell Dev. Biol. 2023, 11, 1141215. [Google Scholar] [CrossRef]
- Saito, H.; Kranz, D.M.; Takagaki, Y.; Hayday, A.C.; Eisen, H.N.; Tonegawa, S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature 1984, 309, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [PubMed]
- Deusch, K.; Lüling, F.; Reich, K.; Classen, M.; Wagner, H.; Pfeffer, K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γ/δ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur. J. Immunol. 1991, 21, 1053–1059. [Google Scholar] [CrossRef]
- Andreu-Ballester, J.C.; García-Ballesteros, C.; Benet-Campos, C.; Amigó, V.; Almela-Quilis, A.; Mayans, J.; Ballester, F. Values for αβ and γδ T-lymphocytes and CD4+, CD8+, and CD56+ subsets in healthy adult subjects: Assessment by age and gender. Cytom. Part B Clin. Cytom. 2012, 82, 238–244. [Google Scholar] [CrossRef]
- Takagaki, Y.; DeCloux, A.; Bonneville, M.; Tonegawa, S. Diversity of γδ T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature 1989, 339, 712–714. [Google Scholar] [CrossRef]
- Kenna, T.; Golden-Mason, L.; Norris, S.; Hegarty, J.E.; O’Farrelly, C.; Doherty, D.G. Distinct subpopulations of γδ T cells are present in normal and tumor-bearing human liver. Clin. Immunol. 2004, 113, 56–63. [Google Scholar]
- Di Marco Barros, R.; Roberts, N.A.; Dart, R.J.; Vantourout, P.; Jandke, A.; Nussbaumer, O.; Deban, L.; Cipolat, S.; Hart, R.; Iannitto, M.L.; et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific γδ T Cell Compartments. Cell 2016, 167, 203–218.e17. [Google Scholar]
- Chien, Y.H.; Meyer, C.; Bonneville, M. γδ T cells: First line of defense and beyond. Annu. Rev. Immunol. 2014, 32, 121–155. [Google Scholar]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wei, Y.L.; Huang, J.; Newell, E.W.; Yu, H.; Kidd, B.A.; Kuhns, M.S.; Waters, R.W.; Davis, M.M.; Weaver, C.T.; et al. γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 2012, 37, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 1999, 190, 1241–1256. [Google Scholar] [CrossRef]
- Wurbel, M.A.; Malissen, M.; Guy-Grand, D.; Meffre, E.; Nussenzweig, M.C.; Richelme, M.; Carrier, A.; Malissen, B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ(+) gut intraepithelial lymphocytes. Blood 2001, 98, 2626–2632. [Google Scholar] [CrossRef] [PubMed]
- Wurbel, M.-A.; Malissen, M.; Guy-Grand, D.; Malissen, B.; Campbell, J.J. Impaired Accumulation of Antigen-Specific CD8 Lymphocytes in Chemokine CCL25-Deficient Intestinal Epithelium and Lamina Propria. J. Immunol. 2007, 178, 7598–7606. [Google Scholar] [CrossRef]
- Suzuki, T.; Hayman, L.; Kilbey, A.; Edwards, J.; Coffelt, S.B. Gut γδ T cells as guardians, disruptors, and instigators of cancer. Immunol. Rev. 2020, 298, 198–217. [Google Scholar] [CrossRef]
- Olivares-Villagómez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Li, G.Q.; Xia, J.; Zeng, W.; Luo, W.; Liu, L.; Zeng, X.; Cao, D. The intestinal γδ T cells: Functions in the gut and in the distant organs. Front. Immunol. 2023, 14, 1206299. [Google Scholar]
- Wang, X.; Sumida, H.; Cyster, J.G. GPR18 is required for a normal CD8αα intestinal intraepithelial lymphocyte compartment. J. Exp. Med. 2014, 211, 2351–2359. [Google Scholar] [CrossRef]
- Becker, A.M.; Callahan, D.J.; Richner, J.M.; Choi, J.; DiPersio, J.F.; Diamond, M.S.; Bhattacharya, D. GPR18 Controls Reconstitution of Mouse Small Intestine Intraepithelial Lymphocytes following Bone Marrow Transplantation. PLoS ONE 2015, 10, e0133854. [Google Scholar] [CrossRef]
- Wu, J.; Song, Y.; Bakker, A.B.H.; Bauer, S.; Spies, T.; Lanier, L.L.; Phillips, J.H. An Activating Immunoreceptor Complex Formed by NKG2D and DAP10. Science 1999, 285, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK Cells and T Cells by NKG2D, a Receptor for Stress-Inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Rhinehart, R.; Secrist, H.; Bauer, S.; Grabstein, K.H.; Spies, T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc. Natl. Acad. Sci. USA 1999, 96, 6879–6884. [Google Scholar] [CrossRef] [PubMed]
- Das, H.; Groh, V.; Kuijl, C.; Sugita, M.; Morita, C.T.; Spies, T.; Bukowski, J.F. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity 2001, 15, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Steinle, A.; Bauer, S.; Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 1998, 279, 1737–1740. [Google Scholar] [CrossRef]
- Marlin, R.; Pappalardo, A.; Kaminski, H.; Willcox, C.R.; Pitard, V.; Netzer, S.; Khairallah, C.; Lomenech, A.-M.; Harly, C.; Bonneville, M.; et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 2017, 114, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Witherden, D.A.; Havran, W.L. EPCR: A stress trigger for γδ T cells. Nat. Immunol. 2012, 13, 812–814. [Google Scholar] [CrossRef]
- Ismail, A.S.; Severson, K.M.; Vaishnava, S.; Behrendt, C.L.; Yu, X.; Benjamin, J.L.; Ruhn, K.A.; Hou, B.; DeFranco, A.L.; Yarovinsky, F.; et al. γδ intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc. Natl. Acad. Sci. USA 2011, 108, 8743–8748. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, I.; Lee, H.K. γδ T Cells in Brain Homeostasis and Diseases. Front. Immunol. 2022, 13, 886397. [Google Scholar] [CrossRef]
- Hamada, S.; Umemura, M.; Shiono, T.; Tanaka, K.; Yahagi, A.; Begum, M.D.; Oshiro, K.; Okamoto, Y.; Watanabe, H.; Kawakami, K.; et al. IL-17A produced by γδ T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol 2008, 181, 3456–3463. [Google Scholar] [CrossRef]
- Martin, B.; Hirota, K.; Cua, D.J.; Stockinger, B.; Veldhoen, M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009, 31, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shibata, K.; Yamada, H.; Guo, Y.; Muta, H.; Podack, E.R.; Yoshikai, Y. CD30L/CD30 is critical for maintenance of IL-17A-producing γδ T cells bearing Vγ6 in mucosa-associated tissues in mice. Mucosal Immunol. 2013, 6, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, W.; Pan, M.; Scully, E.; Girardi, M.; Augenlicht, L.H.; Craft, J.; Yin, Z. γδ T cells provide an early source of interferon γ in tumor immunity. J. Exp. Med. 2003, 198, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Niikura, M.; Mineo, S.; Kobayashi, F. Roles of IFN-γ and γδ T Cells in Protective Immunity against Blood-Stage Malaria. Front. Immunol. 2013, 4, 258. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.J.; Ohtsuki, T.; Kohno, K.; Tanabe, F.; Ushio, S.; Namba, M.; Tanimoto, T.; Torigoe, K.; Fujii, M.; Ikeda, M.; et al. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: Synergism with interleukin-12 for interferon-γ production. Eur. J. Immunol. 1996, 26, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Simonian, P.L.; Wehrmann, F.; Roark, C.L.; Born, W.K.; O’Brien, R.L.; Fontenot, A.P. γδ T cells protect against lung fibrosis via IL-22. J. Exp. Med. 2010, 207, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.-M.E.; Fish, K.; Fu, Y.-X.; Zhou, L. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, K.; Fuchs, E.; Havran, W.L.; Boismenu, R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14338–14343. [Google Scholar] [CrossRef]
- Kolls, J.K.; McCray, P.B., Jr.; Chan, Y.R. Cytokine-mediated regulation of antimicrobial proteins. Nat. Rev. Immunol. 2008, 8, 829–835. [Google Scholar] [CrossRef]
- Sutton, C.E.; Mielke, L.A.; Mills, K.H. IL-17-producing γδ T cells and innate lymphoid cells. Eur. J. Immunol. 2012, 42, 2221–2231. [Google Scholar] [CrossRef]
- Mielke, L.A.; Jones, S.A.; Raverdeau, M.; Higgs, R.; Stefanska, A.; Groom, J.R.; Misiak, A.; Dungan, L.S.; Sutton, C.E.; Streubel, G.; et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 2013, 210, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Shires, J.; Theodoridis, E.; Hayday, A.C. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 2001, 15, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.D.; Golovchenko, N.B.; Burns, G.L.; Nair, P.M.; Kelly, T.J., IV; Agos, J.; Irani, M.Z.; Soh, W.S.; Zeglinski, M.R.; Lemenze, A.; et al. γδ Intraepithelial Lymphocytes Facilitate Pathological Epithelial Cell Shedding Via CD103-Mediated Granzyme Release. Gastroenterology 2022, 162, 877–889.e7. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Mao, H.; Zheng, J.; Sia, S.F.; Liu, Y.; Chan, P.L.; Lam, K.-T.; Peiris, J.S.M.; Lau, Y.-L.; Tu, W. Phosphoantigen-expanded human γδ T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J. Infect. Dis. 2009, 200, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, A.A.; Pawlowski, N.N.; Grollich, K.; Blessenohl, M.; Westermann, J.; Zeitz, M.; Loddenkemper, C.; Hoffmann, J.C. Human peripheral γδ T cells possess regulatory potential. Immunology 2009, 128, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Rampoldi, F.; Prinz, I. Three Layers of Intestinal γδ T Cells Talk Different Languages with the Microbiota. Front. Immunol. 2022, 13, 849954. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Olivares-Villagómez, D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, X.; Shibata, K.; Yamada, H.; Muta, H.; Podack, E.R.; Yoshikai, Y. CD30 is required for activation of a unique subset of interleukin-17A-producing γδ T cells in innate immunity against Mycobacterium bovis Bacillus Calmette-Guerin infection. Infect. Immun. 2013, 81, 3923–3934. [Google Scholar] [CrossRef]
- Boismenu, R.; Havran, W.L. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science 1994, 266, 1253–1255. [Google Scholar] [CrossRef]
- Fahrer, A.M.; Konigshofer, Y.; Kerr, E.M.; Ghandour, G.; Mack, D.H.; Davis, M.M.; Chien, Y.-H. Attributes of γδ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc. Natl. Acad. Sci. USA 2001, 98, 10261–10266. [Google Scholar] [CrossRef]
- Lodolce, J.P.; Burkett, P.R.; Koka, R.M.; Boone, D.L.; Ma, A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev. 2002, 13, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Wesch, D.; Peters, C.; Oberg, H.H.; Pietschmann, K.; Kabelitz, D. Modulation of γδ T cell responses by TLR ligands. Cell. Mol. Life Sci. 2011, 68, 2357–2370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Zeng, B.; Liu, L.; Tardivel, A.; Wei, H.; Han, J.; MacDonald, H.R.; Tschopp, J.; Tian, Z.; et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J. Exp. Med. 2013, 210, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, A.; Mota-Santos, T.; Itohara, S.; Degermann, S.; Heusser, C.; Tonegawa, S.; Coutinho, A. Localization of γ/δ T cells to the intestinal epithelium is independent of normal microbial colonization. J. Exp. Med. 1990, 172, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.M.; Cox, L.M.; Moreira, T.G.; Liu, S.; Boulenouar, S.; Dhang, F.; LeServe, D.S.; Nakagaki, B.N.; Lopes, J.R.; Tatematsu, B.K.; et al. Gamma-delta T cells modulate the microbiota and fecal micro-RNAs to maintain mucosal tolerance. Microbiome 2023, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell. Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Duan, J.; Chung, H.; Troy, E.; Kasper, D.L. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing γ/δ T cells. Cell Host Microbe 2010, 7, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Chen, I.B.; Pham, G.; Shao, T.Y.; Bangar, H.; Way, S.S.; Haslam, D.B. IL-17-producing γδ T cells protect against Clostridium difficile infection. J. Clin. Investig. 2020, 130, 2377–2390. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Hintz, M.; Reichenberg, A.; Altincicek, B.; Bahr, U.; Gschwind, R.M.; Kollas, A.K.; Beck, E.; Wiesner, J.; Eberl, M.; Jomaa, H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001, 509, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.; Cai, Y.; Sun, X.; Jala, V.R.; Xue, F.; Morrissey, S.; Wei, Y.-L.; Chien, Y.-H.; Zhang, H.-G.; Haribabu, B.; et al. Microbiota-activated CD103+ DCs stemming from microbiota adaptation specifically drive γδT17 proliferation and activation. Microbiome 2017, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c− Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005.e8. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef]
- Cha, H.-R.; Chang, S.-Y.; Chang, J.-H.; Kim, J.-O.; Yang, J.-Y.; Kim, C.-H.; Kweon, M.-N. Downregulation of Th17 Cells in the Small Intestine by Disruption of Gut Flora in the Absence of Retinoic Acid. J. Immunol. 2010, 184, 6799–6806. [Google Scholar] [CrossRef]
- Iyer, S.S.; Gensollen, T.; Gandhi, A.; Oh, S.F.; Neves, J.F.; Collin, F.; Lavin, R.; Serra, C.; Glickman, J.; de Silva, P.S.; et al. Dietary and Microbial Oxazoles Induce Intestinal Inflammation by Modulating Aryl Hydrocarbon Receptor Responses. Cell 2018, 173, 1123–1134.e11. [Google Scholar] [CrossRef] [PubMed]
- Barragan, L.C.; Chai, J.; Tianero, M.D.; Di Luccia, B.; Ahern, P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Baixauli, F.; Sanin, D.E.; Edwards-Hicks, J.; Villa, M.; Kabat, A.M.; Kamiński, M.M.; Stanckzak, M.; Weiss, H.J.; Grzes, K.M.; et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 2021, 184, 4186–4202.e20. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wang, Y.; Yu, C.; Qiu, R.; Jiang, Y.; Jia, J.; Tao, Z.; Zhang, L.; Zou, B.; Tang, D. Gut microbiota mediates the inhibition of lymphopoiesis in dietary-restricted mice by suppressing glycolysis. Gut Microbes 2022, 14, 2117509. [Google Scholar] [CrossRef] [PubMed]
- van Konijnenburg, D.P.H.; Reis, B.S.; Pedicord, V.A.; Farache, J.; Victora, G.D.; Mucida, D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell 2017, 171, 783–794.e13. [Google Scholar] [CrossRef]
- Semenkovich, N.P.; Planer, J.D.; Ahern, P.P.; Griffin, N.W.; Lin, C.Y.; Gordon, J.I. Impact of the gut microbiota on enhancer accessibility in gut intraepithelial lymphocytes. Proc. Natl. Acad. Sci. USA 2016, 113, 14805–14810. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Gut microbiota, immune development and function. Pharmacol. Res. 2013, 69, 87–113. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohloolyy, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ching, Y.-H.; Chiu, C.-C.; Liu, J.-Y.; Hung, S.-W.; Huang, W.-C.; Huang, Y.-T.; Chuang, H.-L. TLR2 and interleukin-10 are involved in Bacteroides fragilis-mediated prevention of DSS-induced colitis in gnotobiotic mice. PLoS ONE 2017, 12, e0180025. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Sukawa, Y.; Adachi, Y.; Ito, M.; Mitsuhashi, K.; Kurihara, H.; Kanno, S.; Yamamoto, I.; Ishigami, K.; Igarashi, H.; et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 2016, 22, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Oligschlaeger, Y.; Yadati, T.; Houben, T.; Oliván, C.M.C.; Shiri-Sverdlov, R. Inflammatory Bowel Disease: A Stressed “Gut/Feeling”. Cells 2019, 8, 659. [Google Scholar] [CrossRef]
- Vila, A.V.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- Sun, X.; He, S.; Lv, C.; Sun, X.; Wang, J.; Zheng, W.; Wang, D. Analysis of murine and human Treg subsets in inflammatory bowel disease. Mol. Med. Rep. 2017, 16, 2893–2898. [Google Scholar] [CrossRef]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Meisel, M.; Mayassi, T.; Fehlner-Peach, H.; Koval, J.C.; O’Brien, S.L.; Hinterleitner, R.; Lesko, K.; Kim, S.; Bouziat, R.; Chen, L.; et al. Interleukin-15 promotes intestinal dysbiosis with butyrate deficiency associated with increased susceptibility to colitis. ISME J. 2017, 11, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Heinken, A.; Ravcheev, D.A.; Baldini, F.; Heirendt, L.; Fleming, R.M.T.; Thiele, I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 2019, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M.J.; Comstock, L.E. Type VI Secretion Systems and the Gut Microbiota. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.; Hontecillas, R.; Tubau-Juni, N.; Zoccoli-Rodriguez, V.; Abedi, V.; Bassaganya-Riera, J. NLRX1 Modulates Immunometabolic Mechanisms Controlling the Host–Gut Microbiota Interactions during Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Naik, S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 2013, 14, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef]
- Watanabe, I.K.M.; Andrade-Silva, M.; Foresto-Neto, O.; Felizardo, R.J.F.; Matheus, M.A.C.; Silva, R.C.; Cenedeze, M.A.; Honda, T.S.B.; Perandini, L.A.B.; Volpini, R.A.; et al. Gut Microbiota and Intestinal Epithelial Myd88 Signaling Are Crucial for Renal Injury in UUO Mice. Front. Immunol. 2020, 11, 578623. [Google Scholar] [CrossRef]
- Brown, J.; Robusto, B.; Morel, L. Intestinal Dysbiosis and Tryptophan Metabolism in Autoimmunity. Front. Immunol. 2020, 11, 1741. [Google Scholar] [CrossRef] [PubMed]
- Coquant, G.; Grill, J.-P.; Seksik, P. Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity. Front. Immunol. 2020, 11, 1827. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Goehler, L.E.; Relton, J.K.; Tartaglia, N.; Silbert, L.; Martin, D.; Maier, S.F. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: Evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 1995, 183, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.K.; O’Connor, K.A.; Goehler, L.E.; Watkins, L.R.; Maier, S.F. The contribution of the vagus nerve in interleukin-1β-induced fever is dependent on dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R929–R934. [Google Scholar] [CrossRef]
- Baral, P.; Umans, B.D.; Li, L.; Wallrapp, A.; Bist, M.; Kirschbaum, T.; Wei, Y.; Zhou, Y.; Kuchroo, V.K.; Burkett, P.R.; et al. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 2018, 24, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Chesné, J.; Cardoso, V.; Veiga-Fernandes, H. Neuro-immune regulation of mucosal physiology. Mucosal Immunol. 2019, 12, 10–20. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Pachnis, V. Neuroimmune regulation during intestinal development and homeostasis. Nat. Immunol. 2017, 18, 116–122. [Google Scholar] [CrossRef]
- Brun, P.; Giron, M.C.; Qesari, M.; Porzionato, A.; Caputi, V.; Zoppellaro, C.; Banzato, S.; Grillo, A.R.; Spagnol, L.; De Caro, R.; et al. Toll-Like Receptor 2 Regulates Intestinal Inflammation by Controlling Integrity of the Enteric Nervous System. Gastroenterology 2013, 145, 1323–1333. [Google Scholar] [CrossRef]
- Barajon, I.; Serrao, G.; Arnaboldi, F.; Opizzi, E.; Ripamonti, G.; Balsari, A.; Rumio, C. Toll-like Receptors 3, 4, and 7 Are Expressed in the Enteric Nervous System and Dorsal Root Ganglia. J. Histochem. Cytochem. 2009, 57, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Matheis, F.; Muller, P.A.; Graves, C.L.; Gabanyi, I.; Kerner, Z.J.; Costa-Borges, D.; Ahrends, T.; Rosenstiel, P.; Mucida, D. Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell 2020, 180, 64–78.e16. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Hahn, P.; Kroehling, L.; Nguyen, H.; Li, D.; Littman, D.R. Feeding-dependent VIP neuron–ILC3 circuit regulates the intestinal barrier. Nature 2020, 579, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Powley, T.L. Innervation of the gastrointestinal tract: Patterns of aging. Auton. Neurosci. 2007, 136, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Silva, C.; Domingues, R.G.; Rendas, M.; Raposo, B.; Ribeiro, H.; da Silva, J.A.; Vieira, A.; Costa, R.M.; Barbosa-Morais, N.L.; Carvalho, T.; et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature 2019, 574, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.Y.; Musser, M.A.; Pinho-Ribeiro, F.A.; Baral, P.; Jacobson, A.; Ma, P.; Potts, D.E.; Chen, Z.; Paik, D.; Soualhi, S.; et al. Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 2020, 180, 33–49.e22. [Google Scholar] [CrossRef] [PubMed]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622.e23. [Google Scholar] [CrossRef]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell 2017, 168, 1135–1148.e12. [Google Scholar] [CrossRef]
- Yan, Y.; Ramanan, D.; Rozenberg, M.; McGovern, K.; Rastelli, D.; Vijaykumar, B.; Yaghi, O.; Voisin, T.; Mosaheb, M.; Chiu, I.; et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity 2021, 54, 499–513.e5. [Google Scholar] [CrossRef]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Shibata, S.; et al. The liver-brain-gut neural arc maintains the T(reg) cell niche in the gut. Nature 2020, 585, 591–596. [Google Scholar] [CrossRef]
- Di Giovangiulio, M.; Bosmans, G.; Meroni, E.; Stakenborg, N.; Florens, M.; Farro, G.; Gomez-Pinilla, P.J.; Matteoli, G.; Boeckxstaens, G.E. Vagotomy Affects the Development of Oral Tolerance and Increases Susceptibility to Develop Colitis Independently of α-7 Nicotinic Receptor. Mol. Med. 2016, 22, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Al-Barazie, R.M.; Bashir, G.H.; Qureshi, M.M.; Mohamed, Y.A.; Al-Sbiei, A.; Tariq, S.; Lammers, W.J.; Al-Ramadi, B.K.; Fernandez-Cabezudo, M.J. Cholinergic Activation Enhances Resistance to Oral Salmonella Infection by Modulating Innate Immune Defense Mechanisms at the Intestinal Barrier. Front. Immunol. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota–gut–brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior. PLoS Pathog. 2013, 9, e1003726. [Google Scholar] [CrossRef]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial neuroactive compounds produced by psychobiotics. Adv. Exp. Med. Biol. 2014, 817, 221–239. [Google Scholar]
- Schretter, C.E.; Vielmetter, J.; Bartos, I.; Marka, Z.; Marka, S.; Argade, S.; Mazmanian, S.K. A gut microbial factor modulates locomotor behaviour in Drosophila. Nature 2018, 563, 402–406. [Google Scholar] [CrossRef]
- Chen, W.; Ford, M.S.; Young, K.J.; Zhang, L. The role and mechanisms of double negative regulatory T cells in the suppression of immune responses. Cell. Mol. Immunol. 2004, 1, 328–335. [Google Scholar]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Golpour, F.; Abbasi-Alaei, M.; Babaei, F.; Mirzababaei, M.; Parvardeh, S.; Mohammadi, G.; Nassiri-Asl, M. Short chain fatty acids, a possible treatment option for autoimmune diseases. Biomed. Pharmacother. 2023, 163, 114763. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Dawin, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sakamoto, S.; Ishii, C.; Smith, M.D.; Ito, K.; Obayashi, M.; Unger, L.; Hasegawa, Y.; Kurokawa, S.; Kishimoto, T.; et al. Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nat. Immunol. 2023, 24, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, H. Metagenome-wide association studies: Fine-mining the microbiome. Nat. Rev. Microbiol. 2016, 14, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Shi, D.; Kong, C.; Liu, J.; Liu, G.; Li, X.; Ma, Y. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 2021, 12, 6757. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef]
- Xiang, Z.-B.; Xu, R.-S.; Zhu, Y.; Yuan, M.; Liu, Y.; Yang, F.; Chen, W.-Z.; Xu, Z.-Z. Association between inflammatory bowel diseases and Parkinson’s disease: Systematic review and meta-analysis. Neural Regen. Res. 2022, 17, 344–353. [Google Scholar] [CrossRef]
- Su, Y.; Liu, N.; Zhang, Z.; Li, H.; Ma, J.; Yuan, Y.; Shi, M.; Liu, J.; Zhao, Z.; Zhang, Z.; et al. Cholecystokinin and glucagon-like peptide-1 analogues regulate intestinal tight junction, inflammation, dopaminergic neurons and α-synuclein accumulation in the colon of two Parkinson’s disease mouse models. Eur. J. Pharmacol. 2022, 926, 175029. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Marta, D.; Dănău, A.; Lefter, A.; Tulbă, D.; Cozma, L.; Manole, E.; Gherghiceanu, M.; Ceafalan, L.C.; Popescu, B.O. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front. Neurosci. 2021, 15, 689723. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Del Tredici, K.; Braak, H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J. Neural. Transm. Suppl. 2006, 70, 99–103. [Google Scholar]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; Moloney, R.D.; Clarke, G.; Dinan, T.G.; Crya, J.F. The Impact of Microbiota on Brain and Behavior: Mechanisms & Therapeutic Potential. Adv. Exp. Med. Biol. 2014, 817, 373–403. [Google Scholar] [PubMed]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Daher, J.P. Interaction of LRRK2 and α-Synuclein in Parkinson’s Disease. Adv. Neurobiol. 2017, 14, 209–226. [Google Scholar] [CrossRef]
- Stolzenberg, E.; Berry, D.; Yang, D.; Lee, E.Y.; Kroemer, A.; Kaufman, S.; Wong, G.C.; Oppenheim, J.J.; Sen, S.; Fishbein, T.; et al. A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity. J. Innate Immun. 2017, 9, 456–463. [Google Scholar] [CrossRef]
- Piancone, F.; Saresella, M.; La Rosa, F.; Marventano, I.; Meloni, M.; Navarro, J.; Clerici, M. Inflammatory Responses to Monomeric and Aggregated α-Synuclein in Peripheral Blood of Parkinson Disease Patients. Front. Neurosci. 2021, 15, 639646. [Google Scholar] [CrossRef] [PubMed]
- de Lima, K.A.; Rustenhoven, J.; Kipnis, J. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. Annu. Rev. Immunol. 2020, 38, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the immune system: A focus on cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Alves de Lima, K.; Rustenhoven, J.; Da Mesquita, S.; Wall, M.; Salvador, A.F.; Smirnov, I.; Cebinelli, G.M.; Mamuladze, T.; Baker, W.; Papadopoulos, Z.; et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 2020, 21, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Brigas, H.C.; Temido-Ferreira, M.; Pousinha, P.A.; Regen, T.; Santa, C.; Coelho, J.E.; Marques-Morgado, I.; Valente, C.A.; Omenetti, S.; et al. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci. Immunol. 2019, 4, eaay5199. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Mohebiany, A.N.; Ramphal, N.S.; Karram, K.; Di Liberto, G.; Novkovic, T.; Klein, M.; Marini, F.; Kreutzfeldt, M.; Härtner, F.; Lacher, S.M.; et al. Microglial A20 Protects the Brain from CD8 T-Cell-Mediated Immunopathology. Cell Rep. 2020, 30, 1585–1597.e6. [Google Scholar] [CrossRef]
- Fiszer, U.; Mix, E.; Fredrikson, S.; Kostulas, V.; Olsson, T.; Link, H. γδ+ T cells are increased in patients with Parkinson’s disease. J. Neurol. Sci. 1994, 121, 39–45. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, X.; He, D.; Li, Z.; Xie, X.; Ren, Y. Reduction of Peripheral Blood iNKT and γδT Cells in Patients with Parkinson’s Disease: An Observational Study. Front. Immunol. 2020, 11, 1329. [Google Scholar] [CrossRef]
- Derkow, K.; Krüger, C.; Dembny, P.; Lehnardt, S. Microglia Induce Neurotoxic IL-17+ γδ T Cells Dependent on TLR2, TLR4, and TLR9 Activation. PLoS ONE 2015, 10, e0135898. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Pang, L.; Xie, G.; Welte, T.; Saxena, V.; Wicker, J.; Mann, B.; Soong, L.; Barrett, A.; et al. The co-stimulatory effects of MyD88-dependent Toll-like receptor signaling on activation of murine γδ T cells. PLoS ONE 2014, 9, e108156. [Google Scholar] [CrossRef] [PubMed]

| Gut Microbiota | Target Cells | Effect | References |
|---|---|---|---|
| Ruminococcus gnavus Akermansia muciniphilaas | γδ T cells | ↑ Oral tolerance | [86] |
| Phylum Firmicutes aerobic Bacteroidetes | γδ T cells | ↑ IL-17 | [91] |
| SFB (segmented filamentous bacteria) | γδ T cells | ↑ IL-17 ↑ IL-22 | [89] |
| E. coli Salmonella typhymurium | γδ T cells | ↑ Reg III | [58] |
| Commensal microbiota | γδ T cells | ↑ Mobility in the intestinal epithelium | [105] |
| Microbial metabolites (SCFAs, Propionate) | γδ T cells | ↓ IL-17 ↓ IL-22 | [90] |
| Phosphorylated microbial metabolites | γδ T cells | ↑ Cell activity | [40,93,94] |
| Clostridium spp. Bacteroid fragilis | Tregs | ↓ IL-10 | [87,88] |
| E. coli | NK T cells | ↑ Pro-inflammatory cytokines | [101] |
| Microbial metabolites (SCFAs, retinoid acid, polyamines, tryptophan derivatives) | T cells | ↑ T cell differentiation ↑ T cell activity | [96,97,98,99,100,101,102,103,104,105] |
| Commensal microbiota | IELs | ↑Chromatin accessibility | [106] |
| Lactobacillus reuteri | IELs | ↑ T cell differentiation | [102] |
| Lactobacillus, Bacteroides | IELs | ↑ Lymphopoiesis | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.A.; al-Ramadi, B.K.; Fernandez-Cabezudo, M.J. Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions. Int. J. Mol. Sci. 2024, 25, 1747. https://doi.org/10.3390/ijms25031747
Mohamed AA, al-Ramadi BK, Fernandez-Cabezudo MJ. Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions. International Journal of Molecular Sciences. 2024; 25(3):1747. https://doi.org/10.3390/ijms25031747
Chicago/Turabian StyleMohamed, Alaa A., Basel K. al-Ramadi, and Maria J. Fernandez-Cabezudo. 2024. "Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions" International Journal of Molecular Sciences 25, no. 3: 1747. https://doi.org/10.3390/ijms25031747
APA StyleMohamed, A. A., al-Ramadi, B. K., & Fernandez-Cabezudo, M. J. (2024). Interplay between Microbiota and γδ T Cells: Insights into Immune Homeostasis and Neuro-Immune Interactions. International Journal of Molecular Sciences, 25(3), 1747. https://doi.org/10.3390/ijms25031747





