Chondroitin Sulfate Proteoglycan 4 Provides New Treatment Approach to Preventing Peritoneal Dissemination in Ovarian Cancer
Abstract
1. Introduction
2. Results
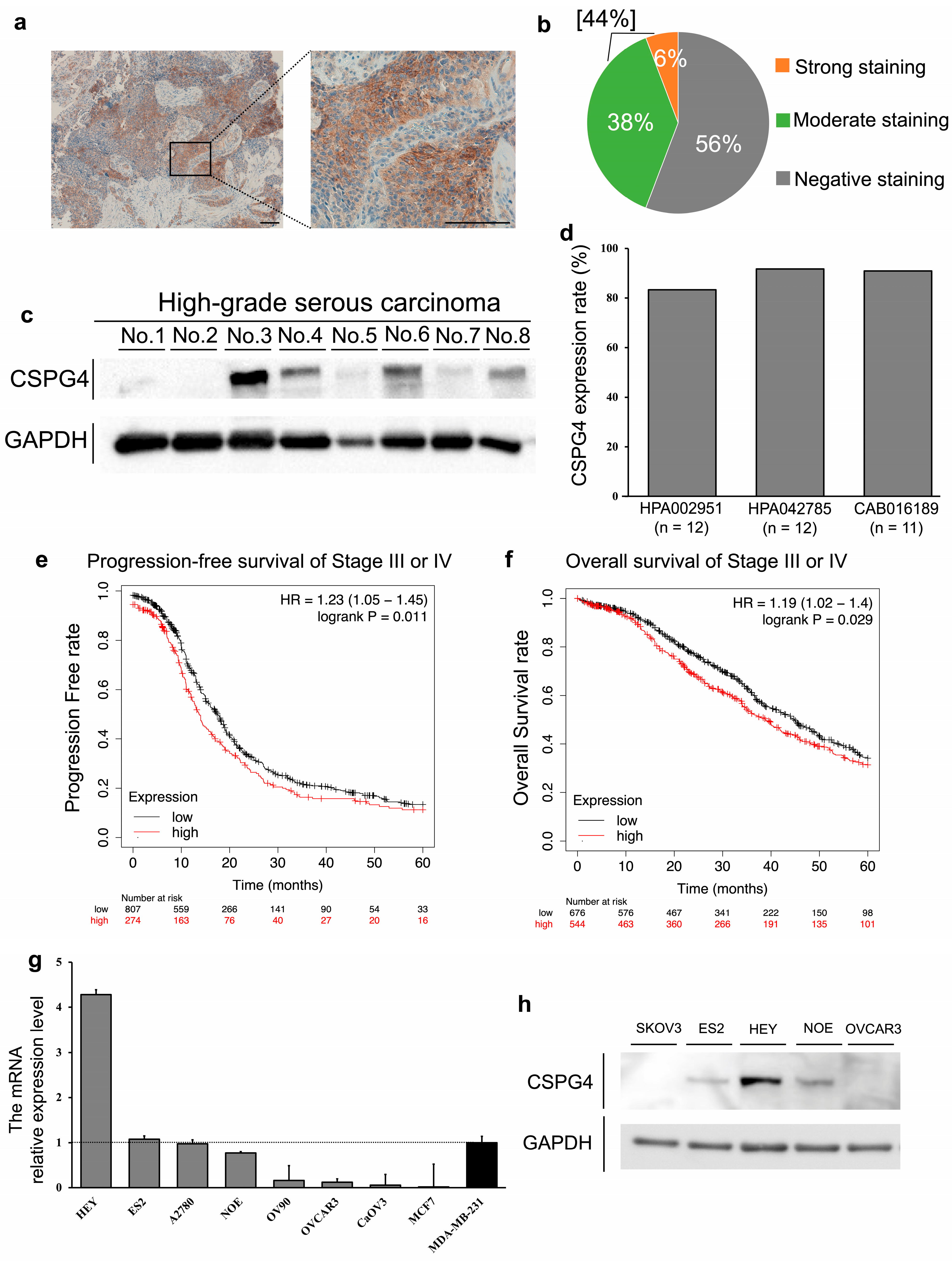
2.1. The Positive Expression of CSPG4 in Clinical Samples and Cell Lines of EOC
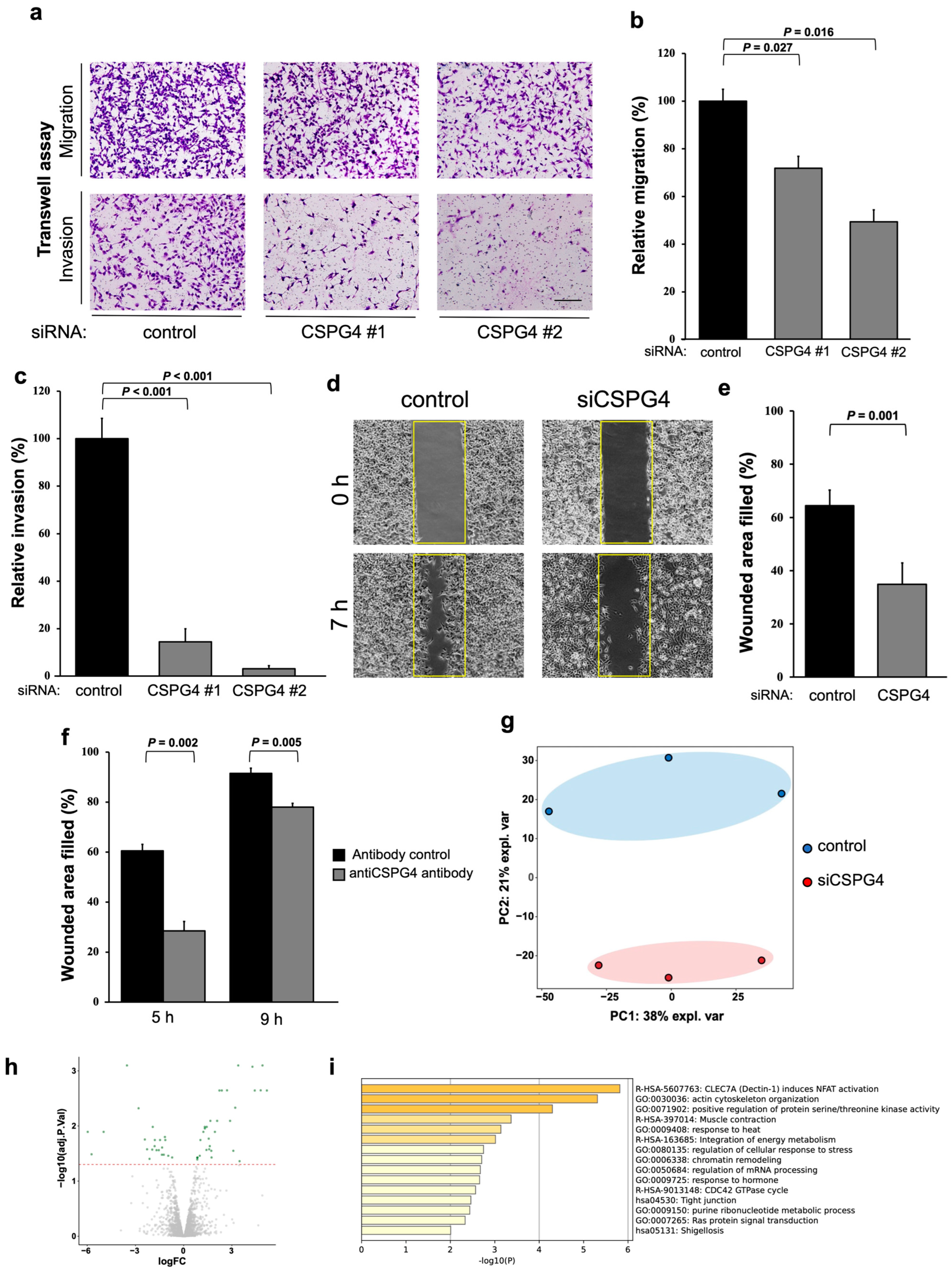
2.2. CSPG4 Downregulation Affects Invasion/Migration Abilities and Changes the Expression of Various Proteins in EOC Cells
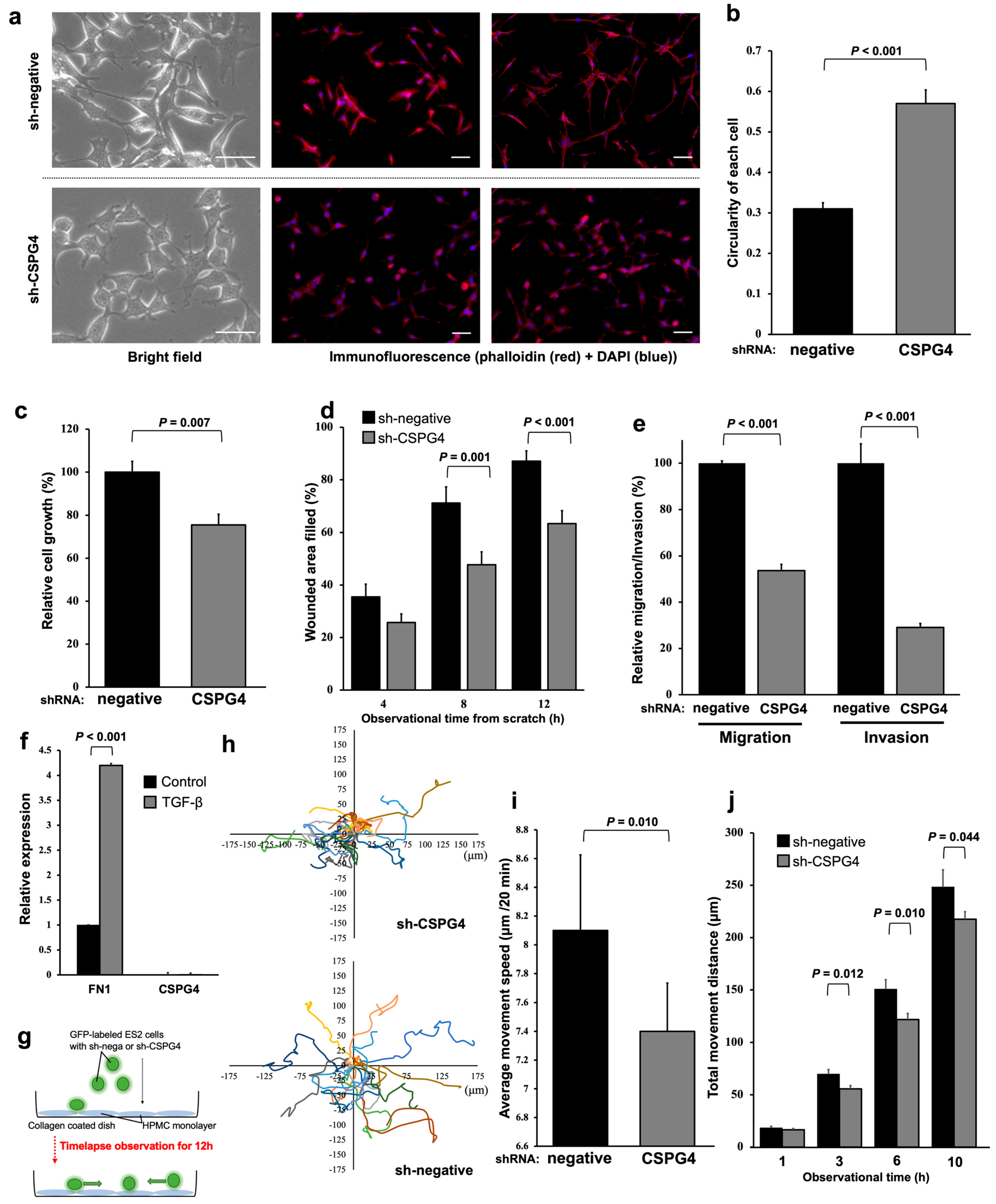
2.3. EOC Cells with CSPG4 Knockdown Have Morphological Changes and Decreased Interactions with Mesothelial Cells
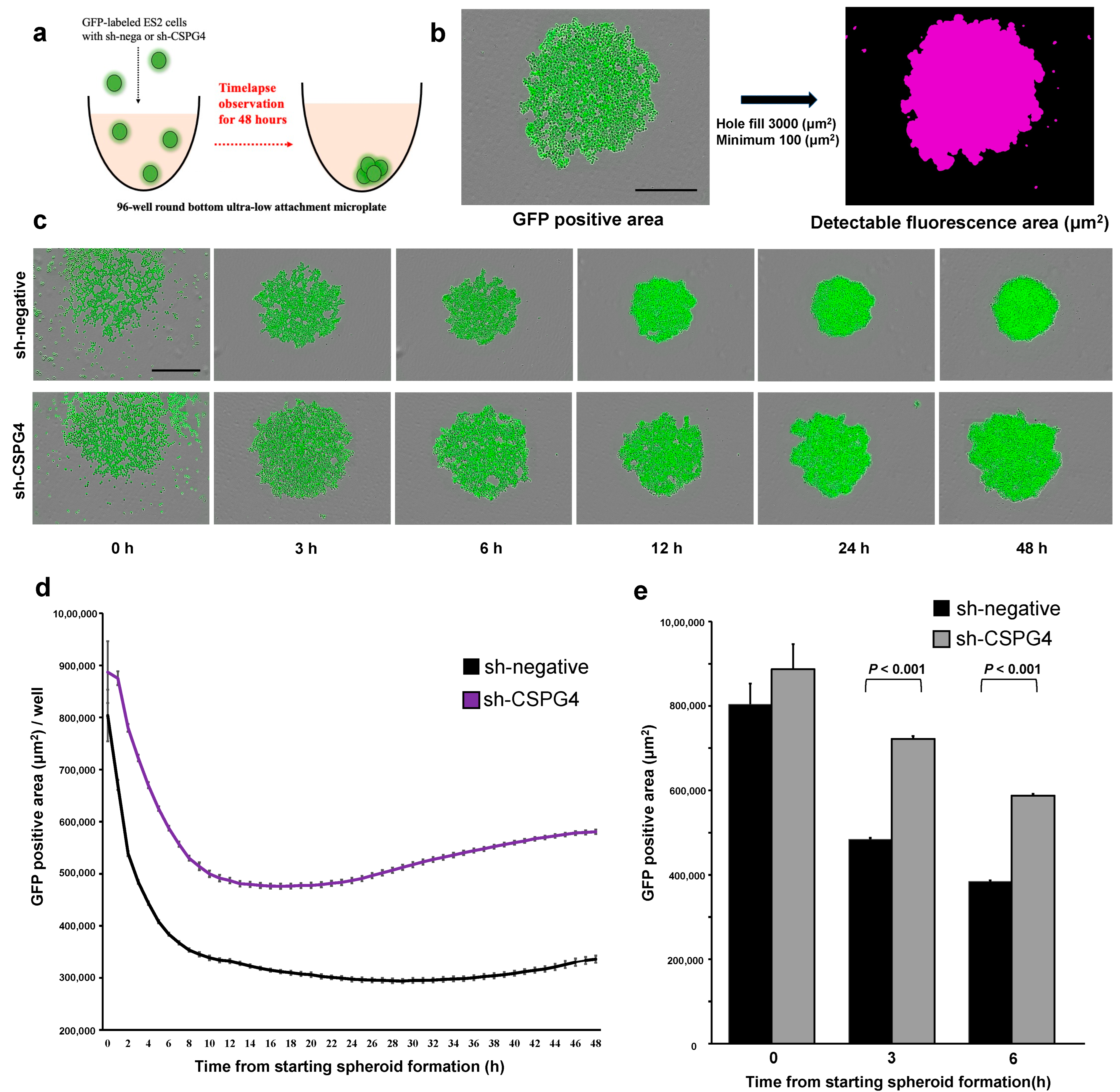
2.4. CSPG4 Downregulation Decreases the Ability to Form Spheroids
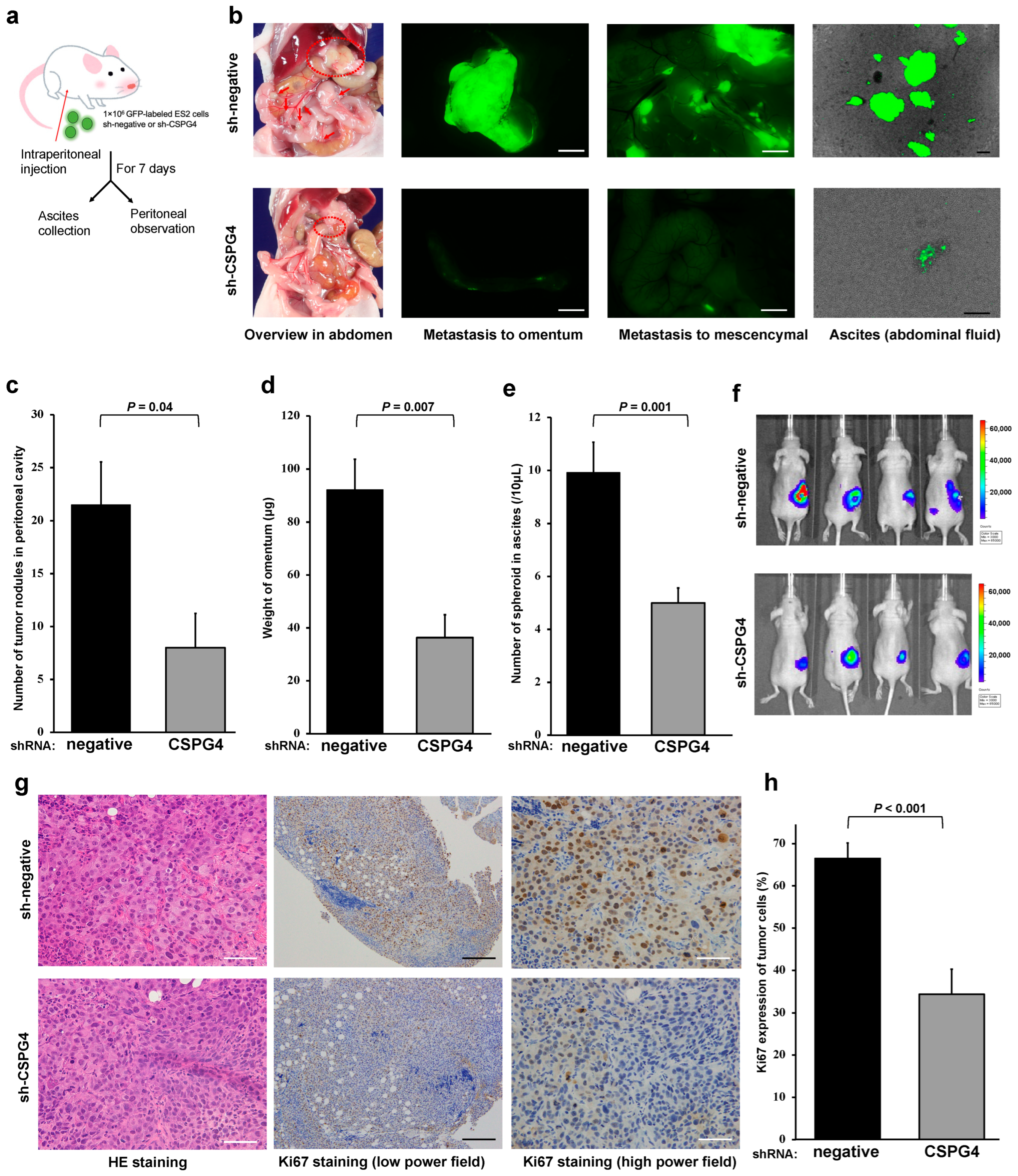
2.5. CSPG4 Is Related to Peritoneal Dissemination and Sphere Formation in Ascites of Mice
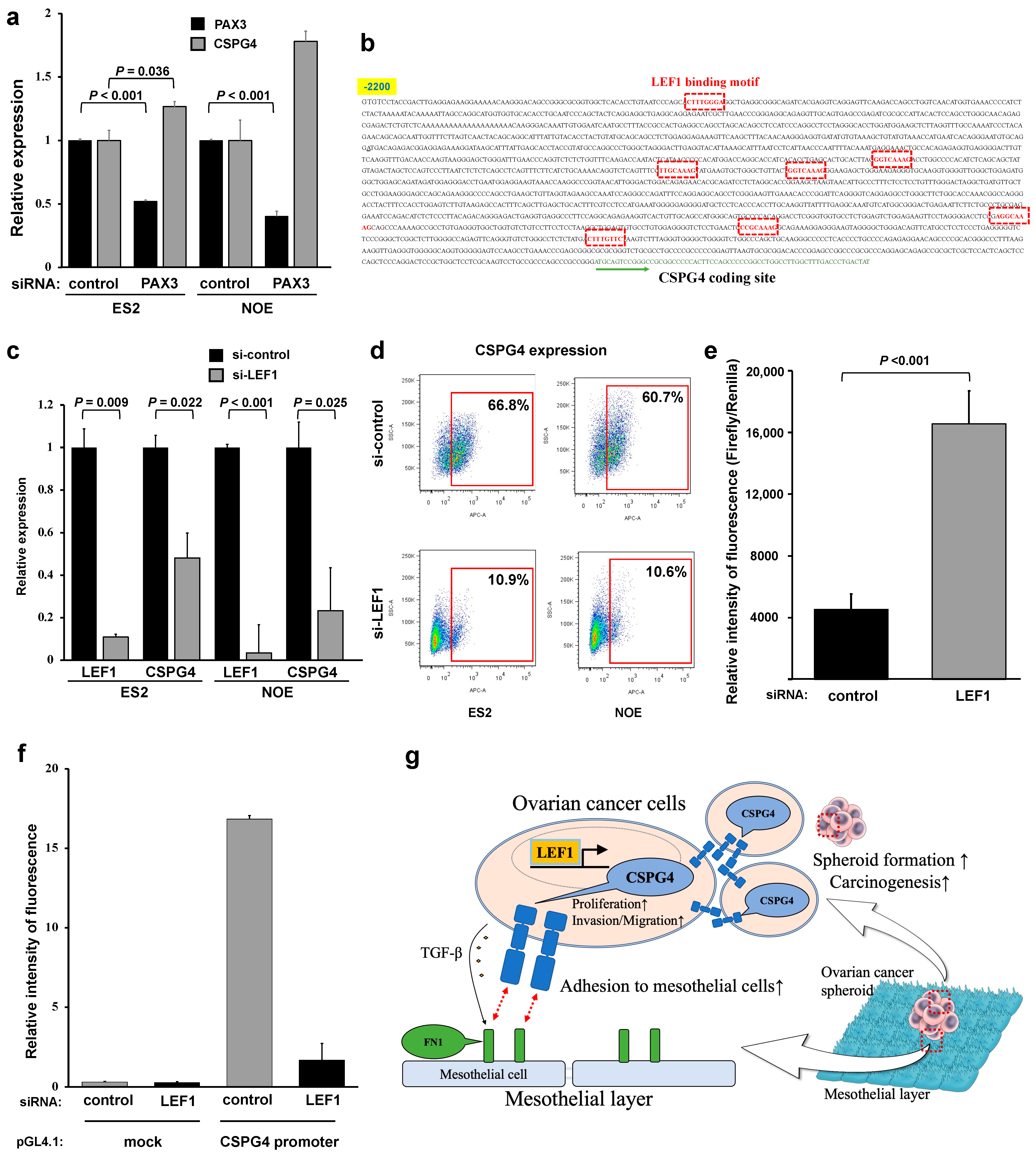
2.6. Lymphoid Enhancer-Binding Factor 1 (LEF1) Regulates CSPG4 Expression in EOC
3. Discussion
4. Materials and Methods
4.1. Patient Sample Collection
4.2. Immunohistochemistry
4.3. Western Blot Analysis
4.4. Public Data Analysis
4.5. Quantitative Real-Time PCR (qPCR)
4.6. Cell Lines
4.7. Knocked-Down CSPG4 Expression
4.8. Transwell Migration and Invasion Assays
4.9. Wound Healing
4.10. Proteomic Analysis
4.11. Immunofluorescence
4.12. Sphere Forming Assay
4.13. Mesothelial Migration Assay
4.14. Mouse Model Studies
4.15. Analysis of Cell Surface Markers
4.16. Promoter Luciferase Assays
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian Cancer Prevention and Screening. Obstet. Gynecol. 2018, 131, 909–927. [Google Scholar] [CrossRef]
- Hilliard, T.S. The impact of Mesothelin in the Ovarian cancer tumor microenvironment. Cancers 2018, 10, 277. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Uno, K.; Iyoshi, S.; Yoshihara, M.; Kitami, K.; Mogi, K.; Fujimoto, H.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Nawa, A.; et al. Metastatic Voyage of Ovarian Cancer Cells in Ascites with the Assistance of Various Cellular Components. Int. J. Mol. Sci. 2022, 23, 4383. [Google Scholar] [CrossRef]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. The regulatory mechanisms of NG2/CSPG4 expression. Cell Mol. Biol. Lett. 2017, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Pochechueva, T.; Jacob, F.; Fedier, A.; Heinzelmann-Schwarz, V. Tumor-associated glycans and their role in gynecological cancers: Accelerating translational research by novel high- throughput approaches. Metabolites 2012, 2, 913–939. [Google Scholar] [CrossRef] [PubMed]
- Rolih, V.; Barutello, G.; Iussich, S.; De Maria, R.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. CSPG4: A prototype oncoantigen for translational immunotherapy studies. J. Transl. Med. 2017, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Harrer, D.C.; Dörrie, J.; Schaft, N. Cspg4 as target for CAR-T-cell therapy of various tumor entities—Merits and challenges. Int. J. Mol. Sci. 2019, 20, 5942. [Google Scholar] [CrossRef] [PubMed]
- Rivera, Z.; Ferrone, S.; Wang, X.; Jube, S.; Yang, H.; Pass, H.I.; Kanodia, S.; Gaudino, G.; Carbone, M. CSPG4 as a Target of Antibody-Based Immunotherapy for Malignant Mesothelioma. Clin. Cancer Res. 2012, 18, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, A.; Verhoeff, J.J.C.; Immervoll, H.; Brøgger, J.C.; Kmiecik, J.; Poli, A.; Netland, N.A.; Prestegarden, L.; Planagumà, J.; Torsvik, A.; et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011, 122, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Mellai, M.; Boldorini, R.; Bisogno, I.; Grifoni, S.; Corona, C.; Bertero, L.; Cassoni, P.; Casalone, C.; Annovazzi, L. The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas. Int. J. Mol. Sci. 2018, 19, 2724. [Google Scholar] [CrossRef] [PubMed]
- Mayayo, S.L.; Prestigio, S.; Maniscalco, L.; Rosa, G.L.; Aricò, A.; Maria, R.D.; Cavallo, F.; Ferrone, S.; Buracco, P.; Iussich, S. Chondroitin sulfate proteoglycan-4: A biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet. J. 2011, 190, e26–e30. [Google Scholar] [CrossRef] [PubMed]
- Dye, D.E.; Medic, S.; Ziman, M.; Coombe, D.R. Melanoma biomolecules: Independently identified but functionally intertwined. Front. Oncol. 2013, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Price, M.A.; Wanshura, L.E.C.; Yang, J.; Carlson, J.; Xiang, B.; Li, G.; Ferrone, S.; Dudek, A.Z.; Turley, E.A.; McCarthy, J.B. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment. Cell Melanoma Res. 2011, 24, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Cooney, C.A.; Jousheghany, F.; Yao-Borengasser, A.; Phanavanh, B.; Gomes, T.; Kieber-Emmons, A.M.; Siegel, E.R.; Suva, L.J.; Ferrone, S.; Kieber-Emmons, T.; et al. Chondroitin sulfates play a major role in breast cancer metastasis: A role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 2011, 13, R58. [Google Scholar] [CrossRef] [PubMed]
- Tsidulko, A.Y.; Kazanskaya, G.M.; Kostromskaya, D.V.; Aidagulova, S.V.; Kiselev, R.S.; Volkov, A.M.; Kobozev, V.V.; Gaitan, A.S.; Krivoshapkin, A.L.; Grigorieva, E.V. Prognostic relevance of NG2/CSPG4, CD44 and Ki-67 in patients with glioblastoma. Tumor Biol. 2017, 39, 1010428317724282. [Google Scholar] [CrossRef]
- Amoury, M.; Mladenov, R.; Nachreiner, T.; Pham, A.T.; Hristodorov, D.; Fiore, S.D.; Helfrich, W.; Pardo, A.; Fey, G.; Schwenkert, M.; et al. A novel approach for targeted elimination of CSPG4-positive triple-negative breast cancer cells using a MAP tau-based fusion protein. Int. J. Cancer 2016, 139, 916–927. [Google Scholar] [CrossRef]
- Yang, J.; Liao, Q.; Price, M.; Moriarity, B.; Wolf, N.; Felices, M.; Miller, J.S.; Geller, M.A.; Bendzick, L.; Hopps, R.; et al. Chondroitin sulfate proteoglycan 4, a targetable oncoantigen that promotes ovarian cancer growth, invasion, cisplatin resistance and spheroid formation. Transl. Oncol. 2021, 16, 101318. [Google Scholar] [CrossRef]
- Yoshihara, M.; Kajiyama, H.; Yokoi, A.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Liu, W.; Nakamura, K.; Moriyama, Y.; Yasui, H.; et al. Ovarian cancer-associated mesothelial cells induce acquired platinum-resistance in peritoneal metastasis via the FN1/Akt signaling pathway. Int. J. Cancer 2020, 146, 2268–2280. [Google Scholar] [CrossRef]
- Yang, J.; Price, M.A.; Gui, Y.L.; Bar-Eli, M.; Salgia, R.; Jagedeeswaran, R.; Carlson, J.H.; Ferrone, S.; Turley, E.A.; McCarthy, J.B. Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition. Cancer Res. 2009, 69, 7538–7547. [Google Scholar] [CrossRef] [PubMed]
- Koya, Y.; Liu, W.; Yamakita, Y.; Senga, T.; Shibata, K.; Yamashita, M.; Nawa, A.; Kikkawa, F.; Kajiyama, K. Hematopoietic lineage cell-specific protein 1 (HS1), a hidden player in migration, invasion, and tumor formation, is overexpressed in ovarian carcinoma cells. Oncotarget 2018, 9, 32609–32623. [Google Scholar] [CrossRef] [PubMed]
- Kitami, K.; Yoshihara, M.; Koya, Y.; Sugiyama, M.; Iyoshi, S.; Uno, K.; Mogi, K.; Tano, S.; Fujimoto, H.; Nawa, A.; et al. Microphthalmia-associated transcription factor-dependent melanoma cell adhesion molecule activation promotes peritoneal metastasis of ovarian cancer. Int. J. Mol. Sci. 2020, 21, 9776. [Google Scholar] [CrossRef]
- Shahrokh, S.; Mansouri, V.; Razzaghi, M. Assessment of the SRC inhibition role in the efficacy of breast cancer radiotherapy. J. Lasers Med. Sci. 2019, 10, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J. Clin. Investig. 2021, 131, e146186. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef]
- Iida, J.; Meijne, A.M.; Spiro, R.C.; Roos, E.; Furcht, L.T.; McCarthy, J.B. Spreading and Focal Contact Formation of Human Melanoma Cells in Response to the Stimulation of Both Melanoma-associated Proteoglycan (NG2) and α4β1 Integrin. Cancer Res. 1995, 55, 2177–2185. [Google Scholar]
- Iida, J.; Skubitz, A.P.; Furcht, L.T.; Wayner, E.A.; J B McCarthy, J.B. Coordinate role for cell surface chondroitin sulfate proteoglycan and alpha 4 beta 1 integrin in mediating melanoma cell adhesion to fibronectin. J. Cell Biol. 1992, 118, 431–444. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, J.-Y.; Choi, J.-H.; Kim, J.-H.; Lee, C.-J.; Singh, P.; Sarkar, S.; Baek, J.-H.; Nam, J.-S. Inhibition of LEF1-Mediated DCLK1 by Niclosamide Attenuates Colorectal Cancer Stemness. Clin. Cancer Res. 2019, 25, 1415–1429. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.; Ye, T.; Ye, Z. High expression of LEF1 correlates with poor prognosis in solid tumors, but not blood tumors: A meta-analysis. Biosci. Rep. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Schmoeckel, E.; Odai-Afotey, A.A.; Schleißheimer, M.; Rottmann, M.; Flesken-Nikitin, A.; Ellenson, L.H.; Kirchner, T.; Mayr, D.; Nikitin, A.Y. LEF1 is preferentially expressed in the tubal-peritoneal junctions and is a reliable marker of tubal intraepithelial lesions. Mod. Pathol. 2017, 30, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Price, M.A.; Wanushura, L.E.; He, J.; Yi, M.; Welch, D.R.; Li, G.; Conner, S.; Sachs, J.; Turley, E.A.; et al. Chondroitin sulfate proteoglycan 4 enhanced melanoma motility and growth requires a cysteine in the core protein transmembrane domain. Melanoma Res. 2019, 29, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Mellai, M.; Casalone, C.; Corona, C.; Crociara, P.; Favole, A.; Cassoni, P.; Schiffer, D.; Boldorini, R. Chondroitin Sulphate Proteoglycans in the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1272, 73–92. [Google Scholar] [PubMed]
- Levy, A.; Alhazzani, K.; Dondapati, P.; Alaseem, A.; Cheema, K.; Thallapureddy, K.; Kaur, P.; Alobid, S.; Rathinavelu, A. Focal Adhesion Kinase in Ovarian Cancer: A Potential Therapeutic Target for Platinum and Taxane-Resistant Tumors. Curr. Cancer Drug Targets 2019, 19, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.; Ren, C. Focal adhesion kinase inhibitor BI 853520 inhibits cell proliferation, migration and EMT process through PI3K/AKT/mTOR signaling pathway in ovarian cancer. Discov. Oncol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Yousif, N.G. Fibronectin promotes migration and invasion of ovarian cancer cells through up-regulation of FAK-PI3K/Akt pathway. Cell Biol. Int. 2014, 38, 85–91. [Google Scholar] [CrossRef]
- Leuci, V.; Donini, C.; Grignani, G.; Rotolo, R.; Mesiano, G.; Fiorino, E.; Gammaitoni, L.; D’Ambrosio, L.; Merlini, A.; Landoni, E.; et al. CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes. Clin. Cancer Res. 2020, 26, 6321–6334. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.W.; et al. The prioritization of cancer antigens: A National Cancer Institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Zheng, C.; Yang, J.; Ding, S.; Tian, C.; Xie, N.; Xue, L.; Wu, M.; Fu, S.; Rao, Z.; et al. Co-Expression and Combined Prognostic Value of CSPG4 and PDL1 in TP53-Aberrant Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 804466. [Google Scholar] [CrossRef]
- Kasten, B.B.; Oliver, P.G.; Kim, H.; Fan, J.; Ferrone, S.; Zinn, K.R.; Buchsbaum, D.J. 212Pb-Labeled Antibody 225.28 Targeted to Chondroitin Sulfate Proteoglycan 4 for Triple-Negative Breast Cancer Therapy in Mouse Models. Int. J. Mol. Sci. 2018, 19, 925. [Google Scholar] [CrossRef]
- Barnes, B.M.; Nelson, L.; Tighe, A.; Burghel, G.J.; Lin, I.H.; Desai, S.; McGrail, J.C.; Morgan, R.D.; Taylor, S.S. Distinct transcriptional programs stratify ovarian cancer cell lines into the five major histological subtypes. Genome Med. 2021, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Warta, R.; Herold-Mende, C.; Chaisaingmongkol, J.; Popanda, O.; Mock, A.; Mogler, C.; Osswald, F.; Herpel, E.; Küstner, S.; Eckstein, V.; et al. Reduced promoter methylation and increased expression of CSPG4 negatively influences survival of HNSCC patients. Int. J. Cancer 2014, 135, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Kajiyama, H.; Terauchi, M.; Shibata, K.; Ino, K.; Nawa, A.; Kikkawa, F. Establishment of a new cell line of endometrioid carcinoma of the ovary and its chemosensitivity. Hum. Cell 2007, 20, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Eng, M.S.; Kaur, J.; Prasmickaite, L.; Engesæter, B.Ø.; Weyergang, A.; Skarpen, E.; Berg, K.; Rosenblum, M.G.; Mælandsmo, G.M.; Høgset, A.; et al. Enhanced targeting of triple-negative breast carcinoma and malignant melanoma by photochemical internalization of CSPG4-targeting immunotoxins. Photochem. Photobiol. Sci. 2018, 17, 539–551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uno, K.; Koya, Y.; Yoshihara, M.; Iyoshi, S.; Kitami, K.; Sugiyama, M.; Miyamoto, E.; Mogi, K.; Fujimoto, H.; Yamakita, Y.; et al. Chondroitin Sulfate Proteoglycan 4 Provides New Treatment Approach to Preventing Peritoneal Dissemination in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 1626. https://doi.org/10.3390/ijms25031626
Uno K, Koya Y, Yoshihara M, Iyoshi S, Kitami K, Sugiyama M, Miyamoto E, Mogi K, Fujimoto H, Yamakita Y, et al. Chondroitin Sulfate Proteoglycan 4 Provides New Treatment Approach to Preventing Peritoneal Dissemination in Ovarian Cancer. International Journal of Molecular Sciences. 2024; 25(3):1626. https://doi.org/10.3390/ijms25031626
Chicago/Turabian StyleUno, Kaname, Yoshihiro Koya, Masato Yoshihara, Shohei Iyoshi, Kazuhisa Kitami, Mai Sugiyama, Emiri Miyamoto, Kazumasa Mogi, Hiroki Fujimoto, Yoshihiko Yamakita, and et al. 2024. "Chondroitin Sulfate Proteoglycan 4 Provides New Treatment Approach to Preventing Peritoneal Dissemination in Ovarian Cancer" International Journal of Molecular Sciences 25, no. 3: 1626. https://doi.org/10.3390/ijms25031626
APA StyleUno, K., Koya, Y., Yoshihara, M., Iyoshi, S., Kitami, K., Sugiyama, M., Miyamoto, E., Mogi, K., Fujimoto, H., Yamakita, Y., Wang, X., Nawa, A., & Kajiyama, H. (2024). Chondroitin Sulfate Proteoglycan 4 Provides New Treatment Approach to Preventing Peritoneal Dissemination in Ovarian Cancer. International Journal of Molecular Sciences, 25(3), 1626. https://doi.org/10.3390/ijms25031626




