Bioactive Bismuth Compounds: Is Their Toxicity a Barrier to Therapeutic Use?
Abstract
1. Introduction
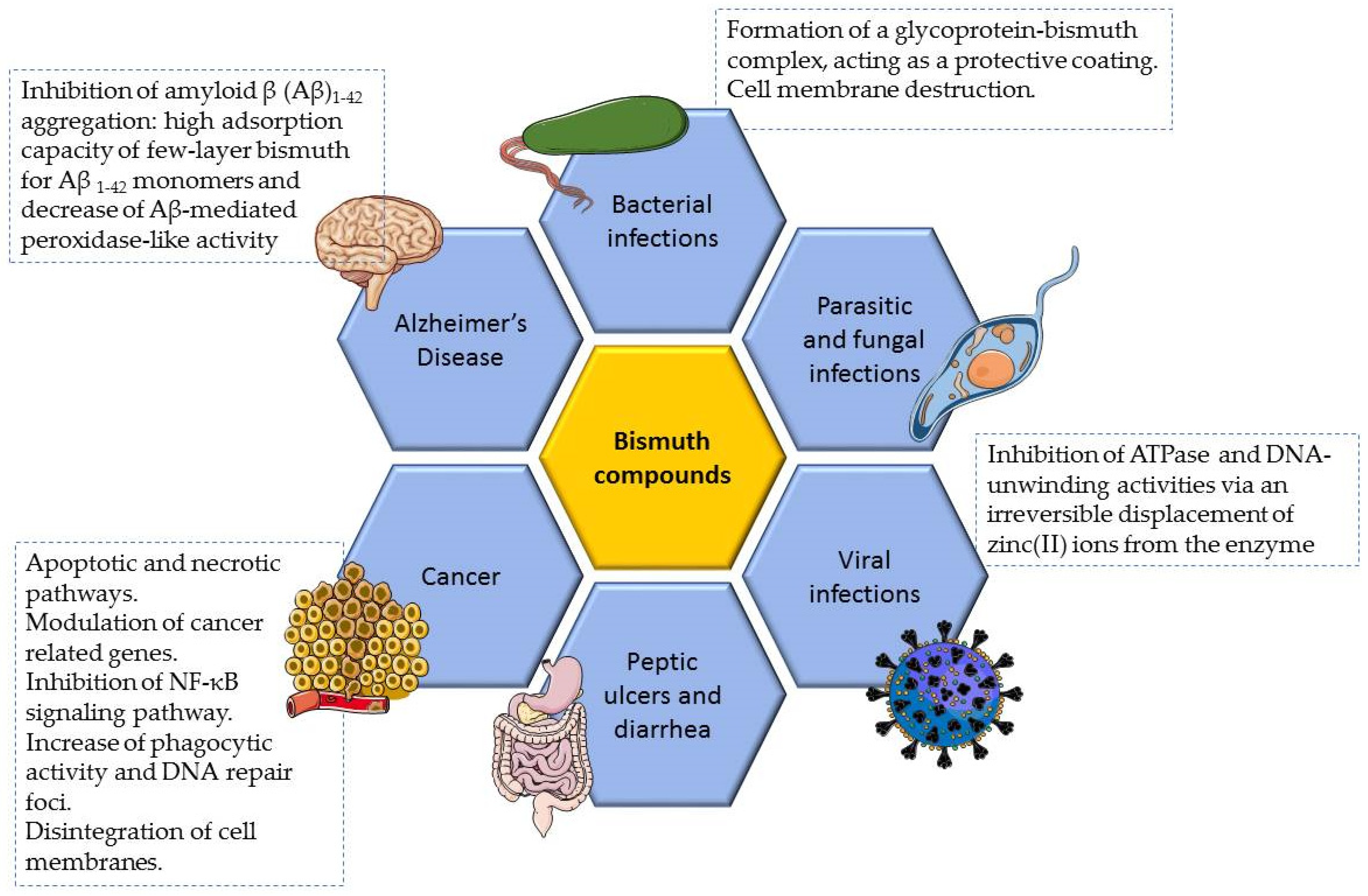
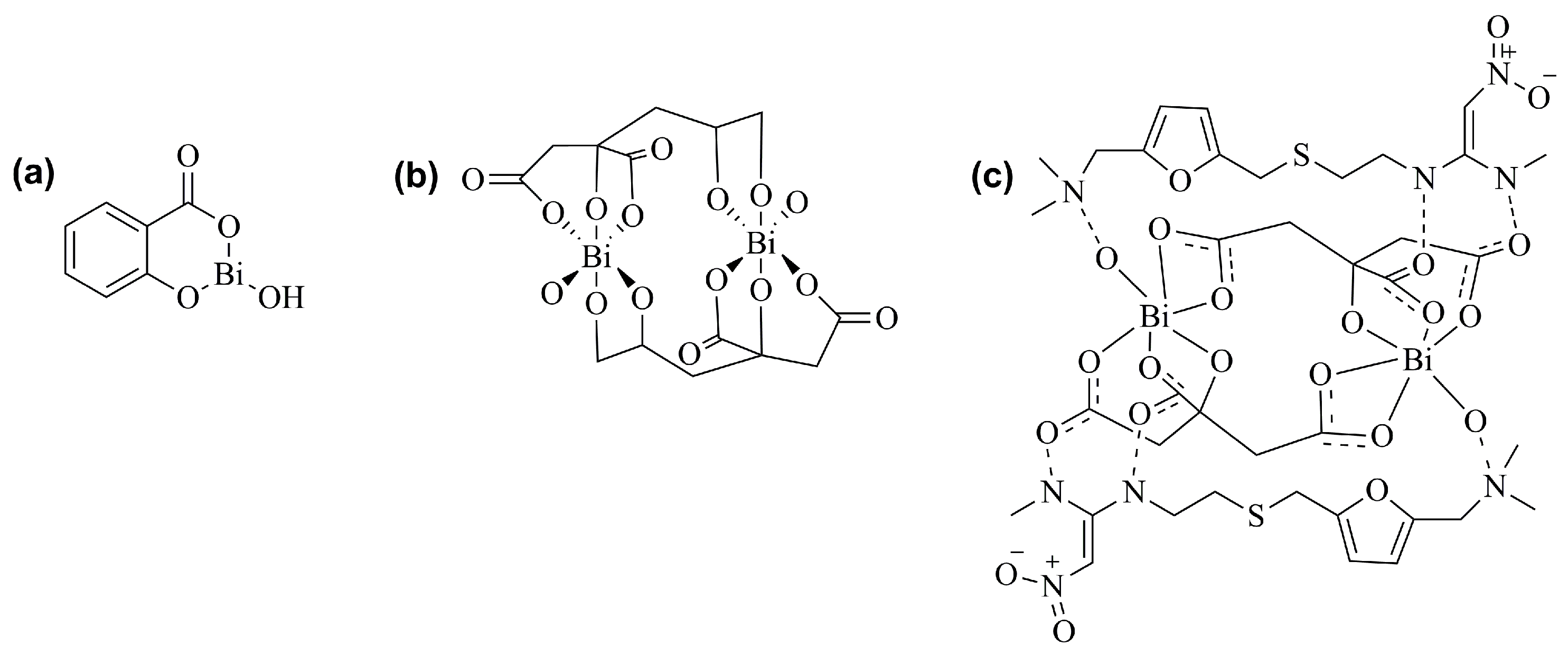
2. Bismuth Compounds with Therapeutic Properties
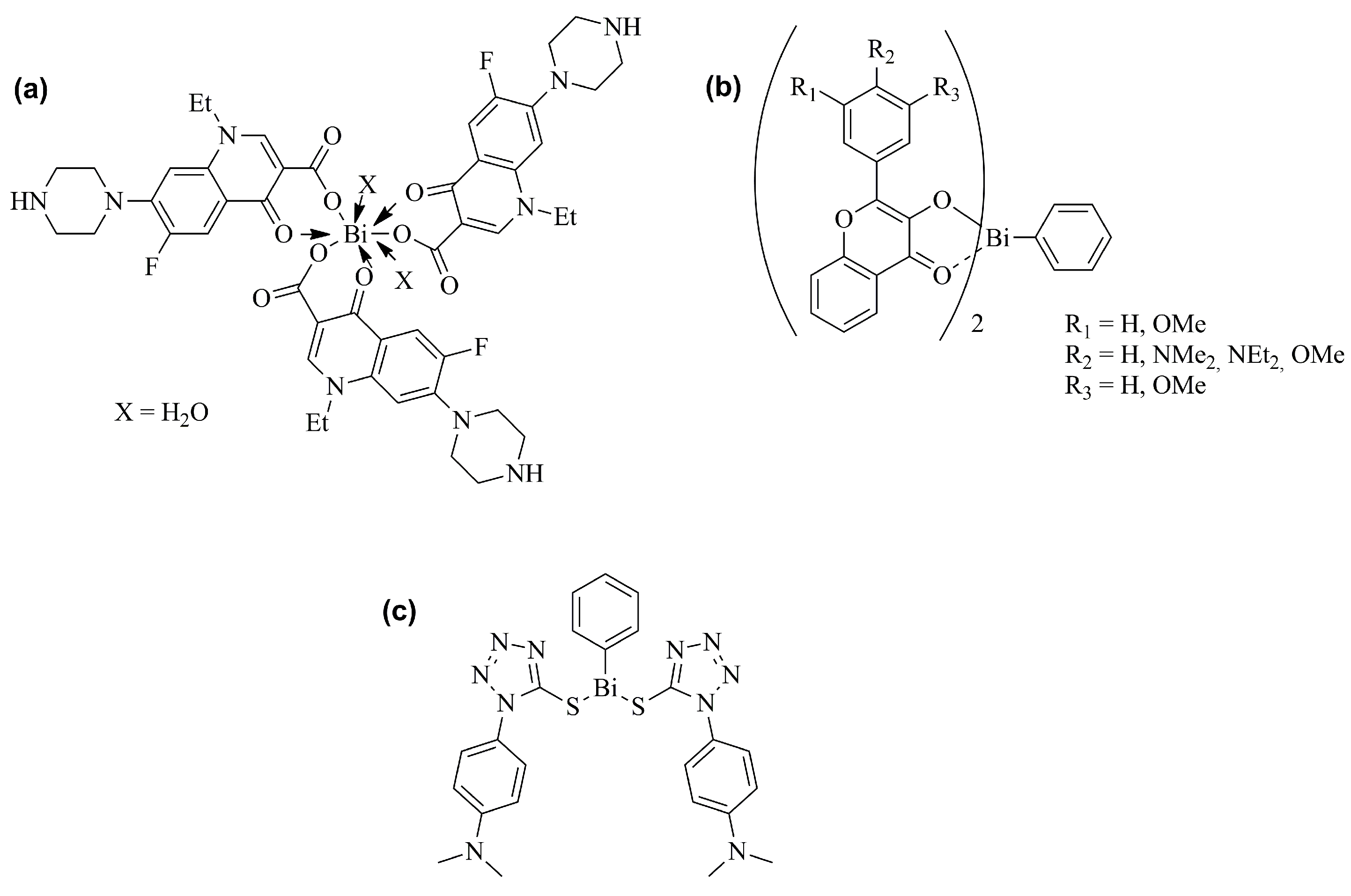
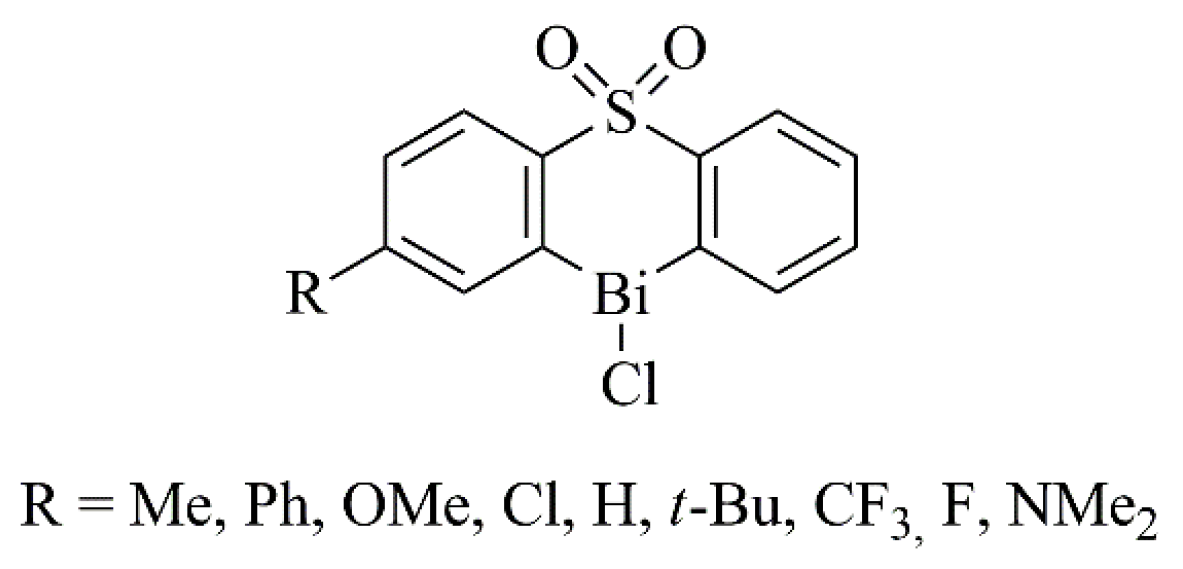
2.1. Antiulcer and Anti-Infective Effects
2.2. Antitumor Effects
2.3. Other Properties
3. Bismuth Toxicity
3.1. Preclinical Studies
3.1.1. In Vitro Studies
3.1.2. In Vivo Studies
3.2. Clinical Evidences
3.2.1. Toxicity after Systemic Inadequate Use of Bismuth-Based Drugs
3.2.2. Toxicity after Local Application of Bismuth Iodoform Paraffin Paste
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohan, R. Green Bismuth. Nat. Chem. 2010, 2, 336. [Google Scholar] [CrossRef] [PubMed]
- Silvestru, C.; Breunig, H.J.; Althaus, H. Structural Chemistry of Bismuth Compounds. I. Organobismuth Derivatives. Chem. Rev. 1999, 99, 3277–3327. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ogawa, T. Organobismuth(III) Compounds. In Organobismuth Chemistry; Elsevier: Amsterdam, The Netherlands, 2001; pp. 21–245. [Google Scholar]
- Matias, M.; Campos, G.; Santos, A.O.; Falcão, A.; Silvestre, S.; Alves, G. Potential Antitumoral 3,4-Dihydropyrimidin-2-(1H)-Ones: Synthesis, in Vitro Biological Evaluation and QSAR Studies. RSC Adv. 2016, 6, 84943–84958. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Moreira, V.M.; Pinto, R.M.A.; Leal, A.S.; Le Roux, C. Bismuth(III) Triflate-Based Catalytic Direct Opening of Oleanolic Hydroxy-γ-Lactones to Afford 12-Oxo-28-Carboxylic Acids. Adv. Synth. Catal 2011, 353, 2637–2642. [Google Scholar] [CrossRef]
- Pinto, R.M.A.; Salvador, J.A.R.; Le Roux, C.; Paixão, J.A. Bismuth(III) Triflate-Catalyzed Direct Conversion of Corticosteroids into Highly Functionalized 17-Ketosteroids by Cleavage of the C17-Dihydroxyacetone Side Chain. J. Org. Chem. 2009, 74, 8488–8491. [Google Scholar] [CrossRef]
- Pinto, R.M.A.; Salvador, J.A.R.; Le Roux, C.; Carvalho, R.A.; Beja, A.M.; Paixão, J.A. Bismuth(III) Triflate-Catalyzed Rearrangement of 16α,17α-Epoxy-20-Oxosteroids. Synthesis and Structural Elucidation of New 16α-Substituted 17α-Alkyl-17β-Methyl-Δ13-18-Norsteroids. Tetrahedron 2009, 65, 6169–6178. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Silvestre, S.M. Bismuth-Catalyzed Allylic Oxidation Using t-Butyl Hydroperoxide. Tetrahedron Lett. 2005, 46, 2581–2584. [Google Scholar] [CrossRef]
- Matias, M.; Campos, G.; Silvestre, S.; Falcão, A.; Alves, G. Early Preclinical Evaluation of Dihydropyrimidin(Thi)Ones as Potential Anticonvulsant Drug Candidates. Eur. J. Pharm. Sci. 2017, 102, 264–274. [Google Scholar] [CrossRef]
- Gorbach, S.L. Bismuth Therapy in Gastrointestinal Diseases. Gastroenterology 1990, 99, 863–875. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Benfield, P.; Monk, J.P. Colloidal Bismuth Subcitrate. A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Its Therapeutic Use in Peptic Ulcer Disease. Drugs 1988, 36, 132–157. [Google Scholar] [CrossRef]
- Oliver, T.E.; Piantavigna, S.; Andrews, P.C.; Holt, S.A.; Dillon, C.T. Interactions of Non-Steroidal Anti-Inflammatory Drugs and Their Bismuth Analogues (BiNSAIDs) with Biological Membrane Mimics at Physiological PH. Langmuir 2021, 37, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, H. Recent Advances in Bioinorganic Chemistry of Bismuth. Curr. Opin. Chem. Biol. 2012, 16, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Von Recklinghausen, U.; Hartmann, L.M.; Rabieh, S.; Hippler, J.; Hirner, A.V.; Rettenmeier, A.W.; Dopp, E. Methylated Bismuth, but Not Bismuth Citrate or Bismuth Glutathione, Induces Cyto- and Genotoxic Effects in Human Cells in Vitro. Chem. Res. Toxicol. 2008, 21, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, H.; Sun, H. Bismuth: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J.B.T.-E., Ed.; Elsevier: Oxford, UK, 2019; pp. 415–423. ISBN 978-0-444-63952-3. [Google Scholar]
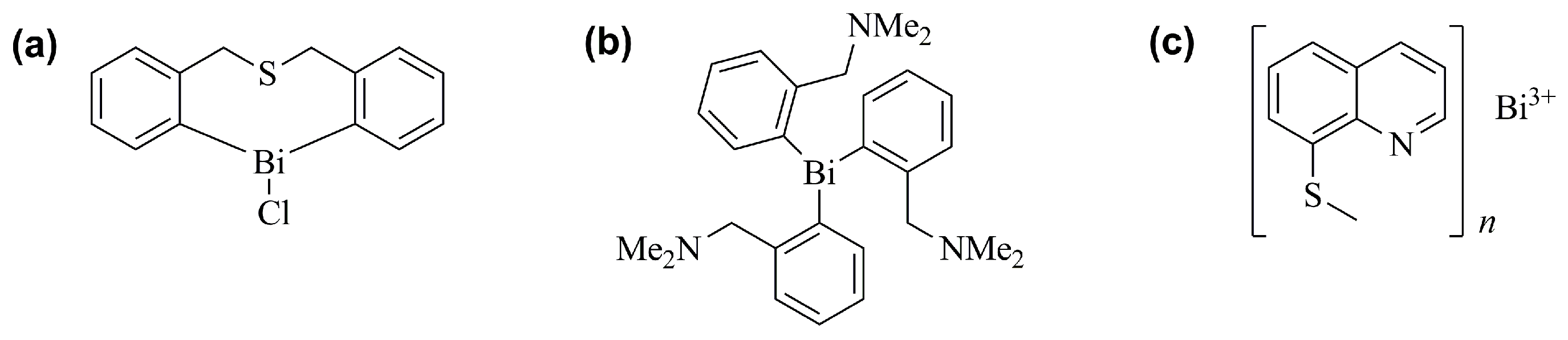
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal Chemistry and Biomedical Applications of Bismuth-Based Compounds and Nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef] [PubMed]
- Badrigilan, S.; Heydarpanahi, F.; Choupani, J.; Jaymand, M.; Samadian, H.; Hoseini-Ghahfarokhi, M.; Webster, T.J.; Tayebi, L. A Review on the Biodistribution, Pharmacokinetics and Toxicity of Bismuth-Based Nanomaterials. Int. J. Nanomed. 2020, 15, 7079–7096. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.B. Bismuth. In Goldfrank’s Toxicologic Emergencies, 10th ed.; Hoffman, R.S., Howland, M.A., Lewin, N.A., Nelson, L.S., Goldfrank, L.R., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Xin, Y.; Wang, Z.; Yao, C.; Shen, H.; Miao, Y. Bismuth, a Previously Less-Studied Element, Is Bursting into New Hotspots. ChemistrySelect 2022, 7, e202201220. [Google Scholar] [CrossRef]
- Lopez, E.; Thorp, S.C.; Mohan, R.S. Bismuth(III) Compounds as Catalysts in Organic Synthesis: A Mini Review. Polyhedron 2022, 222, 115765. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Ip, T.K.-Y.; Sun, H. Chapter Six—Bismuth Drugs as Antimicrobial Agents. In Advances in Inorganic Chemistry; Sadler, P.J., van Eldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 183–205. ISBN 0898-8838. [Google Scholar]
- Işlek, I.; Uysal, S.; Gök, F.; Dündaröz, R.; Küçüködük, Ş. Reversible Nephrotoxicity after Overdose of Colloidal Bismuth Subcitrate. Pediatr. Nephrol. 2001, 16, 510–514. [Google Scholar] [CrossRef]
- Cengiz, N.; Uslu, Y.; Gök, F.; Anarat, A. Acute Renal Failure after Overdose of Colloidal Bismuth Subcitrate. Pediatr. Nephrol. 2005, 20, 1355–1358. [Google Scholar] [CrossRef]
- Erden, A.; Karahan, S.; Bulut, K.; Basak, M.; Aslan, T.; Cetinkaya, A.; Karagoz, H.; Avci, D. A Case of Bismuth Intoxication with Irreversible Renal Damage. Int. J. Nephrol. Renovasc. Dis. 2013, 6, 241–243. [Google Scholar] [CrossRef][Green Version]
- Supino-Viterbo, V.; Sicard, C.; Risvegliato, M.; Rancurel, G.; Buge, A. Toxic Encephalopathy Due to Ingestion of Bismuth Salts: Clinical and EEG Studies of 45 Patients. J. Neurol. Neurosurg. Psychiatry 1977, 40, 748–752. [Google Scholar] [CrossRef]
- Stoltenberg, M.; Danscher, G. Histochemical Differentiation of Autometallographically Traceable Metals (Au, Ag, Hg, Bi, Zn): Protocols for Chemical Removal of Separate Autometallographic Metal Clusters in Epon Sections. Histochem. J. 2000, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Danscher, G.; Rungby, J.; Stoltenberg, M. Tissue Uptake of Bismuth from Shotgun Pellets. Environ. Res. 2000, 82, 258–262. [Google Scholar] [CrossRef]
- Stoltenberg, M.; Schiønning, J.D.; Danscher, G. Retrograde Axonal Transport of Bismuth: An Autometallographic Study. Acta Neuropathol. 2001, 101, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Stoltenberg, M.; Danscher, G.; Pamphlett, R.; Christensen, M.M.; Rungby, J. Histochemical Tracing of Bismuth in Testis from Rats Exposed Intraperitoneally to Bismuth Subnitrate. Reprod. Toxicol. 2000, 14, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Stoltenberg, M.; Larsen, A.; Zhao, M.; Danscher, G.; Brunk, U.T. Bismuth-Induced Lysosomal Rupture in J774 Cells. Apmis 2002, 110, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Sun, H. Bioinorganic Chemistry of Bismuth and Antimony: Target Sites of Metallodrugs. Acc. Chem. Res. 2007, 40, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Biological Chemistry of Arsenic, Antimony and Bismuth; Sun, H., Ed.; Biological Chemistry of Arsenic, Antimony and Bismuth; John Wiley & Sons Ltd.: Singapore, 2011. [Google Scholar]
- Rosário, J.; Moreira, F.; Rosa, L.; Guerra, W.; Silva-Caldeira, P. Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome. Molecules 2023, 28, 5921. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Figueiredo, S.A.C.; Pinto, R.M.A.; Silvestre, S.M. Bismuth Compounds in Medicinal Chemistry. Future Med. Chem. 2012, 4, 1495–1523. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, R.; Chan, J.F.W.; Zhang, A.J.; Cheng, T.; Chik, K.K.H.; Ye, Z.W.; Wang, S.; Lee, A.C.Y.; Jin, L.; et al. Metallodrug Ranitidine Bismuth Citrate Suppresses SARS-CoV-2 Replication and Relieves Virus-Associated Pneumonia in Syrian Hamsters. Nat. Microbiol. 2020, 5, 1439–1448. [Google Scholar] [CrossRef]
- Sandha, G.S.; Leblanc, R.; Veldhuyzen Van Zanten, S.J.O.; Sitland, T.D.; Agocs, L.; Burford, N.; Best, L.; Mahoney, D.; Hoffman, P.; Leddin, D.J. Chemical Structure of Bismuth Compounds Determines Their Gastric Ulcer Healing Efficacy and Anti-Helicobacter pylori Activity. Dig. Dis. Sci. 1998, 43, 2727–2732. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Aldaco, M.G.; Baéz, J.E.; Jiménez-Halla, J.O.C. Bismuth Subsalicylate, a Low-Toxicity Catalyst for the Ring-Opening Polymerization (ROP) of l-Lactide (l-LA) with Aliphatic Diol Initiators: Synthesis, Characterization, and Mechanism of Initiation. RSC Adv. 2020, 10, 30815–30824. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Sun, H. Biocoordination Chemistry of Bismuth: Recent Advances. Coord. Chem. Rev. 2007, 251, 2354–2366. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwak, M.S.; Jeon, J.W.; Cha, J.M. Pretreatment with Ranitidine Bismuth Citrate May Improve Success Rates of Helicobacter pylori Eradication: A Prospective, Randomized, Controlled and Open-Label Study. Tohoku J. Exp. Med. 2021, 255, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.C.; Celiñski, K.; Giguère, M.; Rivière, M.; Mégraud, F. Helicobacter pylori Eradication with a Capsule Containing Bismuth Subcitrate Potassium, Metronidazole, and Tetracycline given with Omeprazole versus Clarithromycin-Based Triple Therapy: A Randomised, Open-Label, Non-Inferiority, Phase 3 Trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.N.; Ho, K.S.; Sun, H.; Chan, W.T. Tracking Bismuth Antiulcer Drug Uptake in Single Helicobacter pylori Cells. J. Am. Chem. Soc. 2011, 133, 7355–7357. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Wong, B.C.Y. Practice Parameters Committee of the American College of Gastroenterology American College of Gastroenterology Guideline on the Management of Helicobacter pylori Infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Sun, H. Systems Approaches for Unveiling the Mechanism of Action of Bismuth Drugs: New Medicinal Applications beyond Helicobacter pylori Infection. Acc. Chem. Res. 2019, 52, 216–227. [Google Scholar] [CrossRef]
- Pathak, A.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Tabor, R.F.; Andrews, P.C. Synthesis and Structural Characterisation of Bismuth(III) Hydroxamates and Their Activity against Helicobacter pylori. Dalton Trans. 2015, 44, 16903–16913. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, G.; So, M.H.; Wu, J.; Lu, Z.; Che, C.M.; Sun, H. Bismuth Subcarbonate Nanoparticles Fabricated by Water-in-Oil Microemulsion-Assisted Hydrothermal Process Exhibit Anti-Helicobacter pylori Properties. Mater. Res. Bull. 2010, 45, 654–658. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Giridhar, R.; Megraud, F.; Yadav, M.R. Metalloantibiotics: Synthesis, Characterization and Antimicrobial Evaluation of Bismuth-Fluoroquinolone Complexes against Helicobacter pylori. Acta Pharm. 2009, 59, 259–271. [Google Scholar] [CrossRef]
- Andrews, P.C.; Deacon, G.B.; Ferrero, R.L.; Junk, P.C.; Karrar, A.; Kumar, I.; MacLellan, J.G. Bismuth(III) 5-Sulfosalicylate Complexes: Structure, Solubility and Activity against Helicobacter pylori. Dalton Trans. 2009, 28, 6377–6384. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Busse, M.; Deacon, G.B.; Ferrero, R.L.; Junk, P.C.; Huynh, K.K.; Kumar, I.; Maclellan, J.G. Structural and Solution Studies of Phenylbismuth(III) Sulfonate Complexes and Their Activity against Helicobacter pylori. Dalton Trans. 2010, 39, 9633–9641. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.H.; Chen, C.C.; Tseng, P.H.; Liou, J.M.; Wu, M.S.; Shun, C.T.; Lee, Y.C.; Graham, D.Y. Bismuth Salts with versus without Acid Suppression for Helicobacter pylori Infection: A Transmission Electron Microscope Study. Helicobacter 2021, 26, e12801. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.J.; Stephens, L.J.; Werrett, M.V.; Andrews, P.C. Bismuth(III) Flavonolates: The Impact of Structural Diversity on Antibacterial Activity, Mammalian Cell Viability and Cellular Uptake. Chem. A Eur. J. 2020, 26, 7657–7671. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.E.; Werrett, M.V.; Andrews, P.C. Aryl Bismuth Phosphinates [BiAr2(O(O)PRR′)]: Structure–Activity Relationships for Antibacterial Activity and Cytotoxicity. Dalton Trans. 2022, 51, 9323–9335. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.J.; Munuganti, S.; Duffin, R.N.; Werrett, M.V.; Andrews, P.C. Is Bismuth Really the “Green” Metal? Exploring the Antimicrobial Activity and Cytotoxicity of Organobismuth Thiolate Complexes. Inorg. Chem. 2020, 59, 3494–3508. [Google Scholar] [CrossRef] [PubMed]
- Rostamifar, S.; Azad, A.; Bazrafkan, A.; Modaresi, F.; Atashpour, S.; Jahromi, Z.K. New Strategy of Reducing Biofilm Forming Bacteria in Oral Cavity by Bismuth Nanoparticles. BioMed Res. Int. 2021, 2021, 6695692. [Google Scholar] [CrossRef] [PubMed]
- Kotani, T.; Nagai, D.; Asahi, K.; Suzuki, H.; Yamao, F.; Kataoka, N.; Yagura, T. Antibacterial Properties of Some Cyclic Organobismuth (III) Compounds. Antimicrob. Agents Chemother. 2005, 49, 2729–2734. [Google Scholar] [CrossRef]
- Tripathi, U.N.; Siddiqui, A.; Solanki, J.S. Synthesis, Spectral Characterization, and Antimicrobial Activity of Arsenic(III) and Bismuth(III) Phenyl)Pyrazolinates]. Turk. J. Chem. 2009, 33, 257–266. [Google Scholar] [CrossRef]
- Chauhan, H.P.S.; Shaik, N.M.; Singh, U.P. Synthetic, Spectroscopic and Antimicrobial Studies of Bis(Dialkyldithiocarbamato)Diorganodithiophosphatobismuth(III) Complexes. Appl. Organomet. Chem. 2005, 19, 1132–1139. [Google Scholar] [CrossRef]
- Solanki, J.S.; Thapak, T.R.; Bhardwaj, A. Synthesis, Structural Characterization, and in Vitro Antimicrobial Properties of Salicylate and Pyrazoline Complexes of Bismuth(III). J. Coord. Chem. 2011, 64, 369–376. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Giridhar, R.; Yadav, M.R. Bismuth-Norfloxacin Complex: Synthesis, Physicochemical and Antimicrobial Evaluation. Int. J. Pharm. 2007, 332, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Frank, R.; Junk, P.C.; Kedzierski, L.; Kumar, I.; MacLellan, J.G. Anti-Leishmanial Activity of Homo- and Heteroleptic Bismuth(III) Carboxylates. J. Inorg. Biochem. 2011, 105, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, S.; Imtiaz-ud-Din; Rauf, M.K.; Azam, S.S.; Haq, I.L.; Tahir, M.N.; Zaman, N. Structural Characterization and Antileishmanial Activity of Newly Synthesized Organo-Bismuth(V) Carboxylates: Experimental and Molecular Docking Studies. J. Biol. Inorg. Chem. 2022, 27, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Murafuji, T.; Miyoshi, Y.; Ishibashi, M.; Rahman, A.F.M.M.; Sugihara, Y.; Miyakawa, I.; Uno, H. Antifungal Activity of Organobismuth Compounds against the Yeast Saccharomyces cerevisiae: Structure-Activity Relationship. J. Inorg. Biochem. 2004, 98, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Murafuji, T.; Fujiwara, Y.; Yoshimatsu, D.; Miyakawa, I.; Migita, K.; Mikata, Y. Bismuth Heterocycles Based on a Diphenyl Sulfone Scaffold: Synthesis and Substituent Effect on the Antifungal Activity against Saccharomyces cerevisiae. Eur. J. Med. Chem. 2011, 46, 519–525. [Google Scholar] [CrossRef] [PubMed]
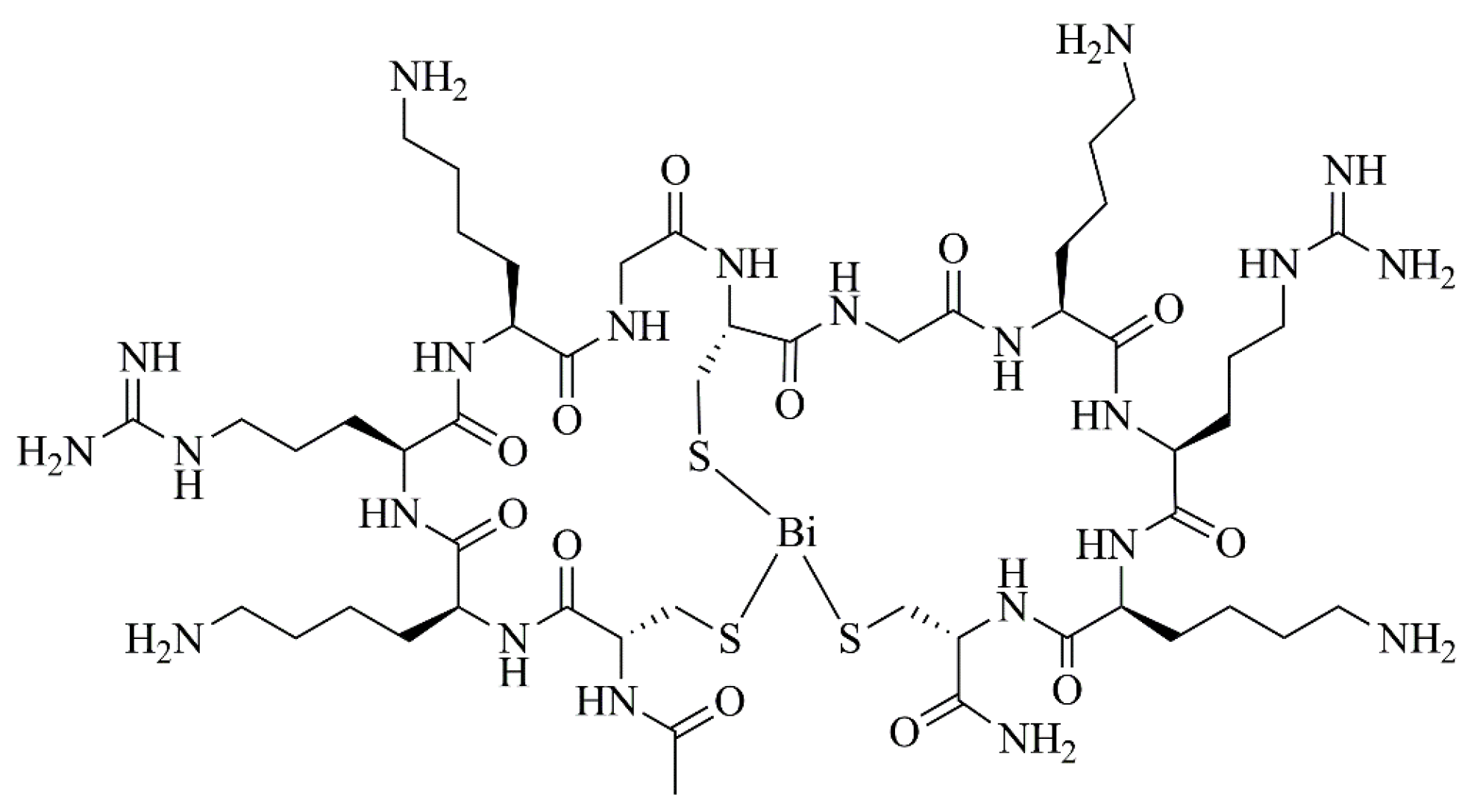
- Voss, S.; Rademann, J.; Nitsche, C. Peptide–Bismuth Bicycles: In Situ Access to Stable Constrained Peptides with Superior Bioactivity. Angew. Chem. Int. Ed. 2022, 61, e202113857. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.C.; Wolf, C.H.; Rubin, D.T. Temporal Improvement of a COVID-19-Positive Crohn’s Disease Patient Treated with Bismuth Subsalicylate. Am. J. Gastroenterol. 2020, 115, 1298. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Ameri, A.; Adeli-Sardou, M.; Jafari, M.; Rahimi, H.R. Cytotoxicity of Biologically Synthesised Bismuth Nanoparticles against HT-29 Cell Line. IET Nanobiotechnol. 2018, 12, 653–657. [Google Scholar] [CrossRef]
- Iuchi, K.; Tasaki, Y.; Shirai, S.; Hisatomi, H. Upregulation of Nuclear Factor (Erythroid-Derived 2)-like 2 Protein Level in the Human Colorectal Adenocarcinoma Cell Line DLD-1 by a Heterocyclic Organobismuth(III) Compound: Effect of Organobismuth(III) Compound on NRF2 Signaling. Biomed. Pharmacother. 2020, 125, 109928. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mitani, M.; Yasuike, S.; Kurita, J.; Kaji, T. An Organobismuth Compound That Exhibits Selective Cytotoxicity to Vascular Endothelial Cells in Vitro. J. Health Sci. 2005, 51, 333–340. [Google Scholar] [CrossRef]
- Lukevics, E.; Shestakova, I.; Domracheva, I.; Nesterova, A.; Zaruma, D.; Ashaks, J. Cytotoxicity of Metal 8-Quinolinethiolates. Chem. Heterocycl. Compd. 2006, 42, 761–764. [Google Scholar] [CrossRef]
- García-Cuellar, C.M.; Cabral-Romero, C.; Hernández-Delgadillo, R.; Solis-Soto, J.M.; Meester, I.; Sánchez-Pérez, Y.; Nakagoshi-Cepeda, S.E.; Pineda-Aguilar, N.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; et al. Bismuth Lipophilic Nanoparticles (BisBAL NP) Inhibit the Growth of Tumor Cells in a Mouse Melanoma Model. Anticancer. Agents Med. Chem. 2022, 22, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.F.; Ang, K.P.; Hamid, R.A. A Bismuth Diethyldithiocarbamate Compound Induced Apoptosis via Mitochondria-Dependent Pathway and Suppressed Invasion in MCF-7 Breast Cancer Cells. BioMetals 2021, 34, 365–391. [Google Scholar] [CrossRef] [PubMed]
- da Luz, J.Z.; Machado, T.N.; Bezerra, A.G.; de Oliveira Ribeiro, C.A.; Neto, F.F. Cytotoxicity of Bismuth Nanoparticles in the Murine Macrophage Cell Line RAW 264.7. J. Mater. Sci. Mater. Med. 2020, 31, 95. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Ikeda, K.; Sugiyama, H. Cytotoxicity of Bismuth Compounds to Cultured Cancer Cells. J. Environ. Anal. Toxicol. 2017, 7, 1000462. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.; Qiao, Y.; Hossain, M.; Ma, L.; Su, M. In Vitro Cytotoxicity of Surface Modified Bismuth Nanoparticles. J. Mater. Sci. Mater. Med. 2012, 23, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, Y.; Jiang, Z.; Tang, M.; Li, N.; Wei, F.; Cheng, G. The Acute Cytotoxicity of Bismuth Ferrite Nanoparticles on PC12 Cells. J. Nanoparticle Res. 2014, 16, 2408. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, J.; Zhang, X.; Yue, C.; Zhu, N.; Yang, L.; Wang, Y.; Chen, T.; Wang, Y.; Zhang, L.W. Autophagy Associated Cytotoxicity and Cellular Uptake Mechanisms of Bismuth Nanoparticles in Human Kidney Cells. Toxicol. Lett. 2017, 275, 39–48. [Google Scholar] [CrossRef]
- Abudayyak, M.; Öztaş, E.; Arici, M.; Özhan, G. Investigation of the Toxicity of Bismuth Oxide Nanoparticles in Various Cell Lines. Chemosphere 2017, 169, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Brechbiel, M.W. An Overview of Targeted Alpha Therapy. Tumor Biol. 2012, 33, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Brechbiel, M.W. Targeted Alpha-Therapy: Past, Present, Future? Dalton Trans. 2007, 43, 4918–4928. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.P.; Beyler, M.; Oukhatar, F.; Le Saec, P.; Faivre-Chauvet, A.; Platas-Iglesias, C.; Delgado, R.; Tripier, R. H2Me-Do2pa: An Attractive Chelator with Fast, Stable and Inert (Nat)Bi3+ and 213Bi3+ Complexation for Potential α-Radioimmunotherapy Applications. Chem. Commun. 2014, 50, 12371–12374. [Google Scholar] [CrossRef]
- Chan, S.; Wang, R.; Man, K.; Nicholls, J.; Li, H.; Sun, H.; Chan, G.C.F. A Novel Synthetic Compound, Bismuth Zinc Citrate, Could Potentially Reduce Cisplatin-Induced Toxicity Without Compromising the Anticancer Effect Through Enhanced Expression of Antioxidant Protein. Transl. Oncol. 2019, 12, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hong, Y.; Fan, G. Bismuth Reduces Cisplatin-Induced Nephrotoxicity Via Enhancing Glutathione Conjugation and Vesicular Transport. Front. Pharmacol. 2022, 13, 887876. [Google Scholar] [CrossRef] [PubMed]
- Brum, J.M.; Gibb, R.D.; Ramsey, D.L.; Balan, G.; Yacyshyn, B.R. Systematic Review and Meta-Analyses Assessment of the Clinical Efficacy of Bismuth Subsalicylate for Prevention and Treatment of Infectious Diarrhea. Dig. Dis. Sci. 2021, 66, 2323–2335. [Google Scholar] [CrossRef]
- Goldman, R.D. Bismuth Salicylate for Diarrhea in Children. Can. Fam. Physician 2013, 59, 843–844. [Google Scholar]
- Bowen, A.; Agboatwalla, M.; Pitz, A.; Salahuddin, S.; Brum, J.; Plikaytis, B. Effect of Bismuth Subsalicylate vs Placebo on Use of Antibiotics among Adult Outpatients with Diarrhea in Pakistan: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e199441. [Google Scholar] [CrossRef]
- Peng, J.; Xiong, Y.; Lin, Z.; Sun, L.; Weng, J. Few-Layer Bismuth Selenides Exfoliated by Hemin Inhibit Amyloid-β 1-42 Fibril Formation. Sci. Rep. 2015, 5, 10171. [Google Scholar] [CrossRef]
- Yang, N.; Sun, H. Bismuth: Environmental Pollution and Health Effects. Encycl. Environ. Health 2011, 414–420. [Google Scholar] [CrossRef]
- Bradley, B.; Singleton, M.; Po, A.L.W. Bismuth Toxicity—A Reassessment. J. Clin. Pharm. Ther. 1989, 14, 423–441. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Peng, S.; Yue, B.; Fan, C.; Chen, W.; Li, X. Comparative Toxicities of Bismuth Oxybromide and Titanium Dioxide Exposure on Human Skin Keratinocyte Cells. Chemosphere 2015, 135, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, X.; Wang, Y.; Fan, C. Effects of Morphology and Surface Hydroxyl on the Toxicity of BiOCl in Human HaCaT Cells. Chemosphere 2016, 163, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Dopp, E.; von Recklinghausen, U.; Hippler, J.; Diaz-Bone, R.A.; Richard, J.; Zimmermann, U.; Rettenmeier, A.W.; Hirner, A.V. Toxicity of Volatile Methylated Species of Bismuth, Arsenic, Tin, and Mercury in Mammalian Cells in Vitro. J. Toxicol. 2011, 2011, 503576. [Google Scholar] [CrossRef]
- Liman, R. Genotoxic Effects of Bismuth (III) Oxide Nanoparticles by Allium and Comet Assay. Chemosphere 2013, 93, 269–273. [Google Scholar] [CrossRef]
- Larsen, A.; Martiny, N.; Stoltenberg, M.; Danscher, G.; Rungby, J. Gastrointestinal and Systemic Uptake of Bismuth in Mice after Oral Exposure. Pharmacol. Toxicol. 2003, 93, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Satoh, H.; Chiba, M.; Okamoto, M.; Serizawa, K.; Nakashima, H.; Omae, K. Oral Toxicity of Bismuth in Rat: Single and 28-Day Repeated Administration Studies. J. Occup. Health 2005, 47, 293–298. [Google Scholar] [CrossRef]
- Sano, Y.; Satoh, H.; Chiba, M.; Shinohara, A.; Okamoto, M.; Serizawa, K.; Nakashima, H.; Omae, K. A 13-Week Toxicity Study of Bismuth in Rats by Intratracheal Intermittent Administration. J. Occup. Health 2005, 47, 242–248. [Google Scholar] [CrossRef]
- Dorso, L.; Bigot-Corbel, E.; Abadie, J.; Diab, M.; Gouard, S.; Bruchertseifer, F.; Morgenstern, A.; Maurel, C.; Chérel, M.; Davodeau, F. Long-Term Toxicity of 213Bi-Labelled BSA in Mice. PLoS ONE 2016, 11, e0151330. [Google Scholar] [CrossRef]
- Omouri, Z.; Hawari, J.; Fournier, M.; Robidoux, P.Y. Bioavailability and Chronic Toxicity of Bismuth Citrate to Earthworm Eisenia Andrei Exposed to Natural Sandy Soil. Ecotoxicol. Environ. Saf. 2018, 147, 1–8. [Google Scholar] [CrossRef]
- He, N.; Li, X.; Feng, D.; Wu, M.; Chen, R.; Chen, T.; Chen, D.; Feng, X. Exploring the Toxicity of a Bismuth-Asparagine Coordination Polymer on the Early Development of Zebrafish Embryos. Chem. Res. Toxicol. 2013, 26, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.A.; Sullivan, D.W., Jr.; Sexton, M.J. Chapter 31—Bismuth. Vol. I, Handbook on the Toxicology of Metals, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 1307–1345. [Google Scholar]
- Pelepenko, L.E.; Janini, A.C.P.; Gomes, B.P.F.A.; de-Jesus-Soares, A.; Marciano, M.A. Effects of Bismuth Exposure on the Human Kidney—A Systematic Review. Antibiotics 2022, 11, 1741. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.; Mowat, N. A Reversible Toxicity in Poisoning with Colloidal Bismuth Subcitrate. BMJ 1989, 299, 159. [Google Scholar] [CrossRef]
- Taylor, E.G.; Klenerman, P. Acute Renal Failure after Colloidal Bismuth Subcitrate Overdose. Lancet 1990, 335, 670–671. [Google Scholar] [CrossRef]
- Playford, R.J.; Matthews, C.H.; Campbell, M.J.; Delves, H.T.; Hla, K.K.; Hodgson, H.J.; Calam, J. Bismuth Induced Encephalopathy Caused by Tri Potassium Dicitrato Bismuthate in a Patient with Chronic Renal Failure. Gut 1990, 31, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Huwez, F.; Pall, A.; Lyons, D.; Stewart, M.J. Acute Renal Failure after Overdose of Colloidal Bismuth Subcitrate. Lancet 1992, 353, 1298. [Google Scholar] [CrossRef]
- Akpolat, I.; Kahraman, H.; Arik, N.; Akpolat, T.; Kandemir, B.; Cengiz, K. Acute Renal Failure Due to Overdose of Colloidal Bismuth. Nephrol. Dial. Transpl. 1996, 11, 1890–1891. [Google Scholar] [CrossRef]
- Summers, W.K. Bismuth Toxicity Masquerading as Alzheimer’s Dementia. J. Alzheimers Dis. 1998, 1, 57–59. [Google Scholar] [CrossRef]
- Hruz, P.; Mayr, M.; Löw, R.; Drewe, J.; Huber, G. Fanconi’s Syndrome, Acute Renal Failure, and Tonsil Ulcerations after Colloidal Bismuth Subcitrate Intoxication. Am. J. Kidney Dis. 2002, 39, E18. [Google Scholar] [CrossRef]
- Reynolds, P.T.; Abalos, K.; Hopp, J.; Williams, M.E. Bismuth Toxicity: A Rare Cause of Neurologic Dysfunction. Int. J. Clin. Med. 2012, 3, 46–48. [Google Scholar] [CrossRef]
- Akinci, E.; Koylu, R.; Yortanli, M.; Gumus, H.; Koylu, O.; Altintepe, L.; Cander, B. Acute Bismuth Intoxication: Acute Renal Failure, Tonsillar Ulceration and Posterior Reversible Encephalopathy Syndrome. Hong Kong J. Emerg. Med. 2015, 22, 121–125. [Google Scholar] [CrossRef]
- Sampognaro, P.; Vo, K.T.; Richie, M.; Blanc, P.D.; Keenan, K. Bismuth Subgallate Toxicity in the Age of Online Supplement Use. Neurologist 2017, 22, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Disel, N.R.; Açikalin, A.; Sebe, A.; Gokel, Y. Utilization of Plasmapheresis in the Management of Bismuth Intoxication with Acute Renal Failure. Saudi J. Kidney Dis. Transpl. 2017, 28, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Borbinha, C.; Serrazina, F.; Salavisa, M.; Viana-Baptista, M. Bismuth Encephalopathy—A Rare Complication of Long-Standing Use of Bismuth Subsalicylate. BMC Neurol. 2019, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.B.; Harbidge, C.; Duncan, A. Bismuth Toxicity Presenting as Declining Mobility and Falls. Can. Geriatr. J. 2018, 21, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Eustaquio, N.; Calello, D.P.; Ruck, B.E.; Nelson, L.S.; Santos, C. Bismuth Subsalicylate Coagulopathy in a Patient with Chronic Liver Disease. J. Med. Toxicol. 2019, 15, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.R.; Cast, I.P.; Redfern, R.M.; O’Brien, C. Extradural Application of Bismuth Iodoform Paraffin Paste Causing Relapsing Bismuth Encephalopathy: A Case Report with CT and MRI Studies. J. Neurol. Neurosurg. Psychiatry 1994, 57, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Poole, A. Beware of Bismuth: Post Maxillectomy Delirium. ANZ J. Surg. 2002, 72, 846–847. [Google Scholar] [CrossRef]
- Roest, M.A.B.; Shaw, S.; Orton, D.I. Allergic Contact Otitis Externa Due to Iodoform in BIPP Cavity Dressings. Contact Dermat. 2002, 46, 360. [Google Scholar] [CrossRef]
- Youngman, L.; Harris, S. BIPP Madness; an Iatrogenic Cause of Acute Confusion. Age Ageing 2004, 33, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Ovaska, H.; Wood, D.M.; House, I.; Dargan, P.I.; Jones, A.L.; Murray, S. Severe Iatrogenic Bismuth Poisoning with Bismuth Iodoform Paraffin Paste Treated with DMPS Chelation. Clin. Toxicol. 2008, 46, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Atwal, A.; Cousin, G.C.S. Bismuth Toxicity in Patients Treated with Bismuth Iodoform Paraffin Packs. Br. J. Oral. Maxillofac. Surg. 2016, 54, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Psaltis, A.J.; Curragh, D.S.; Selva, D. Neurotoxicity Secondary to Bismuth Iodoform Paraffin Paste Packing in an Orbital Exenteration Cavity. Ophthalmic. Plast. Reconstr. Surg. 2018, 34, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Neo, S.; Gan, J.; Fu, E.; Lim, M.Y.; Li, H. Myoclonus from Intoxication by Bismuth Iodoform Paraffin Paste (BIPP) Nasopharyngeal Packing. Cureus 2021, 13, e18530. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Pinho, J.O.; Penetra, M.J.; Campos, G.; Reis, C.P.; Gaspar, M.M. The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells 2021, 10, 3088. [Google Scholar] [CrossRef]
- Guiard, E.; Lelievre, B.; Rouyer, M.; Zerbib, F.; Diquet, B.; Mégraud, F.; Tison, F.; Bignon, E.; Lassalle, R.; Droz-Perroteau, C.; et al. Bismuth Concentrations in Patients Treated in Real-Life Practice with a Bismuth Subcitrate-Metronidazole-Tetracycline Preparation: The SAPHARY Study. Drug Saf. 2019, 42, 993–1003. [Google Scholar] [CrossRef]
| Entry | Gender (Age [Years]) | Quantity Consumed | Time from Ingestion to Hospitalization | Symptoms | Bismuth Concentration before Therapy | Bismuth Concentration after Therapy | Main Findings | Treatment | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/F (24–80) | 5–20 g bismuth subnitrate daily | 4 weeks–30 years | Depression, anxiety, irritability, delusions, phobias, somnolence, hallucinations, anorexia, sleep disorder, motor incoordination, jerky movements | Blood: 150–1600 µg/L Urine: 200–9600 µg/L | - | Monomorphic waves at 3 to 5 Hz; diffuse beta rhythm of low voltage | - | [25] |
| 2 | M (27) | 100 De-Nol® Tablets (12 g colloidal bismuth) | 10 days | Anorexia, vomiting, nausea, legs weakness, blurring of vision, thirst, poor urinary output | Blood: 260 µg/L Urine: 120 µg/L Stools: 26.9 mg/g | 96 days after ingestion: Blood: 8 µg/g | Opacification of the colon; non-specific slow-wave changes to both hemispheres | Purgation (magnesium sulfate), rehydration, hemodialysis | [100] |
| 3 | M (76) | 80 De-Nol® Tablets | 4 h | Confusion, epigastric tenderness | Blood: 1600 µg/L | - | Opacification of the colon; acute tubular necrosis | Ranitidine, antacid, magnesium sulfate enemas, dialysis (3 days) | [101] |
| 4 | M (68) | Twice the recommended dose of DeNol® (864 mg daily) for 2 months | - | Cerebral dysfunction, incontinence, bilateral grasps reflexes, hallucinations, ataxia | Blood: 880 µg/L Urine: 230 µg/L | - | Loss of alpha rhythm and diffuse slow waves consistent with a metabolic encephalopathy | Heavy-metal chelator 2–3 dimercapto-1 propane sulphonic acid (DMPS) | [102] |
| 5 | M (21) | 39 tablets of bismuth subcitrate | - | Epigastric pain | Blood: ~200 µg/L Serum: ~1500 µg/L | Blood: ~125 Serum: ~10 | Acute tubular necrosis | Intravenous furosemide, dopamine, mannitol, crystalloids | [103] |
| 6 | F (16) | 10–15 tablets of tripotassium dicitrato bismuthane | 1 week | Nausea, vomiting, dizziness, oliguria | - | - | Acute tubular necrosis | Hemodialysis, protein restriction, metoclopramide, aluminum hydroxide | [104] |
| 7 | F (76) | Pepto-Bismol® (4.14 mg daily for 7 years) | - | Confusion, poor appetite, disturbed sleep, muscle twitching | On day 6: Serum—242 µg/L | After 30 days: Serum: 90 µg/L After 76 days: Serum: 14 µg/L | Normal X-ray; moderate atrophy; ventricular enlargement; ischemic white matter disease | Penicillamine, oral fluids, salt tablets, Cognex (Tacrine) | [105] |
| 8 | M (2) | 28 De-Nol® tablets (8.4 g of colloidal bismuth subcitrate) | 6 h | - | On day 10: Blood: 739 µg/L Urine: 693 µg/L | Day 105: Blood: 12 µg/L | Opacification of the intestine and colon; normal magnetic resonance imaging (MRI) | Gastric lavage, intravenous saline, mannitol, furosemide | [22] |
| 9 | F (22) | 5.4 g of colloidal bismuth subcitrate | 2 h | - | Day 3: Serum: 640 µg/L | Day 11: Serum: 12 µg/L | Enlarged and edematous kidneys with thinning of the cortical area | DMPS, hemodialysis, hemodiafiltrations | [106] |
| 10 | F (16) | 60 De-Nol® tablets | 10 days | Nausea, vomiting, facial paresthesia | Day 12: Serum: 495 µg/L | Day 64: Serum: 260 µg/L | Normal MRI | Hemodialysis, penicillamine | [23] |
| 11 | F (56) | 45 mL (thrice per day) of bismuth subsalicylate (262 mg/15 mL) | - | Psychomotor retardation, decreased concentration, tremor of the hands, visual hallucinations, postural instability | Blood: 397.3 ng/mL Urine: 292.5 ng/mL | - | Moderate but nonspecific encephalopathy | Medication was held (bismuth subsalicylate) | [107] |
| 12 | F (21) | 20 colloidal bismuth subcitrate tablets (300 mg of colloidal bismuth subcitrate) | 4 h | - | - | - | Normal X-ray and MRI | Gastric lavage, intravenous fluids, DMPS, hemodialysis | [24] |
| 13 | F (16) | 19 g of De-Nol® | 1 h | - | - | - | Opacities in the left side of abdomen; intermittent rhythmic waves in the frontal region; hyper-intense signal alterations at bilateral parietal vertices of both cerebellar hemispheres | - | [108] |
| 14 | F (50) | 200 mg of bismuth subgallate, 3 to 5 times a day | - | Disorientation, inattention, memory loss, tremors, myoclonic jerks, hyperreflexic with bilateral ankle clonus. | Serum: 44.4 µg/L Urine: 57.8 µg/L | - | Excessive theta activity | No specific treatment. Patient continued to improve | [109] |
| 15 | F (34) | 8 De-Nol® tablets (2400 mg bismuth citrate) | 2 days | Nausea, vomiting, apathy, blue-black discoloration in the teeth and gums, proteinuria, glucosuria, hemoglobinuria | - | - | - | Plasmapheresis | [110] |
| 16 | F (44) | Pepto-Bismol® (tablets of 150 mg bismuth subsalicylate) | 20 years | Greyish discoloration of teeth, confusion, generalized myoclonic jerks, which worsened, reduction in alertness | Eight days after admission: Urine: 375 μg/L Serum: 260 μg/L Cerebrospinal fluid: 21.4 μg/L | One month after admission: Urine: 33 μg/L Serum: 13.1 μg/L | Diffuse and nonspecific cerebral dysfunction; no abnormalities reported | Supportive treatment | [111] |
| 17 | F (77) | Pepto-Bismol® (bismuth subsalicylate 262.5 mg) one tablet three times daily | Around 1 year | Falls, tremors | Urine: 2117 nmol/L | - | - | No specific treatment | [112] |
| 18 | F (62) | Pepto-Bismol® (half bottle per day) | 5 days | 1 week of watery non-bloody diarrhea and confusion | Blood: 4 µg/L Urine: 147.6 µg/L | - | - | Intravenous sodium bicarbonate, N-acetylcysteine infusions, one unit of fresh frozen plasma, two doses of 10 mg vitamin K intravenous | [113] |
| Entry | Gender (Age [Years]) | Surgery | Symptoms after Packing with BIPP | Bismuth Levels | Observations | References |
|---|---|---|---|---|---|---|
| 1 | F (57) | Removal of a basal cell carcinoma | Agitation, confusion, restlessness | 52 ng/L | - | [114] |
| 2 | F (86) | Partial maxillectomy | Exhaustion, lightheadedness, poor appetite, tremor | Day 14: 146 nmol/L Day 22: 81 nmol/L | - | [115] |
| 3 | F (16) | Myringoplasty | Mild erythema and swelling of the concha | - | Allergic contact otitis externa due to BIPP | [116] |
| F (13) | Myringoplasty | - | - | Allergic contact otitis externa due to BIPP | ||
| F (52) | Myringoplasty | Florid eczematous reaction | - | Allergic contact otitis externa due to BIPP | ||
| 4 | M (81) | Epistaxis treatment with BIPP packing | Acute confusion, dysphagia | 250 µg/L | - | [117] |
| 5 | M (67) | Resection of a sacral chondroma | Acute confusion, disorientation, delusions, aggressive, abdominal discomfort, nausea, tremor | Blood: 240 µg/L Urine: 2800 µg/L | - | [118] |
| 6 | M (59) | Marsupialisation and packing with BIPP of a keratocystic odontogenic tumor | Fatigue, confusion, apathy, forgetfulness, and spasms in the quadriceps | Blood: 109.9 nmol/L | After 18 months, blood bismuth concentration was 0.02 nmol/L | [119] |
| F (92) | Right hemimaxillectomy | Confusion | Blood: 144.0 nmol/L | After 4 months, blood bismuth concentration was 8.9 nmol/L | ||
| 7 | F (72) | Partial maxillectomy | Confusion, depressed mood, disorientation, aggression, behavioral change | Serum: 391 nmol/L | After 4 months, the serum bismuth concentration was 120 nmol/L | [120] |
| 8 | M (74) | Endoscopic nasopharyngectomy | Agitation, drowsiness, negative myoclonus | Day 7: Urine: 37,094 µg/L | Day 26—Urine bismuth levels: 457 µg/L Blood bismuth levels were not obtained in the early postoperative period, but were normal (13.6 ng/L) on Day 18 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, Â.; Matias, M.; Salvador, J.A.R.; Silvestre, S. Bioactive Bismuth Compounds: Is Their Toxicity a Barrier to Therapeutic Use? Int. J. Mol. Sci. 2024, 25, 1600. https://doi.org/10.3390/ijms25031600
Gonçalves Â, Matias M, Salvador JAR, Silvestre S. Bioactive Bismuth Compounds: Is Their Toxicity a Barrier to Therapeutic Use? International Journal of Molecular Sciences. 2024; 25(3):1600. https://doi.org/10.3390/ijms25031600
Chicago/Turabian StyleGonçalves, Ângela, Mariana Matias, Jorge A. R. Salvador, and Samuel Silvestre. 2024. "Bioactive Bismuth Compounds: Is Their Toxicity a Barrier to Therapeutic Use?" International Journal of Molecular Sciences 25, no. 3: 1600. https://doi.org/10.3390/ijms25031600
APA StyleGonçalves, Â., Matias, M., Salvador, J. A. R., & Silvestre, S. (2024). Bioactive Bismuth Compounds: Is Their Toxicity a Barrier to Therapeutic Use? International Journal of Molecular Sciences, 25(3), 1600. https://doi.org/10.3390/ijms25031600









