GT-00AxIL15, a Novel Tumor-Targeted IL-15-Based Immunocytokine for the Treatment of TA-MUC1-Positive Solid Tumors: Preclinical In Vitro and In Vivo Pharmacodynamics and Biodistribution Studies
Abstract
1. Introduction
2. Results
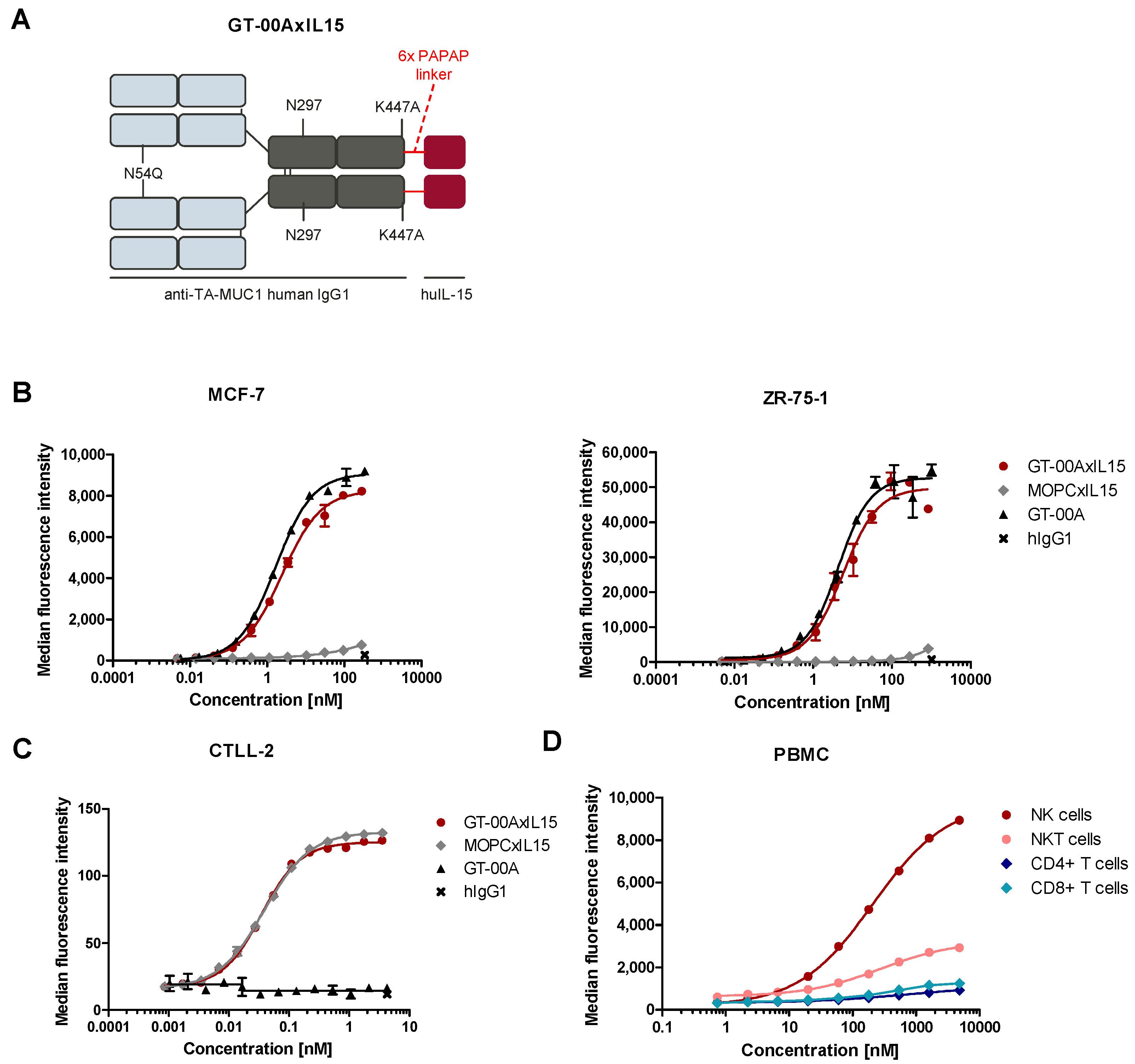
2.1. GT-00AxIL15 Specifically Binds to Its Targets TA-MUC1, IL-15R and FcγRIIIa on Human Tumor Cell Lines and Primary Immune Cells
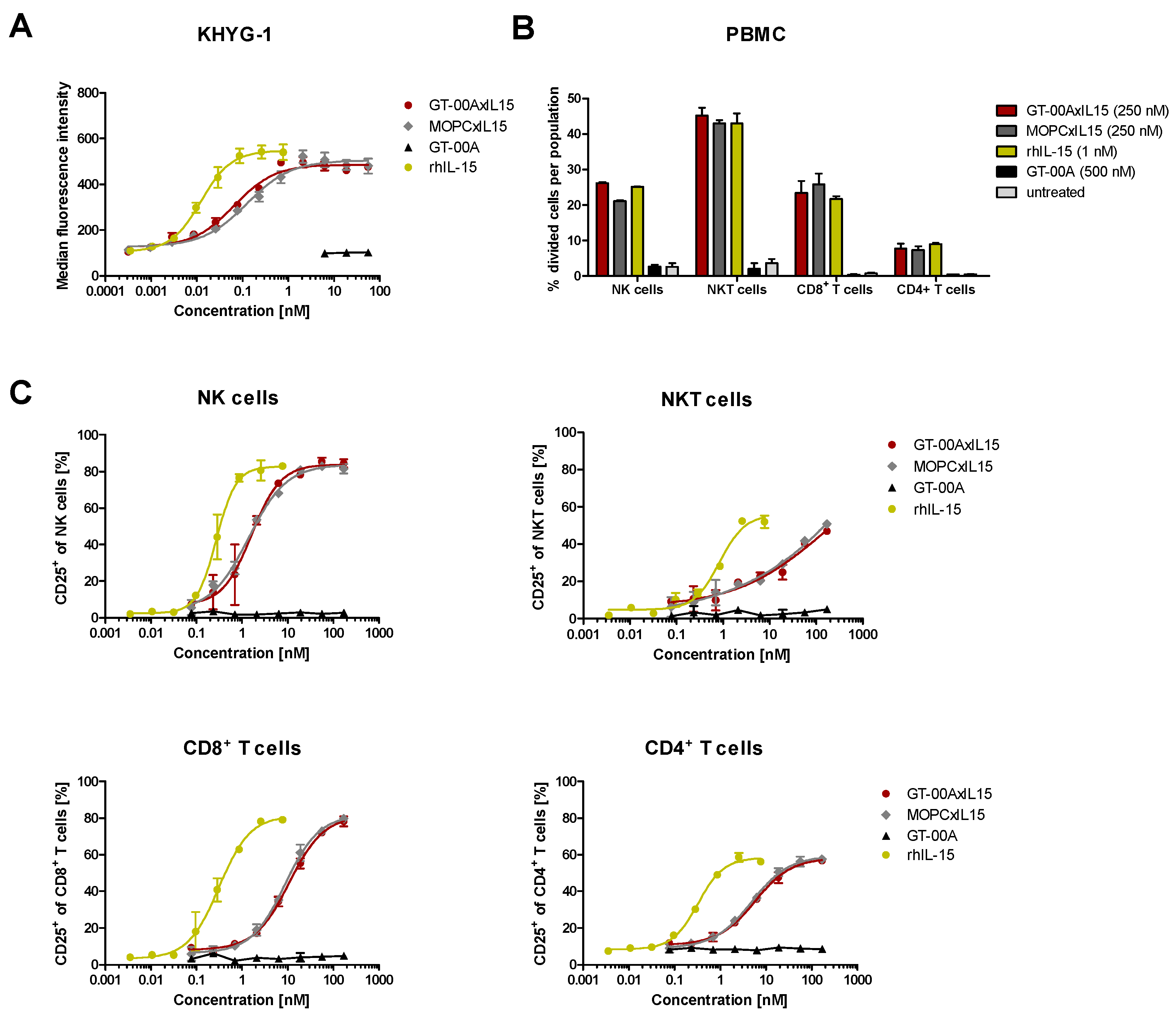
2.2. GT-00AxIL15 Induces Proliferation of a Human NK Cell Line and Potently Activates and Expands Primary Immune Effector Cells In Vitro
2.3. GT-00AxIL15 Mediates Tumor Immune Infiltration and Elicits Enhanced Anti-Tumor Cytotoxicity In Vitro
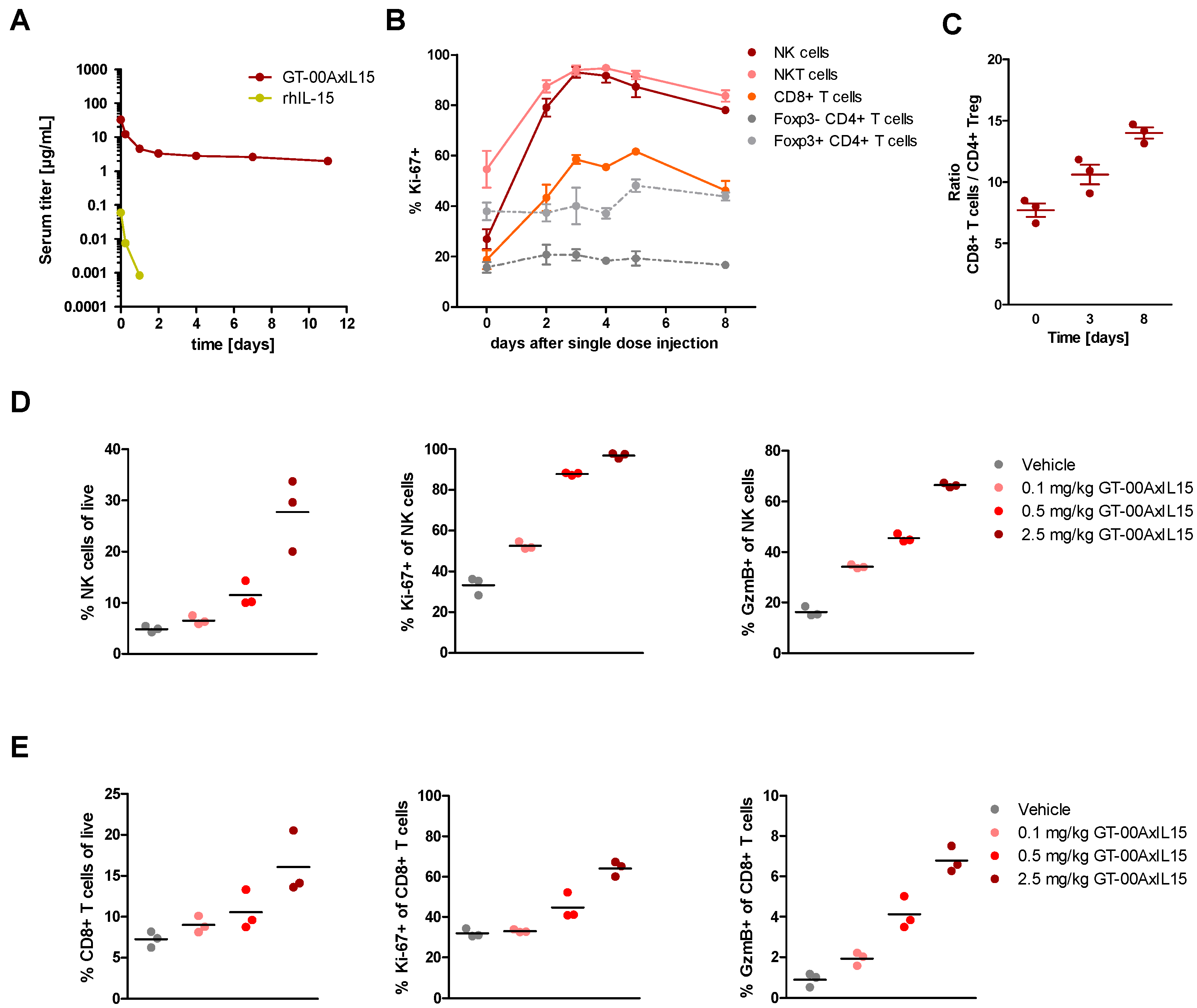
2.4. GT-00AxIL15 Elicits a Prolonged Half-Life and Induces Expansion of NK, NKT and CD8+ T Cells In Vivo after Single and Multiple Injections in Tumor-Free Mice
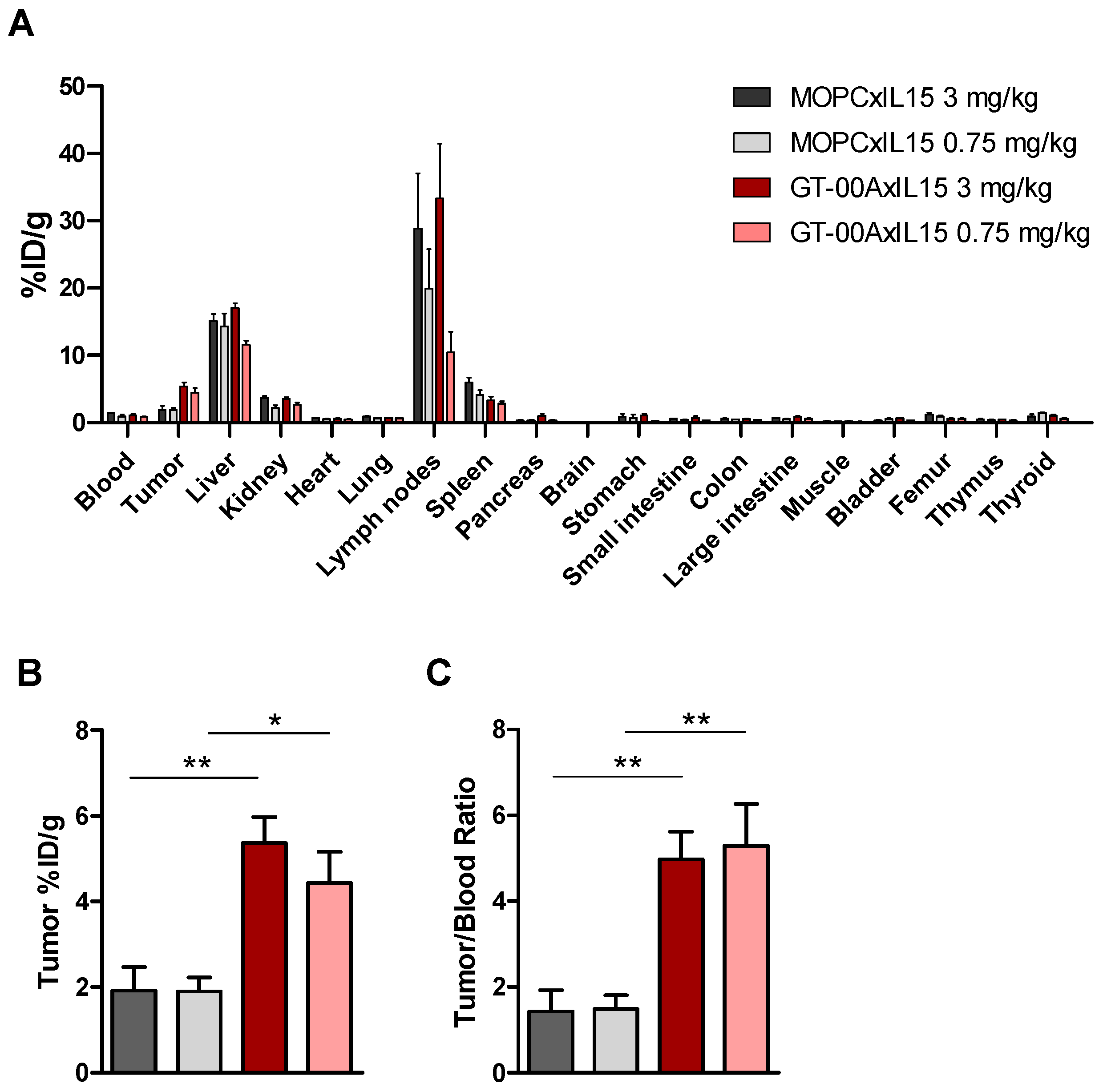
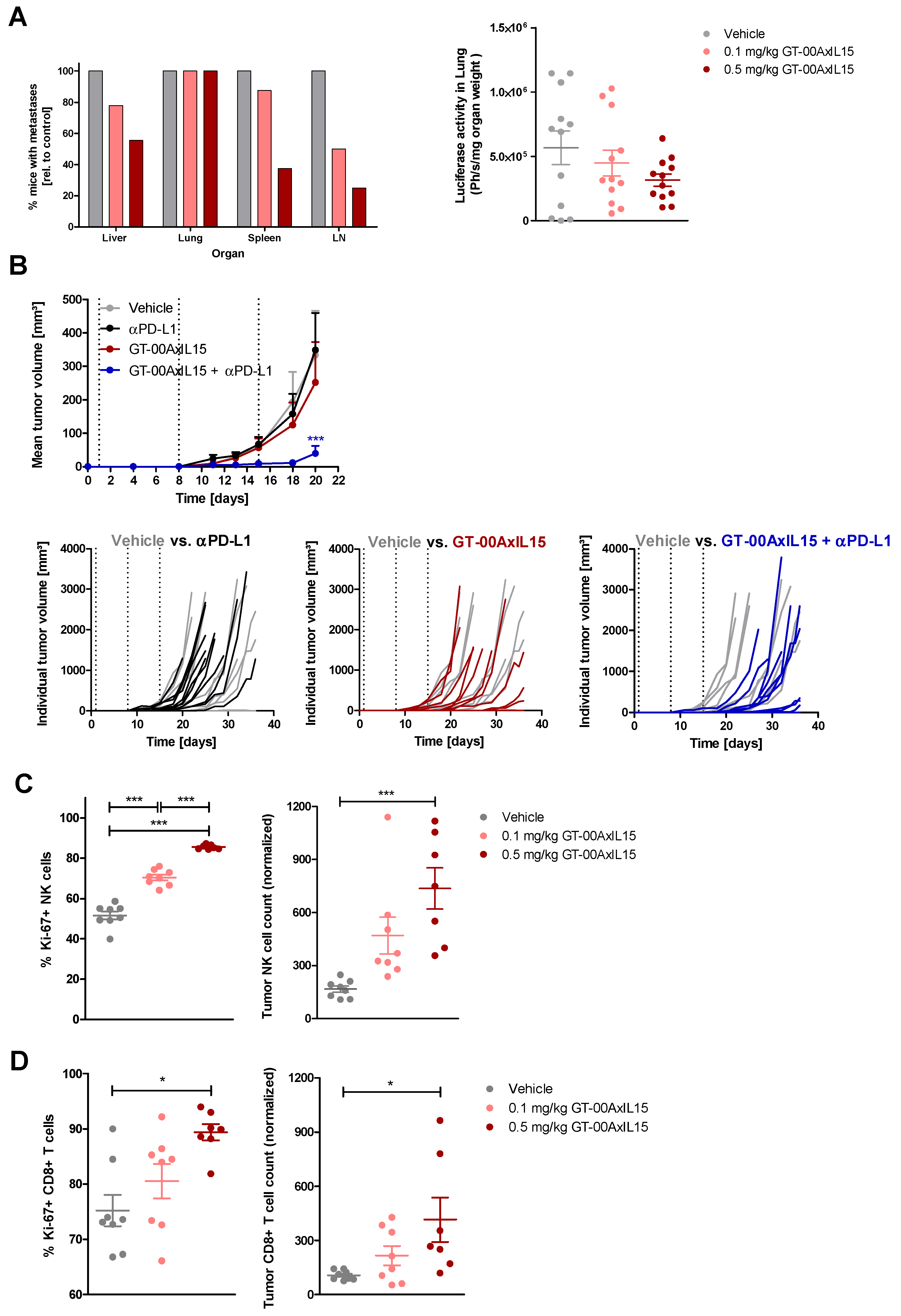
2.5. GT-00AxIL15 Accumulates in TA-MUC1+ Tumors, Selectively Expands Tumor-Infiltrating Lymphocytes (TIL) and Reduces Primary Tumors and Metastatic Load In Vivo
2.6. GT-00AxIL15 Shows In Vivo Efficacy and Elicits PD Effects in a Humanized DU-145 Prostate Cancer Model
3. Discussion
4. Materials and Methods
4.1. Test and Control Articles
4.2. Cells and Cell Culture
4.3. Flow Cytometry
4.4. IL-15 Bioactivity Assay
4.5. Cytotoxicity Assays
4.6. In Vivo PK and PD Properties in Tumor-Free Mice
4.7. Biodistribution and In Vivo Efficacy in Syngeneic Mouse Tumor Models
4.8. In Vivo Efficacy in Humanized DU-145 Prostate Cancer Model (Challenge Setting)
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldmann, T.A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol. Res. 2015, 3, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Sullivan, L.; Caligiuri, M.A. Molecular pathways: Interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 2014, 20, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Stonier, S.W.; Schluns, K.S. Trans-presentation: A novel mechanism regulating IL-15 delivery and responses. Immunol. Lett. 2010, 127, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A. Twelve immunotherapy drugs that could cure cancers. Immunol. Rev. 2008, 222, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.; Watson, D.C.; Waldmann, T.A.; Valentin, A.; Bergamaschi, C.; Felber, B.K.; Peer, C.J.; Figg, W.D.; Potter, E.L.; Roederer, M.; et al. Phase I study of single agent NIZ985, a recombinant heterodimeric IL-15 agonist, in adult patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2021, 9, e003388. [Google Scholar] [CrossRef]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef]
- Waldmann, T.A. Cytokines in Cancer Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028472. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Miljkovic, M.D.; Conlon, K.C. Interleukin-15 (dys)regulation of lymphoid homeostasis: Implications for therapy of autoimmunity and cancer. J. Exp. Med. 2020, 217, e20191062. [Google Scholar] [CrossRef]
- Knudson, K.M.; Hicks, K.C.; Alter, S.; Schlom, J.; Gameiro, S.R. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunother. Cancer 2019, 7, 82. [Google Scholar] [CrossRef]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef]
- Hangasky, J.A.; Waldmann, T.A.; Santi, D.V. Interleukin 15 Pharmacokinetics and Consumption by a Dynamic Cytokine Sink. Front. Immunol. 2020, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Bessard, A.; Sole, V.; Bouchaud, G.; Quemener, A.; Jacques, Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol. Cancer Ther. 2009, 8, 2736–2745. [Google Scholar] [CrossRef] [PubMed]
- Champiat, S.; Marabelle, A.; Galvao, V.; LoRusso, P.; Grell, P.; Cassier, P.; Gomez-Roca, C.; Korakis, I.; Naing, A.; Jelinkova, L.P.; et al. Abstract CT040: SOT101, an IL-2/IL-15 Rβγ superagonist, in combination with pembrolizumab in patients with advanced solid tumors: Interim safety and efficacy results from the AURELIO-03 dose escalation trial. Cancer Res. 2022, 82, CT040-CT. [Google Scholar] [CrossRef]
- Margolin, K.; Morishima, C.; Velcheti, V.; Miller, J.S.; Lee, S.M.; Silk, A.W.; Holtan, S.G.; Lacroix, A.M.; Fling, S.P.; Kaiser, J.C.; et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 5552–5561. [Google Scholar] [CrossRef] [PubMed]
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol. Res. 2016, 4, 49–60. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Velcheti, V.; Patel, M.R.; Garrett-Mayer, E.; Hill, E.G.; Ravenel, J.G.; Miller, J.S.; Farhad, M.; Anderton, K.; Lindsey, K.; et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: A non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018, 19, 694–704. [Google Scholar] [CrossRef]
- Chertova, E.; Bergamaschi, C.; Chertov, O.; Sowder, R.; Bear, J.; Roser, J.D.; Beach, R.K.; Lifson, J.D.; Felber, B.K.; Pavlakis, G.N. Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer. J. Biol. Chem. 2013, 288, 18093–18103. [Google Scholar] [CrossRef]
- Castillo, E.F.; Schluns, K.S. Regulating the immune system via IL-15 transpresentation. Cytokine 2012, 59, 479–490. [Google Scholar] [CrossRef]
- Dubois, S.; Patel, H.J.; Zhang, M.; Waldmann, T.A.; Muller, J.R. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 2008, 180, 2099–2106. [Google Scholar] [CrossRef]
- Kobayashi, H.; Dubois, S.; Sato, N.; Sabzevari, H.; Sakai, Y.; Waldmann, T.A.; Tagaya, Y. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 2005, 105, 721–727. [Google Scholar] [CrossRef]
- Bernett, M.J.; Varma, R.; Bonzon, C.; Rashid, R.; Bogaert, L.; Liu, K.; Schubbert, S.; Avery, K.N.; Leung, I.W.; Rodriguez, N.; et al. Abstract 5565: Potency-reduced IL15/IL15Rα heterodimeric Fc-fusions display enhanced in vivo activity through increased exposure. Cancer Res. 2018, 78, 5565. [Google Scholar] [CrossRef]
- Miyazaki, T.; Maiti, M.; Hennessy, M.; Chang, T.; Kuo, P.; Addepalli, M.; Obalapur, P.; Sheibani, S.; Wilczek, J.; Pena, R.; et al. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J. Immunother. Cancer 2021, 9, e002024. [Google Scholar] [CrossRef] [PubMed]
- Martomo, S.A.; Lu, D.; Polonskaya, Z.; Luna, X.; Zhang, Z.; Feldstein, S.; Lumban-Tobing, R.; Almstead, D.K.; Miyara, F.; Patel, J. Single-Dose Anti-PD-L1/IL-15 Fusion Protein KD033 Generates Synergistic Antitumor Immunity with Robust Tumor-Immune Gene Signatures and Memory Responses. Mol. Cancer Ther. 2021, 20, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, Z.; Jiang, X.; Huang, Z.; Wu, C.; Jiang, D.; Yin, L. Preclinical evaluation of IAP0971, a novel immunocytokine that binds specifically to PD1 and fuses IL15/IL15Ralpha complex. Antib. Ther. 2023, 6, 38–48. [Google Scholar] [PubMed]
- Jochems, C.; Tritsch, S.R.; Knudson, K.M.; Gameiro, S.R.; Rumfield, C.S.; Pellom, S.T.; Morillon, Y.M.; Newman, R.; Marcus, W.; Szeto, C.; et al. The multi-functionality of N-809, a novel fusion protein encompassing anti-PD-L1 and the IL-15 superagonist fusion complex. Oncoimmunology 2019, 8, e1532764. [Google Scholar] [CrossRef] [PubMed]
- Adkins, I.; Antosova, Z.; Danova, K.; Hladikova, K.; Augustynkova, K.; Sajnerova, K.; Podzimkova, N.; Marasek, P.; de Martynoff, G.; Bechard, D.; et al. Abstract 3510: SOT201 is a novel targeted IL-15Rbg agonist to alleviate PD-1-mediated immune cell suppression and potentiate anti-tumor efficacy. Cancer Res. 2022, 82, 3510. [Google Scholar] [CrossRef]
- Bernett, M.J.; Schubbert, S.; Hackett, M.; Ochyl, L.J.; Scott, L.E.; Bonzon, C.; Rashid, R.; Avery, K.N.; Leung, I.W.; Rodriguez, N.; et al. Abstract 2080: LAG3-targeted IL15/IL15Rα-Fc (LAG3 × IL15) fusion proteins for preferential TIL expansion. Cancer Res. 2022, 82, 2080. [Google Scholar] [CrossRef]
- Liu, B.; Kong, L.; Marcus, W.D.; Chen, X.; Han, K.; Rhode, P.R.; Wong, H.C. Evaluation of a novel CD20-targeted IL-15 immunotherapeutic with potent activity against B cell lymphoma. J. Immunother. Cancer 2014, 2 (Suppl. 3), P122. [Google Scholar] [CrossRef][Green Version]
- Fiedler, W.; DeDosso, S.; Cresta, S.; Weidmann, J.; Tessari, A.; Salzberg, M.; Dietrich, B.; Baumeister, H.; Goletz, S.; Gianni, L.; et al. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur. J. Cancer 2016, 63, 55–63. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Zurawski, B.; Raspagliesi, F.; De Giorgi, U.; Arija, J.A.; Marin, M.R.; Lisyanskaya, A.; Póka, R.L.; Markowska, J.; Cebotaru, C.; et al. Maintenance therapy of patients with recurrent epithelial ovarian carcinoma with the anti-tumor-associated-mucin-1 antibody gatipotuzumab: Results from a double-blind, placebo-controlled, randomized, phase II study. ESMO Open 2022, 7, 100311. [Google Scholar] [CrossRef]
- Ochsenreither, S.; Fiedler, W.M.; Conte, G.D.; Macchini, M.; Matos, I.; Habel, B.; Ahrens-Fath, I.; Raspagliesi, F.; Lorusso, D.; Keilholz, U.; et al. Safety and preliminary activity results of the GATTO study, a phase Ib study combining the anti-TA-MUC1 antibody gatipotuzumab with the anti-EGFR tomuzotuximab in patients with refractory solid tumors. ESMO Open 2022, 7, 100447. [Google Scholar] [CrossRef] [PubMed]
- Danielczyk, A.; Stahn, R.; Faulstich, D.; Löffler, A.; Märten, A.; Karsten, U.; Goletz, S. PankoMab: A potent new generation anti-tumour MUC1 antibody. Cancer Immunol. Immunother. 2006, 55, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Dian, D.; Lenhard, M.; Mayr, D.; Heublein, S.; Karsten, U.; Goletz, S.; Kuhn, C.; Wiest, I.; Friese, K.; Weissenbacher, T.; et al. Staining of MUC1 in ovarian cancer tissues with PankoMab-GEX detecting the tumour-associated epitope, TA-MUC1, as compared to antibodies HMFG-1 and 115D8. Histol. Histopathol. 2013, 28, 239–244. [Google Scholar] [PubMed]
- Fan, X.N.; Karsten, U.; Goletz, S.; Cao, Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TA-MUC1) with various human carcinomas. Pathol. Res. Pract. 2010, 206, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Diotel, C.; Klich, G.; Paulsen, H.; Goletz, S.; Müller, S.; Hanisch, F.G. Enhanced binding of antibodies to the DTR motif of MUC1 tandem repeat peptide is mediated by site-specific glycosylation. Cancer Res. 1998, 58, 2541–2549. [Google Scholar] [PubMed]
- Karsten, U.; Serttas, N.; Paulsen, H.; Danielczyk, A.; Goletz, S. Binding patterns of DTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC1). Glycobiology 2004, 14, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; von Mensdorff-Pouilly, S.; Goletz, S. What makes MUC1 a tumor antigen? Tumour Biol. 2005, 26, 217–220. [Google Scholar] [CrossRef]
- Kuemmel, A.; Single, K.; Bittinger, F.; Faldum, A.; Schmidt, L.H.; Sebastian, M.; Micke, P.; Taube, C.; Buhl, R.; Wiewrodt, R. TA-MUC1 epitope in non-small cell lung cancer. Lung Cancer 2009, 63, 98–105. [Google Scholar] [CrossRef]
- Wiest, I.; Alexiou, C.; Friese, K.; Betz, P.; Tuebel, J.; Goletz, S.; Dian, D.; Jeschke, U. Expression of the Tumor-associated Mucin 1 Epitope Analyzed with the Humanized PankoMab-GEX Antibody in Malignant and Normal Tissues of the Head and Neck. Anticancer Res. 2016, 36, 3179–3184. [Google Scholar]
- Mosely, S.I.; Prime, J.E.; Sainson, R.C.; Koopmann, J.O.; Wang, D.Y.; Greenawalt, D.M.; Ahdesmaki, M.J.; Leyland, R.; Mullins, S.; Pacelli, L.; et al. Rational Selection of Syngeneic Preclinical Tumor Models for Immunotherapeutic Drug Discovery. Cancer Immunol. Res. 2017, 5, 29. [Google Scholar] [CrossRef]
- Epardaud, M.; Elpek, K.G.; Rubinstein, M.P.; Yonekura, A.R.; Bellemare-Pelletier, A.; Bronson, R.; Hamerman, J.A.; Goldrath, A.W.; Turley, S.J. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008, 68, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Dubois, S.; Miljkovic, M.D.; Conlon, K.C. IL-15 in the Combination Immunotherapy of Cancer. Front. Immunol. 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Sibinovic, K.H.; Potter, N.; Hoostelaere; Rode, B.; Wax, J. Catalogue of Plasmacytomas and Other Tumors of the Lymphoreticular System, 3rd ed.; Litton Bionetics, Inc.: Kensington, MD, USA, 1976; pp. 1–33. [Google Scholar]

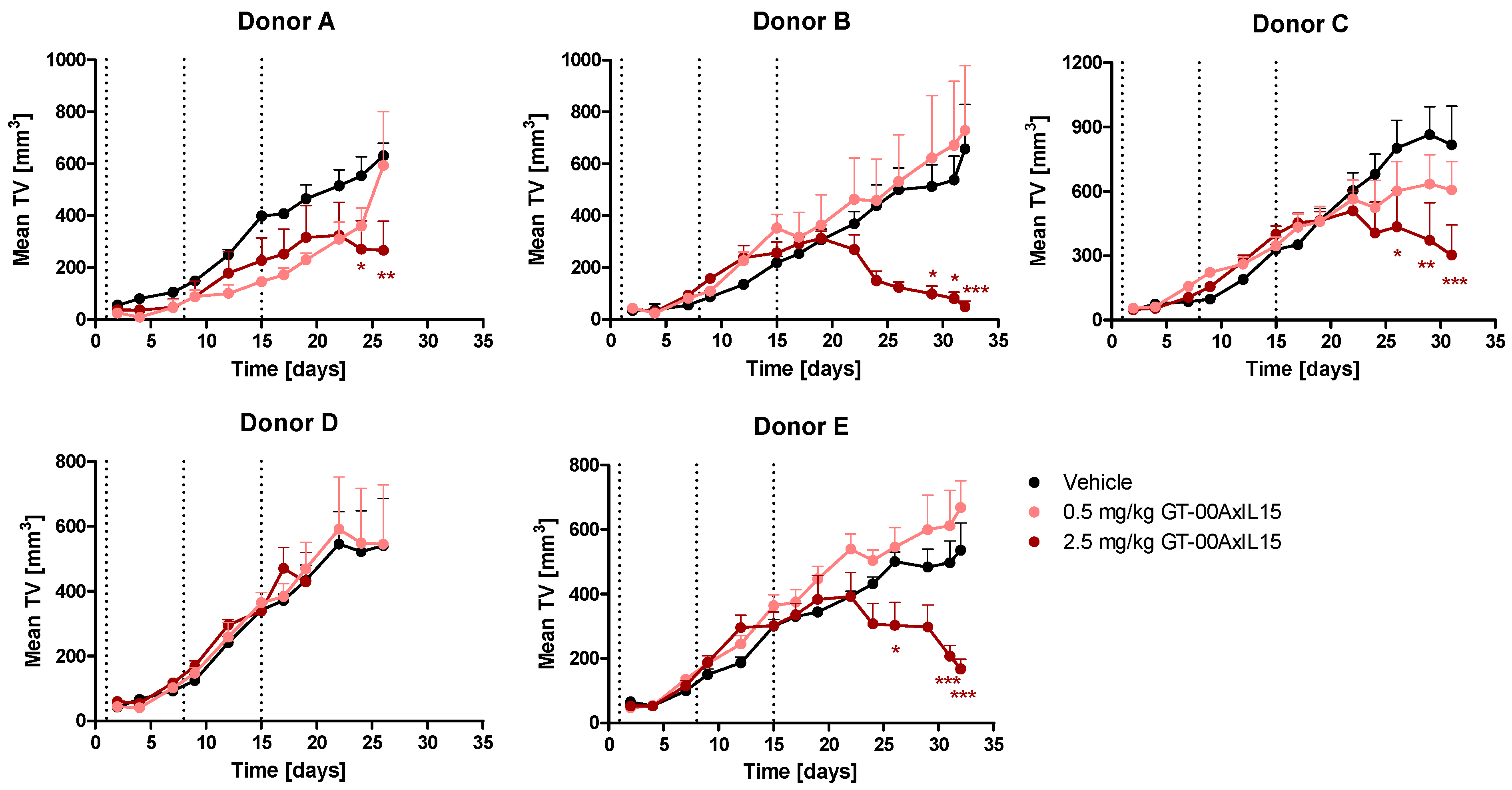
| PBMC Donor | TGI [%] by GT-00AxIL15 | ||
|---|---|---|---|
| Day | 2.5 mg/kg | 0.5 mg/kg | |
| A | 26 | 57.7 | 5.9 |
| B | 32 | 92.5 | −10.9 |
| C | 31 | 62.9 | 25.6 |
| D | 19 | 0.9 | −8.3 |
| E | 32 | 68.7 | −24.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gellert, J.; Jäkel, A.; Danielczyk, A.; Goletz, C.; Lischke, T.; Flechner, A.; Dix, L.; Günzl, A.; Kehler, P. GT-00AxIL15, a Novel Tumor-Targeted IL-15-Based Immunocytokine for the Treatment of TA-MUC1-Positive Solid Tumors: Preclinical In Vitro and In Vivo Pharmacodynamics and Biodistribution Studies. Int. J. Mol. Sci. 2024, 25, 1406. https://doi.org/10.3390/ijms25031406
Gellert J, Jäkel A, Danielczyk A, Goletz C, Lischke T, Flechner A, Dix L, Günzl A, Kehler P. GT-00AxIL15, a Novel Tumor-Targeted IL-15-Based Immunocytokine for the Treatment of TA-MUC1-Positive Solid Tumors: Preclinical In Vitro and In Vivo Pharmacodynamics and Biodistribution Studies. International Journal of Molecular Sciences. 2024; 25(3):1406. https://doi.org/10.3390/ijms25031406
Chicago/Turabian StyleGellert, Johanna, Anika Jäkel, Antje Danielczyk, Christoph Goletz, Timo Lischke, Anke Flechner, Laura Dix, Alexandra Günzl, and Patrik Kehler. 2024. "GT-00AxIL15, a Novel Tumor-Targeted IL-15-Based Immunocytokine for the Treatment of TA-MUC1-Positive Solid Tumors: Preclinical In Vitro and In Vivo Pharmacodynamics and Biodistribution Studies" International Journal of Molecular Sciences 25, no. 3: 1406. https://doi.org/10.3390/ijms25031406
APA StyleGellert, J., Jäkel, A., Danielczyk, A., Goletz, C., Lischke, T., Flechner, A., Dix, L., Günzl, A., & Kehler, P. (2024). GT-00AxIL15, a Novel Tumor-Targeted IL-15-Based Immunocytokine for the Treatment of TA-MUC1-Positive Solid Tumors: Preclinical In Vitro and In Vivo Pharmacodynamics and Biodistribution Studies. International Journal of Molecular Sciences, 25(3), 1406. https://doi.org/10.3390/ijms25031406







