Optimizing Yeast Homologous Recombination for Splicing Large Coronavirus Genome Fragments
Abstract
1. Introduction
2. Results
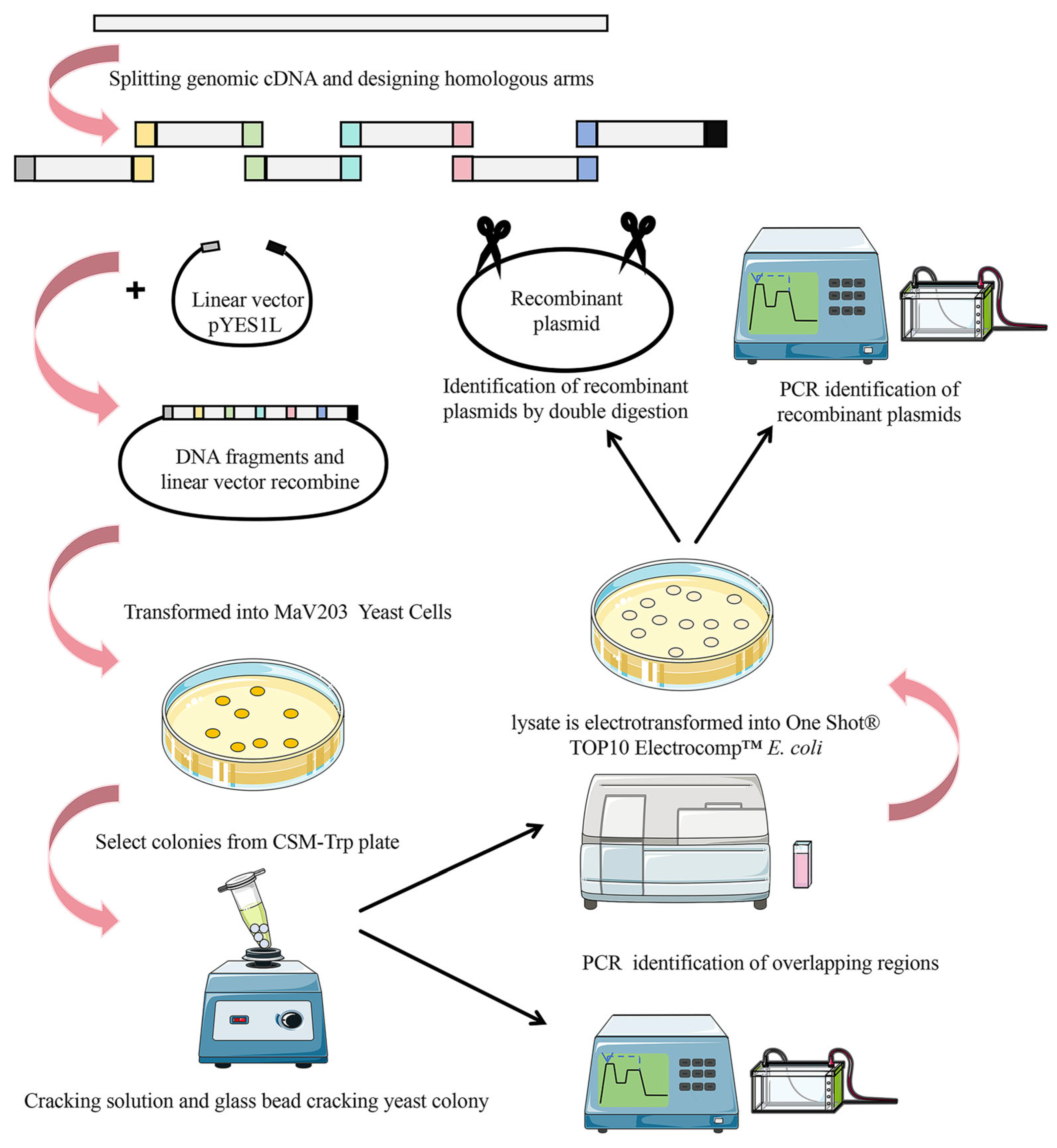
2.1. Selection of Homologous Arms for cDNA Fragments from Viral Genomes
2.2. Examining the Influence of Different Lengths of Homologous Arms and Various Fragment Carriers on Splicing Efficiency
2.3. Identification of the YAC Plasmid
3. Discussion
4. Materials and Methods
4.1. Plasmids and Bacterial Strains
4.2. Sequence Splitting, Homologous Arm Design, and Primer Design Synthesis
4.3. Amplification of Viral Genome Fragments and Homologous Recombination Splicing in Yeast
4.3.1. Viral Genome Fragment Amplification
4.3.2. Yeast Homologous Recombination Splicing of Viral Genome Fragments
4.4. PCR Identification of Yeast Homologous Recombination Products
4.4.1. Preparation of Yeast Cell Lysate Diluent
4.4.2. PCR System
4.5. Evaluation of Genome Splicing Efficiency
4.6. Transformation and Identification of the YAC Plasmid into Escherichia coli
4.6.1. Transformation of YAC Plasmid into Escherichia coli
4.6.2. YAC Plasmid PCR Identification
4.6.3. Identification of YAC Plasmid Double Enzyme Digestion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, H.-L.; Chen, Z.-L.; He, M.-J.; Cui, J.-Z.; Cheng, H.; Wang, Q.-Y.; Xiong, X.-H.; Liu, G.; Chen, H.-P. The Chimeric Chaoyang-Zika Vaccine Candidate Is Safe and Protective in Mice. Vaccines 2024, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Polonio, C.M.; Peron, J.P.S. ZIKV Infection and miRNA Network in Pathogenesis and Immune Response. Viruses 2021, 13, 1992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lv, P.; Li, M.; Chen, Z.; Xin, H.; Reilly, S.; Zhang, X. SARS-CoV-2 E protein: Pathogenesis and potential therapeutic development. Biomed. Pharmacother. 2023, 159, 114242. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Elsayed, S.; Bondy, L.; Hanage, W.P. Monkeypox Virus Infections in Humans. Clin. Microbiol. Rev. 2022, 35, e0009222. [Google Scholar] [CrossRef]
- Peng, Q.; Fang, L.; Ding, Z.; Wang, D.; Peng, G.; Xiao, S. Rapid manipulation of the porcine epidemic diarrhea virus genome by CRISPR/Cas9 technology. J. Virol. Methods 2019, 276, 113772. [Google Scholar] [CrossRef]
- Ma, S.; Tang, N.; Tian, J. DNA synthesis, assembly and applications in synthetic biology. Curr. Opin. Chem. Biol. 2012, 16, 260–267. [Google Scholar] [CrossRef]
- Engler, C.; Kandzia, R.; Marillonnet, S. A One Pot, One Step, Precision Cloning Method with High Throughput Capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef]
- Li, M.Z.; Elledge, S.J. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 2007, 4, 251–256. [Google Scholar] [CrossRef]
- de Kok, S.; Stanton, L.H.; Slaby, T.; Durot, M.; Holmes, V.F.; Patel, K.G.; Platt, D.; Shapland, E.B.; Serber, Z.; Dean, J.; et al. Rapid and Reliable DNA Assembly via Ligase Cycling Reaction. ACS Synth. Biol. 2014, 3, 97–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Werling, U.; Edelmann, W. SLiCE: A novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res. 2012, 40, e55. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Tian, J. Circular Polymerase Extension Cloning of Complex Gene Libraries and Pathways. PLoS ONE 2009, 4, e6441. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A., III; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; Ajioka, J.W. High molecular weight DNA assembly in vivo for synthetic biology applications. Crit. Rev. Biotechnol. 2016, 37, 277–286. [Google Scholar] [CrossRef]
- Shizuya, H.; Birren, B.; Kim, U.J.; Mancino, V.; Slepak, T.; Tachiiri, Y.; Simon, M. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl. Acad. Sci. USA 1992, 89, 8794–8797. [Google Scholar] [CrossRef]
- Kaneko, S.; Tsuge, K.; Takeuchi, T.; Itaya, M. Conversion of sub-megasized DNA to desired structures using a novel Bacillus subtilis genome vector. Nucleic Acids Res. 2003, 31, e112. [Google Scholar] [CrossRef]
- Burke, D.T.; Carle, G.F.; Olson, M.V. Cloning of Large Segments of Exogenous DNA into Yeast by Means of Artificial Chromosome Vectors. Science 1987, 236, 806–812. [Google Scholar] [CrossRef]
- Ogawa, T.; Iwata, T.; Kaneko, S.; Itaya, M.; Hirota, J. An inducible recA expression Bacillus subtilis genome vector for stable manipulation of large DNA fragments. BMC Genom. 2015, 16, 209. [Google Scholar] [CrossRef]
- Gibson, D.G. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic Acids Res. 2009, 37, 6984–6990. [Google Scholar] [CrossRef]
- Lin, Q.; Jia, B.; Mitchell, L.A.; Luo, J.; Yang, K.; Zeller, K.I.; Zhang, W.; Xu, Z.; Stracquadanio, G.; Bader, J.S.; et al. RADOM, an Efficient In Vivo Method for Assembling Designed DNA Fragments up to 10 kb Long in Saccharomyces cerevisiae. ACS Synth. Biol. 2014, 4, 213–220. [Google Scholar] [CrossRef]
- Jakočiūnas, T.; Rajkumar, A.S.; Zhang, J.; Arsovska, D.; Rodriguez, A.; Jendresen, C.B.; Skjødt, M.L.; Nielsen, A.T.; Borodina, I.; Jensen, M.K.; et al. CasEMBLR: Cas9-Facilitated Multiloci Genomic Integration of in Vivo Assembled DNA Parts in Saccharomyces cerevisiae. ACS Synth. Biol. 2015, 4, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Benders, G.A.; Axelrod, K.C.; Zaveri, J.; Algire, M.A.; Moodie, M.; Montague, M.G.; Venter, J.C.; Smith, H.O.; Hutchison, C.A. One-step assembly in yeast of 25 overlapping DNA fragments to form a complete synthetic Mycoplasma genitalium genome. Proc. Natl. Acad. Sci. USA 2008, 105, 20404–20409. [Google Scholar] [CrossRef] [PubMed]
- Thao, T.T.N.; Labroussaa, F.; Ebert, N.; V’Kovski, P.; Stalder, H.; Portmann, J.; Kelly, J.; Steiner, S.; Holwerda, M.; Kratzel, A.; et al. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature 2020, 582, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lokugamage, K.G.; Zhang, X.; Vu, M.N.; Muruato, A.E.; Menachery, V.D.; Shi, P.-Y. Engineering SARS-CoV-2 using a reverse genetic system. Nat. Protoc. 2021, 16, 1761–1784. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Hu, S.; Wan, W.; Xu, M.; Du, R.; Zhao, W.; Gao, X.; Liu, J.; Liu, H.; Hong, J. Effective Optimization of Antibody Affinity by Phage Display Integrated with High-Throughput DNA Synthesis and Sequencing Technologies. PLoS ONE 2015, 10, e0129125. [Google Scholar] [CrossRef]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Lundin, K.E.; Gissberg, O.; Smith, C.E. Oligonucleotide Therapies: The Past and the Present. Hum. Gene Ther. 2015, 26, 475–485. [Google Scholar] [CrossRef]
- Hillson, N.J.; Rosengarten, R.D.; Keasling, J.D. j5 DNA Assembly Design Automation Software. ACS Synth. Biol. 2011, 1, 14–21. [Google Scholar] [CrossRef]
- Storch, M.; Haines, M.C.; Baldwin, G.S. DNA-BOT: A low-cost, automated DNA assembly platform for synthetic biology. Synth. Biol. 2020, 5, ysaa010. [Google Scholar] [CrossRef]
- McInerney, P.; Adams, P.; Hadi, M.Z. Error Rate Comparison during Polymerase Chain Reaction by DNA Polymerase. Mol. Biol. Int. 2014, 2014, 287430. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-L.; Huang, Y.-W. Reverse genetics systems for SARS-CoV-2: Development and applications. Virol. Sin. 2023, 38, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Cello, J.; Paul, A.V.; Wimmer, E. Chemical synthesis of poliovirus cDNA: Generation of infectious virus in the absence of natural template. Science 2002, 297, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.O.; Hutchison, C.A.; Pfannkoch, C.; Venter, J.C. Generating a synthetic genome by whole genome assembly: phiX174 bacteriophage from synthetic oligonucleotides. Proc. Natl. Acad. Sci. USA 2003, 100, 15440–15445. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Benders, G.A.; Andrews-Pfannkoch, C.; Denisova, E.A.; Baden-Tillson, H.; Zaveri, J.; Stockwell, T.B.; Brownley, A.; Thomas, D.W.; Algire, M.A.; et al. Complete Chemical Synthesis, Assembly, and Cloning of a Mycoplasma genitalium Genome. Science 2008, 319, 1215–1220. [Google Scholar] [CrossRef]
- Gibson, D.G.; Glass, J.I.; Lartigue, C.; Noskov, V.N.; Chuang, R.-Y.; Algire, M.A.; Benders, G.A.; Montague, M.G.; Ma, L.; Moodie, M.M.; et al. Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science 2010, 329, 52–56. [Google Scholar] [CrossRef]
- Hillson, N.; Caddick, M.; Cai, Y.; Carrasco, J.A.; Chang, M.W.; Curach, N.C.; Bell, D.J.; Le Feuvre, R.; Friedman, D.C.; Fu, X.; et al. Building a global alliance of biofoundries. Nat. Commun. 2019, 10, 2040. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Fu, L.; Guo, E.; Wang, B.; Dai, L.; Si, T. Accelerating strain engineering in biofuel research via build and test automation of synthetic biology. Curr. Opin. Biotechnol. 2021, 67, 88–98. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Cheng, L.; Luo, Y. An overview and metanalysis of machine and deep learning-based CRISPR gRNA design tools. RNA Biol. 2019, 17, 13–22. [Google Scholar] [CrossRef]
- Carbonell, P.; Radivojevic, T.; Martín, H.G. Opportunities at the Intersection of Synthetic Biology, Machine Learning, and Automation. ACS Synth. Biol. 2019, 8, 1474–1477. [Google Scholar] [CrossRef]
- Gibson, D.G. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011, 498, 349–361. [Google Scholar] [CrossRef]
- SantaLucia, J., Jr.; Allawi, H.T.; Seneviratne, P.A. Improved Nearest-Neighbor Parameters for Predicting DNA Duplex Stability. Biochemistry 1996, 35, 3555–3562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, G.; Huang, X.; Hu, A.; Meng, Z.; Cui, J.; Feng, Y.; Chen, Z.; Lu, Y.; Yang, Q.; Liu, G. Optimizing Yeast Homologous Recombination for Splicing Large Coronavirus Genome Fragments. Int. J. Mol. Sci. 2024, 25, 13742. https://doi.org/10.3390/ijms252413742
Xiong G, Huang X, Hu A, Meng Z, Cui J, Feng Y, Chen Z, Lu Y, Yang Q, Liu G. Optimizing Yeast Homologous Recombination for Splicing Large Coronavirus Genome Fragments. International Journal of Molecular Sciences. 2024; 25(24):13742. https://doi.org/10.3390/ijms252413742
Chicago/Turabian StyleXiong, Guoqing, Xuan Huang, Ao Hu, Zhixin Meng, Jiazhen Cui, Yuzhong Feng, Zhili Chen, Yuanyuan Lu, Qi Yang, and Gang Liu. 2024. "Optimizing Yeast Homologous Recombination for Splicing Large Coronavirus Genome Fragments" International Journal of Molecular Sciences 25, no. 24: 13742. https://doi.org/10.3390/ijms252413742
APA StyleXiong, G., Huang, X., Hu, A., Meng, Z., Cui, J., Feng, Y., Chen, Z., Lu, Y., Yang, Q., & Liu, G. (2024). Optimizing Yeast Homologous Recombination for Splicing Large Coronavirus Genome Fragments. International Journal of Molecular Sciences, 25(24), 13742. https://doi.org/10.3390/ijms252413742




