Multi-Objective Optimization Accelerates the De Novo Design of Antimicrobial Peptide for Staphylococcus aureus
Abstract
1. Introduction
2. Results
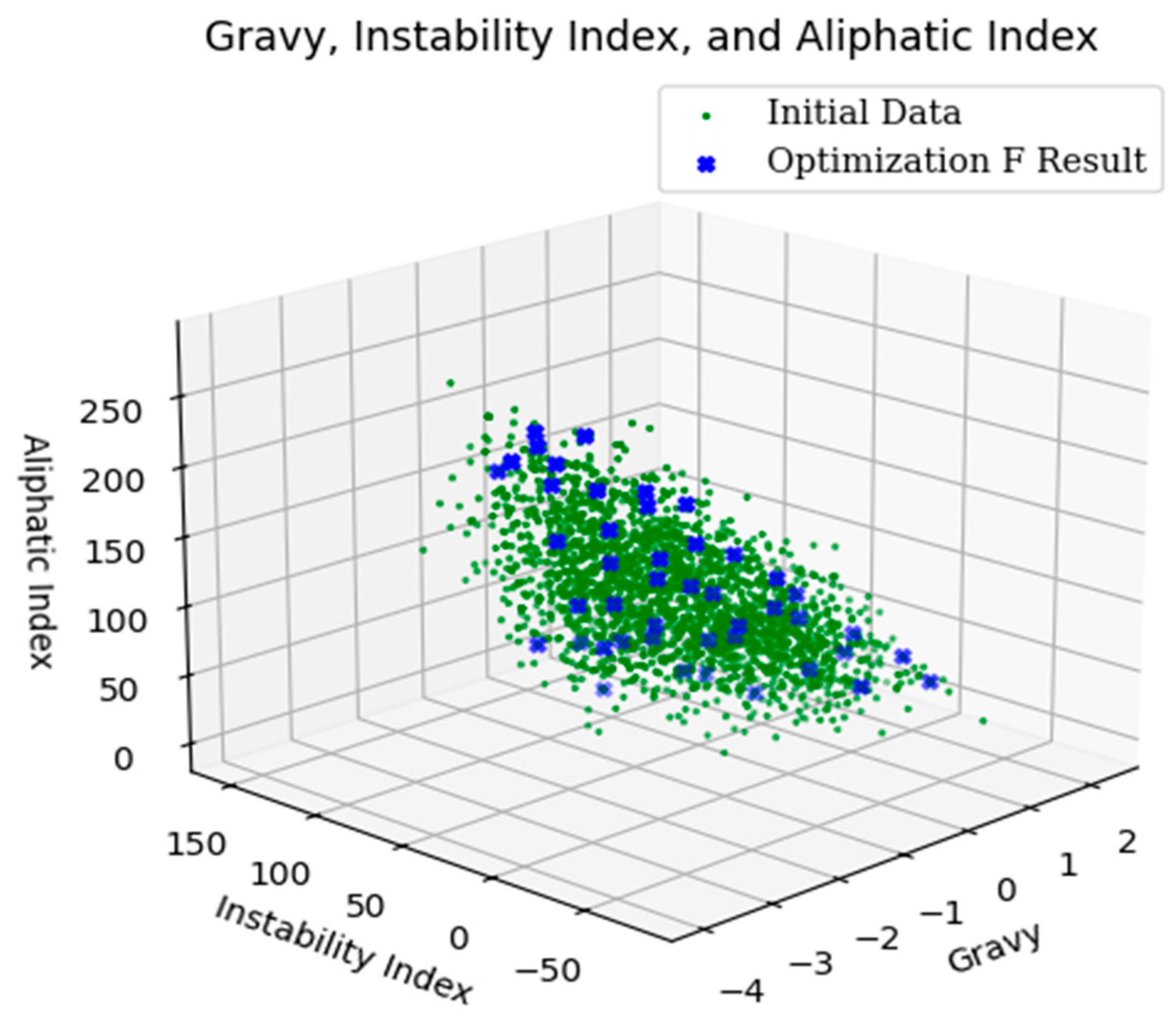
2.1. Multi-Objective Optimization Result
2.2. Neural Networks Result
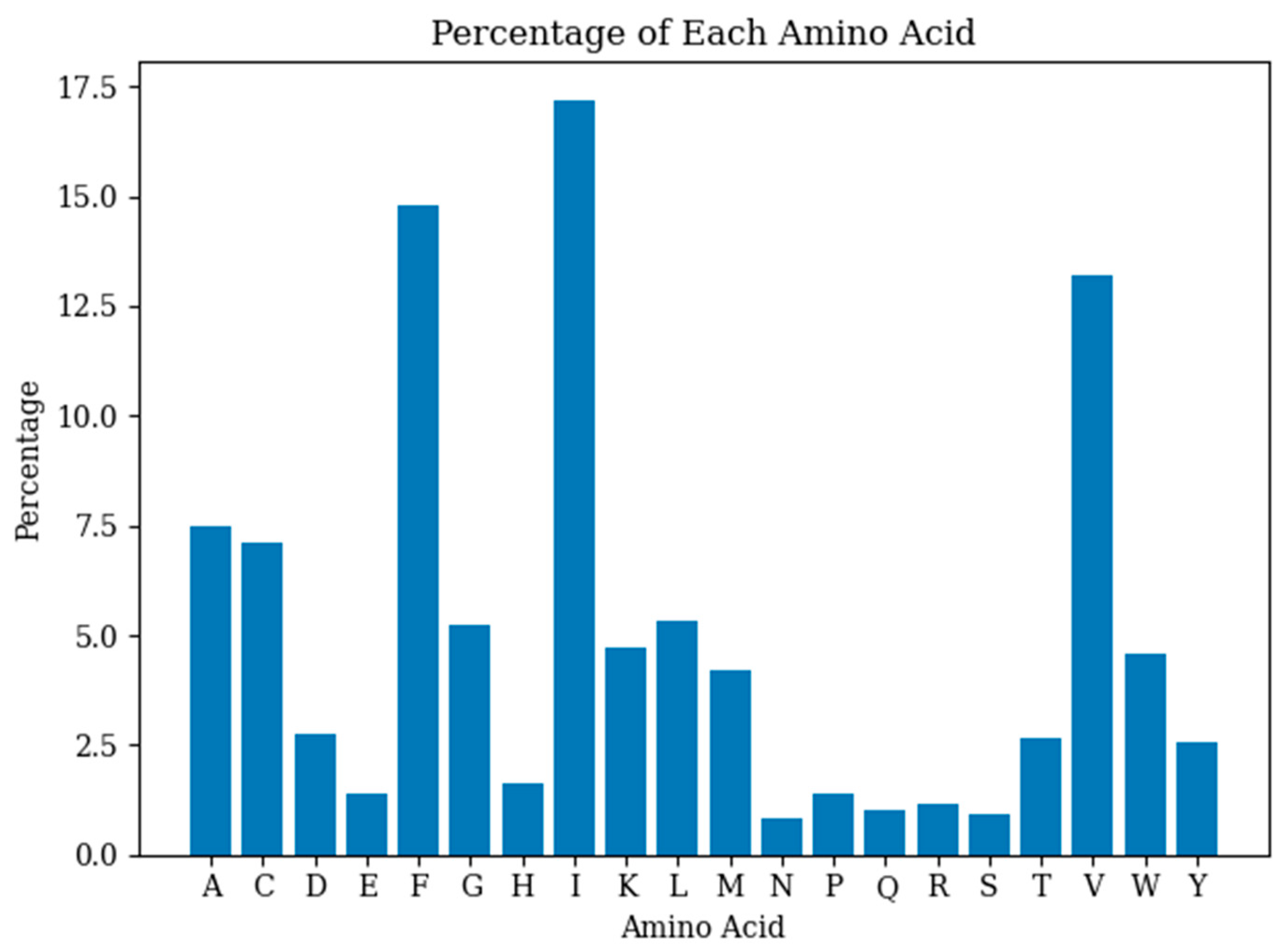
2.3. Antimicrobial Peptides Design Metrics
2.4. Antibacterial Activity of the Designed Peptides
3. Discussion
3.1. Key Limitations of AMPs
3.2. Considered Physicochemical Properties
3.3. MIC Prediction
3.4. Techniques Application
3.5. Future Directions in AMP Optimization
4. Materials and Methods
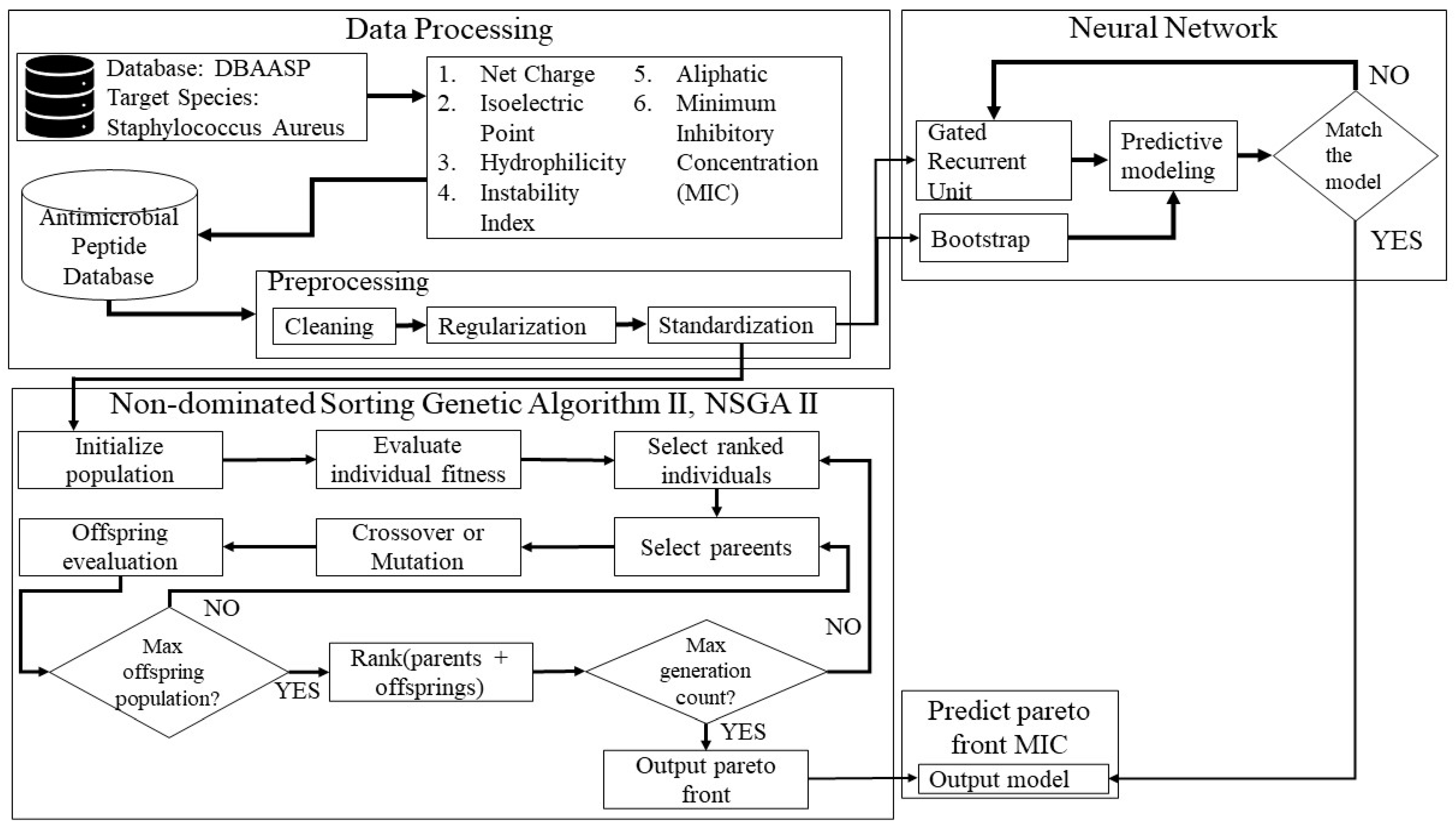
4.1. Proposed Model
4.2. Dataset
4.3. Methods
4.3.1. Non-Dominated Sorting Genetic Algorithm II
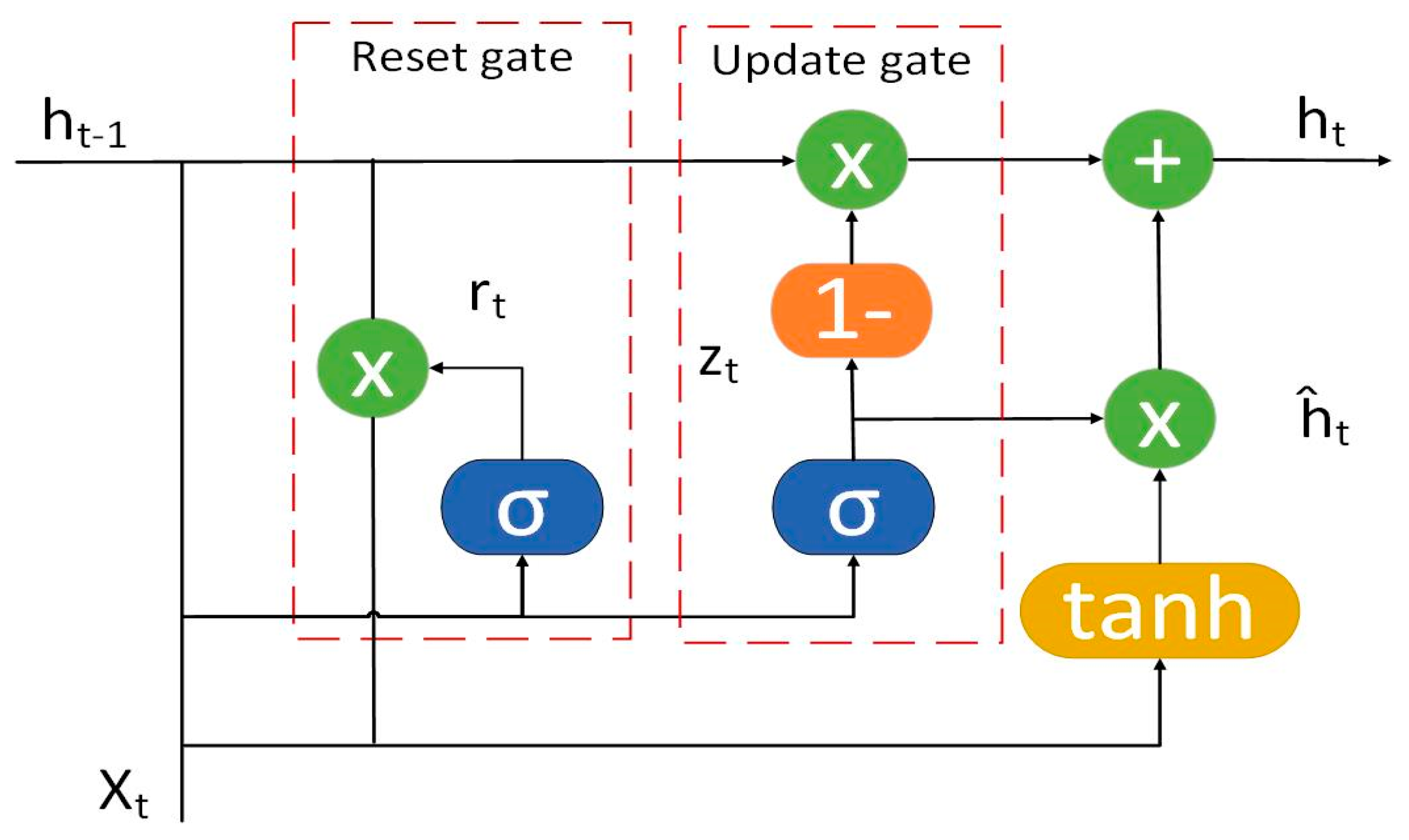
4.3.2. Neural Networks
4.4. Evaluation of Antibacterial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, K.; Thwood, D.; Elgheriani, H.; Salem, M.; Elgadiym, Z.; Zaghdani, A.; Alhudiri, I.; Habibi, A.; Elfahem, A.; Belaid, S.; et al. Prevalence of multi-drug resistant bacteria in intensive care units at Tripoli University Hospital, Tripoli, Libya. Libyan J. Med. 2024, 19, 2348235. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Bello-Madruga, R.; Burgas, M.T. The limits of prediction: Why intrinsically disordered regions challenge our understanding of antimicrobial peptides. Comput. Struct. Biotechnol. J. 2024, 23, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef]
- Büyükkiraz, M.E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2021, 132, 1573–1596. [Google Scholar] [CrossRef]
- de Oliveira, K.B.S.; Leite, M.L.; Cunha, V.A.; da Cunha, N.B.; Franco, O.L. Challenges and advances in antimicrobial peptide development. Drug Discov. Today 2023, 28, 103629. [Google Scholar] [CrossRef]
- Arenas, I.; Villegas, E.; Walls, O.; Barrios, H.; Rodríguez, R.; Corzo, G. Antimicrobial Activity and Stability of Short and Long Based Arachnid Synthetic Peptides in the Presence of Commercial Antibiotics. Molecules 2016, 21, 225. [Google Scholar] [CrossRef]
- de Oliveira, K.B.S.; Leite, M.L.; Rodrigues, G.R.; Duque, H.M.; da Costa, R.A.; Cunha, V.A.; Costa, L.S.d.L.; da Cunha, N.B.; Franco, O.L.; Dias, S.C. Strategies for recombinant production of antimicrobial peptides with pharmacological potential. Expert Rev. Clin. Pharmacol. 2020, 13, 367–390. [Google Scholar] [CrossRef]
- Nuti, R.; Goud, N.S.; Saraswati, A.P.; Alvala, R.; Alvala, M. Antimicrobial Peptides: A Promising Therapeutic Strategy in Tackling Antimicrobial Resistance. Curr. Med. Chem. 2017, 24, 4303–4314. [Google Scholar] [CrossRef]
- Ghimire, J.; Hart, R.J.; Soldano, A.; Chen, C.H.; Guha, S.; Hoffmann, J.P.; Hall, K.M.; Sun, L.; Nelson, B.J.; Lu, T.K.; et al. Optimization of Host Cell-Compatible, Antimicrobial Peptides Effective against Biofilms and Clinical Isolates of Drug-Resistant Bacteria. ACS Infect. Dis. 2023, 9, 952–965. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Silva, A.F.; Andrade, G.P.; Pedron, C.N.; Cerchiaro, G.; Ribeiro, A.O.; Oliveira, V.X., Jr.; de la Fuente-Nunez, C. The wasp venom antimicrobial peptide polybia-cp and its synthetic derivatives display antiplasmodial and anticancer properties. Bioeng. Transl. Med. 2020, 5, e10167. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Subrahmanyam, B.R.; Janani, G.K.; Kalyani, D. PCSPred: Prediction of Short Chain Antimicrobial Peptides using Machine Learning Algorithms. In Proceedings of the 2023 International Conference on Next Generation Electronics (NEleX), Vellore, India, 14–16 December 2023; pp. 1–5. [Google Scholar]
- Yin, K.; Xu, W.; Ren, S.; Xu, Q.; Zhang, S.; Zhang, R.; Jiang, M.; Zhang, Y.; Xu, D.; Li, R. Machine Learning Accelerates De Novo Design of Antimicrobial Peptides. Interdiscip. Sci. Comput. Life Sci. 2024, 16, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Cai, J.; Zhang, B.; Wang, Y.; Wong, D.F.; Siu, S.W.I. Recent Progress in the Discovery and Design of Antimicrobial Peptides Using Traditional Machine Learning and Deep Learning. Antibiotics 2022, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.; Lee, I.; Nam, H. AMP-BERT: Prediction of antimicrobial peptide function based on a BERT model. Protein Sci. 2022, 32, e4529. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Ge, C.; Wang, X.; Harvey, P.J.; Zhang, Z.; Ma, Y.; Wang, X.; Jia, X.; Mobli, M.; Craik, D.J.; et al. Designing antimicrobial peptides using deep learning and molecular dynamic simulations. Briefings Bioinform. 2023, 24, bbad058. [Google Scholar] [CrossRef]
- Teimouri, H.; Medvedeva, A.; Kolomeisky, A.B. Bacteria-Specific Feature Selection for Enhanced Antimicrobial Peptide Activity Predictions Using Machine-Learning Methods. J. Chem. Inf. Model. 2023, 63, 1723–1733. [Google Scholar] [CrossRef]
- Chung, C.-R.; Liou, J.-T.; Wu, L.-C.; Horng, J.-T.; Lee, T.-Y. Multi-label classification and features investigation of antimicrobial peptides with various functional classes. iScience 2023, 26, 108250. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2020, 49, D288–D297. [Google Scholar] [CrossRef]
- Blank, J.; Deb, K. Pymoo: Multi-Objective Optimization in Python. IEEE Access 2020, 8, 89497–89509. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of Peptide Hydrophobicity in the Mechanism of Action of α-Helical Antimicrobial Peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef]
- Guruprasad, K.; Reddy, B.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. Des. Sel. 1990, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ikai, A. Thermostability and Aliphatic Index of Globular Proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- A Blair, J.M.; Zeth, K.; Bavro, V.N.; Sancho-Vaello, E. The role of bacterial transport systems in the removal of host antimicrobial peptides in Gram-negative bacteria. FEMS Microbiol. Rev. 2022, 46, fuac032. [Google Scholar] [CrossRef] [PubMed]
- Kocourková, L.; Novotná, P.; Čujová, S.; Čeřovský, V.; Urbanová, M.; Setnička, V. Conformational study of melectin and antapin antimicrobial peptides in model membrane environments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 170, 247–255. [Google Scholar] [CrossRef]
- Gagat, P.; Ostrówka, M.; Duda-Madej, A.; Mackiewicz, P. Enhancing Antimicrobial Peptide Activity through Modifications of Charge, Hydrophobicity, and Structure. Int. J. Mol. Sci. 2024, 25, 10821. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Pept. Sci. 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Miszkiewicz, J.; Trylska, J. Conformational changes of anoplin, w-mreb1–9, and (kff)3k peptides near the membranes. Int. J. Mol. Sci. 2020, 21, 9672. [Google Scholar] [CrossRef]
- Mourtada, R.; Herce, H.D.; Yin, D.J.; Moroco, J.A.; Wales, T.E.; Engen, J.R.; Walensky, L.D.J.N.B. Design of stapled antimicrobial peptides that overcome antibiotic resistance and in vivo toxicity. Nat. Biotechnol. 2019, 37, 1186. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta BBA-Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Veltri, D.; Kamath, U.; Shehu, A. Deep learning improves antimicrobial peptide recognition. Bioinformatics 2018, 34, 2740–2747. [Google Scholar] [CrossRef]
- Luukkonen, S.; Maagdenberg, H.W.v.D.; Emmerich, M.T.; van Westen, G.J. Artificial intelligence in multi-objective drug design. Curr. Opin. Struct. Biol. 2023, 79, 102537. [Google Scholar] [CrossRef] [PubMed]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Verma, S.; Pant, M.; Snasel, V. A Comprehensive Review on NSGA-II for Multi-Objective Combinatorial Optimization Problems. IEEE Access 2021, 9, 57757–57791. [Google Scholar] [CrossRef]
- Fathi, F.; Ghobeh, M.; Shirazi, F.H.; Tabarzad, M. Design and Evaluation of a Novel Anti-microbial Peptide from Cathelicidin-2: Selectively Active Against Acinetobacter baumannii. Iran. J. Pharm. Res. 2023, 22, e141920. [Google Scholar] [CrossRef]


| Population size | 100 |
| Number of iterations | 50 |
| Crossover probability | 0.9 |
| Mutation probability | 0.9 |
| Crossover index | 15 |
| Mutation index | 20 |
| Sequence | Gravy | Instability Index | Aliphatic Index | Isoelectric Point | Net Charge | MIC |
|---|---|---|---|---|---|---|
| CVVRCRCVFR | 0.94 | −6.08 | 87 | 9.307123 | 3 | 3.732 |
| CVVVDRIVDR | 0.78 | −52.17 | 155 | 5.954078 | 0 | 6.47 |
| RCVCCRIVRF | 0.97 | −7.03 | 97 | 9.307123 | 3 | 3.822 |
| RCVCVRIVTR | 0.79 | −23.06 | 126 | 10.41282 | 3 | 4.727 |
| RCVTRVVIVR | 0.96 | −15.52 | 155 | 11.70032 | 3 | 5.3 |
| RCVVTRIVRF | 0.82 | −15.52 | 126 | 11.70032 | 3 | 4.797 |
| RIVDRIVVVR | 0.88 | −30.55 | 194 | 11.69903 | 2 | 6.11 |
| RIVDRVIVVR | 0.88 | −30.55 | 194 | 11.69903 | 2 | 6.11 |
| RIVDRVVIVR | 0.88 | −30.55 | 194 | 11.69903 | 2 | 6.11 |
| RIVVDRIVVR | 0.88 | −30.55 | 194 | 11.69903 | 2 | 6.11 |
| RIVVDRVIVR | 0.88 | −30.55 | 194 | 11.69903 | 2 | 6.11 |
| VCVCVRMRCR | 0.85 | −13.62 | 87 | 9.305834 | 3 | 4.19 |
| VCVDRFVDRV | 0.61 | −43.68 | 116 | 5.92526 | 0 | 4.805 |
| VCVDRVVDRF | 0.61 | −43.68 | 116 | 5.92526 | 0 | 4.805 |
| VCVRCRCVFR | 0.94 | −6.08 | 87 | 9.305834 | 3 | 3.736 |
| VCVVDRIVDR | 0.78 | −52.17 | 155 | 5.92526 | 0 | 6.473 |
| VRVRIVYKVC | 0.96 | −15.52 | 155 | 10.05334 | 3 | 5.426 |
| VRVTRCVIVR | 0.96 | −15.52 | 155 | 11.70032 | 3 | 5.29 |
| VVDRCVDRIV | 0.78 | −52.17 | 155 | 5.92526 | 0 | 6.473 |
| Peptides | MIC * (μg/mL) | |||
|---|---|---|---|---|
| Bacteria Strains | Predicted | |||
| Sa 6538P | Sa 1075 | Sa 2803 | ||
| 1. CVVRCRCVFR | 12.5 | >100 | >100 | 3.732 |
| 2. RCVCCRIVRF | 12.5 | >100 | 100 | 3.822 |
| 3. RCVCVRIVTR | 100 | >100 | >100 | 4.727 |
| 4. RCVTRVVIVR | 12.5 | >100 | >100 | 5.300 |
| 5. RCVVTRIVRF | 12.5 | >100 | >100 | 4.797 |
| 6. RIVDRIVVVR | >100 | >100 | >100 | 6.110 |
| 7. RIVDRVIVVR | >100 | >100 | >100 | 6.110 |
| 8. RIVDRVVIVR | >100 | >100 | >100 | 6.110 |
| 9. VCVCVRMRCR | >100 | >100 | 25 | 6.110 |
| 10. VCVRCRCVFR | >100 | >100 | 12.5 | 4.805 |
| 11. VRVRIVYKVC | >100 | 12.5 | 3.125 | 6.473 |
| 12. VRVTRCVIVR | >100 | >100 | 6.25 | 5.426 |
| colistin | >100 | >100 | 1.56 | ND |
| polymyxin B | 100 | >100 | 12.5 | ND |
| poly-L-lysine hydrobromide | 50 | 100 | 50 | ND |
| Tetracycline | 1250 | 1250 | 1250 | ND |
| Sequence | Class | Probability | Sequence | Class | Probability |
|---|---|---|---|---|---|
| CVVRCRCVFR | AMP | 0.9989 | RIVVDRVIVR | Non-AMP | 0.0283 |
| CVVVDRIVDR | Non-AMP | 0.0129 | VCVCVRMRCR | AMP | 0.9996 |
| RCVCCRIVRF | AMP | 0.9469 | VCVDRFVDRV | Non-AMP | 0.0268 |
| RCVCVRIVTR | AMP | 0.9947 | VCVDRVVDRF | Non-AMP | 0.089 |
| RCVTRVVIVR | AMP | 0.9385 | VCVRCRCVFR | AMP | 0.9916 |
| RCVVTRIVRF | AMP | 0.9885 | VCVVDRIVDR | Non-AMP | 0.0257 |
| RIVDRIVVVR | Non-AMP | 0.0643 | VRVRIVYKVC | AMP | 0.997 |
| RIVDRVIVVR | Non-AMP | 0.0699 | VRVTRCVIVR | AMP | 0.7774 |
| RIVDRVVIVR | Non-AMP | 0.0818 | VVDRCVDRIV | Non-AMP | 0.0085 |
| RIVVDRIVVR | Non-AMP | 0.0103 |
| Epochs | 2000 |
| Batch size | 1024 |
| Hidden layer activation | Linear |
| Layer | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Chen, Y.-L.; Cheung, T.-H.; Chuang, L.-Y. Multi-Objective Optimization Accelerates the De Novo Design of Antimicrobial Peptide for Staphylococcus aureus. Int. J. Mol. Sci. 2024, 25, 13688. https://doi.org/10.3390/ijms252413688
Yang C-H, Chen Y-L, Cheung T-H, Chuang L-Y. Multi-Objective Optimization Accelerates the De Novo Design of Antimicrobial Peptide for Staphylococcus aureus. International Journal of Molecular Sciences. 2024; 25(24):13688. https://doi.org/10.3390/ijms252413688
Chicago/Turabian StyleYang, Cheng-Hong, Yi-Ling Chen, Tin-Ho Cheung, and Li-Yeh Chuang. 2024. "Multi-Objective Optimization Accelerates the De Novo Design of Antimicrobial Peptide for Staphylococcus aureus" International Journal of Molecular Sciences 25, no. 24: 13688. https://doi.org/10.3390/ijms252413688
APA StyleYang, C.-H., Chen, Y.-L., Cheung, T.-H., & Chuang, L.-Y. (2024). Multi-Objective Optimization Accelerates the De Novo Design of Antimicrobial Peptide for Staphylococcus aureus. International Journal of Molecular Sciences, 25(24), 13688. https://doi.org/10.3390/ijms252413688







