Epigenetic Biomarkers in Thrombophilia-Related Pregnancy Complications: Mechanisms, Diagnostic Potential, and Therapeutic Implications: A Narrative Review
Abstract
1. Introduction
2. Physiology of the Coagulation System During Pregnancy
2.1. Adaptations of the Coagulation Cascade During Pregnancy
2.2. Role of Coagulation in Trophoblast Formation and Invasion
2.3. Angiogenesis and the Coagulation Cascade
2.4. Immune Regulation and Coagulation
3. The Impact of Thrombophilias During Pregnancy
3.1. Complications of Thrombophilias During Pregnancy
3.1.1. Intra-Uterine Growth Restriction (IUGR)
3.1.2. Recurrent Pregnancy Loss (RPL)
3.1.3. Stillbirth
3.1.4. Pre-Eclampsia
3.1.5. Venous Thromboembolism (VTE)
3.2. Congenital Thrombophilias in Pregnancy
3.2.1. Factor V Leiden Mutation
3.2.2. Prothrombin Gene Mutation (G20210A)
3.2.3. Deficiencies in Natural Anticoagulants
3.2.4. MTHFR Mutation
3.3. Acquired Thrombophilias in Pregnancy
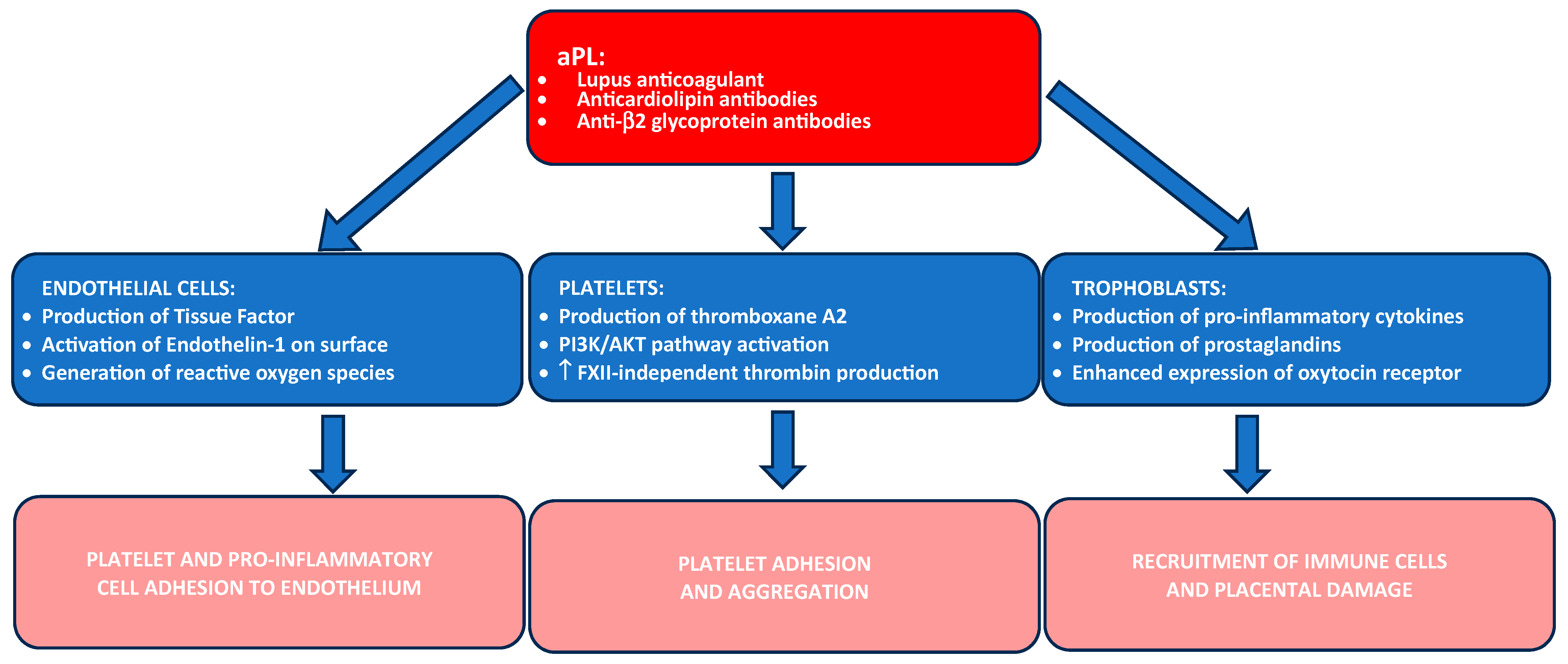
Antiphospholipid Syndrome (APS)
4. Epigenetic Regulation in Coagulation During Pregnancy
4.1. Role of miRNAs in Coagulation and Vascular Health
4.2. Role of lncRNAs in Coagulation and Immune Modulation
4.3. Extracellular Vesicles and Their Role in Coagulation and Pregnancy
5. Epigenetic Biomarkers as Diagnostic Tools for Thrombophilia-Related Complications
5.1. MiRNAs as Non-Invasive Diagnostic Biomarkers
- miR-223: Known for its role in tissue factor regulation, miR-223 is associated with increased thrombin generation and a hypercoagulable state, making it a potential biomarker for assessing thrombotic risk related to APS, Factor V Leiden mutation, and protein C and S deficiencies [65].
- miR-210: Often upregulated under hypoxic conditions, miR-210 is involved in trophoblast invasion and placental development. Several studies have shown its involvement in processes associated with venous thrombosis, making it a good candidate as a biomarker for high-risk pregnancies associated with thrombophilias, especially with APS [56,93].
5.2. LncRNAs as Emerging Diagnostic Biomarkers
- MALAT1: Known for its role in promoting endothelial cell proliferation and angiogenesis, MALAT1 is downregulated in cases of pre-eclampsia and IUGR, indicating vascular insufficiency. Monitoring MALAT1 could offer insights into placental health and predict complications related to endothelial dysfunction [98].
- H19: This lncRNA is crucial for trophoblast invasion and placental development. Low H19 expression in maternal blood correlates with placental insufficiency, recurrent miscarriage, and pre-eclampsia, making it a valuable early marker of placental dysfunction in patients with congenital or acquired thrombophilias [99].
5.3. Extracellular Vesicles as Diagnostic Biomarkers
- Tissue factor-bearing EVs: The presence of tissue factor on EVs contributes to a hypercoagulable state in thrombophilic pregnancies [100]. Elevated levels of these EVs in maternal blood correlate with a higher risk of placental thrombosis and vascular insufficiency, suggesting their utility as biomarkers for thrombotic complications [101,102].
- EVs as miRNA and lncRNA cargo: EVs carrying miRNAs and lncRNAs involved in coagulation and immune regulation can provide a non-invasive means of monitoring immune-mediated and vascular-related risks in pregnancy. For example, EVs enriched with miR-210 may reflect placental hypoxia, while EVs with high levels of HOTAIR could indicate immune dysregulation in APS [103].
6. Epigenetic Biomarkers for Therapy Assessment and Future Directions in Managing Thrombophilia-Related Pregnancy Complications
6.1. The Role of Epigenetic Biomarkers in the Assessment of Anticoagulant Therapy Efficacy
6.1.1. MiRNAs as Therapeutic Biomarkers
6.1.2. LncRNAs and Therapeutic Monitoring
6.1.3. Extracellular Vesicles in Therapy Assessment
6.2. Personalized Medicine: Tailoring Treatment Based on Biomarker Profiles
6.2.1. Risk Stratification Based on Biomarkers
6.2.2. Targeting Epigenetic Mechanisms for Therapeutic Intervention
6.3. Use of Emerging Technologies for Biomarker Discovery and Validation
6.3.1. Next-Generation Sequencing (NGS) and RNA Sequencing (RNA-seq)
6.3.2. Single-Cell Transcriptomics
6.3.3. Potential Utility of Artificial Intelligence
6.4. Possible Study Limitations
6.5. Future Directions in Clinical Translation and Biomarker Standardization
6.5.1. Validation in Large and Diverse Populations
6.5.2. Standardization of Testing Protocols
6.5.3. Incorporating Biomarkers into Clinical Decision-Making Algorithms
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Dipietro, J.A. Maternal stress in pregnancy: Considerations for fetal development. J. Adolesc. Health 2012, 51, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Lanir, N.; Aharon, A.; Brenner, B. Procoagulant and anticoagulant mechanisms in human placenta. Semin. Thromb. Hemost. 2003, 29, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Samfireag, M.; Potre, C.; Potre, O.; Tudor, R.; Hoinoiu, T.; Anghel, A. Approach to Thrombophilia in Pregnancy—A Narrative Review. Medicina 2022, 58, 692. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.A.; Kelton, J.G. Congenital thrombophilic states associated with venous thrombosis: A qualitative overview and proposed classification system. Ann. Intern. Med. 2003, 138, 128–134. [Google Scholar] [CrossRef]
- Kupferminc, M.J. Thrombophilia and pregnancy. Reprod. Biol. Endocrinol. 2003, 1, 111. [Google Scholar] [CrossRef]
- Greer, I.A.; Aharon, A.; Brenner, B.; Gris, J.C. Coagulation and placenta-mediated complications. Rambam Maimonides Med. J. 2014, 5, e0034. [Google Scholar] [CrossRef]
- Patsouras, M.D.; Vlachoyiannopoulos, P.G. Evidence of epigenetic alterations in thrombosis and coagulation: A systematic review. J. Autoimmun. 2019, 104, 102347. [Google Scholar] [CrossRef]
- Tsikouras, P.; Deftereou, T.; Anthoulaki, X.; Bothou, A.; Chalkidou, A.; Christoforidou, A.; Chatzimichael, E.; Gaitatzi, F.; Tsirkas, I.; Bourazan, A.C.; et al. Thrombophilia and Pregnancy: Diagnosis and Management. In Embolic Diseases—Evolving Diagnostic and Management Approaches; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Antonijevic, N.; Gosnjic, N.; Marjanovic, M.; Antonijevic, J.; Culafic, M.; Starcevic, J.; Plavsic, M.; Mostic Stanisic, D.; Uscumlic, A.; Lekovic, Z.; et al. Antiplatelet Drugs Use in Pregnancy—Review of the Current Practice and Future Implications. J. Pers. Med. 2024, 14, 560. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsueh, Y.W.; Chang, C.W.; Hsu, H.C.; Yang, T.C.; Lin, W.C.; Chang, H.M. Establishment of the fetal-maternal interface: Developmental events in human implantation and placentation. Front. Cell Dev. Biol. 2023, 11, 1200330. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Hellgren, M. Hemostasis during normal pregnancy and puerperium. Semin. Thromb. Hemost. 2003, 29, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B. Thrombophilia in pregnancy and its role in abortion. Womens Health 2005, 1, 35–38. [Google Scholar] [CrossRef]
- Battinelli, E.M.; Marshall, A.; Connors, J.M. The role of thrombophilia in pregnancy. Thrombosis 2013, 2013, 516420. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Koren, S.; Greer, W.L.; Fortin, P.R.; Rauch, J.; Fortin, I.; Senécal, J.L.; Docherty, P.; Hanly, J.G. Factor V Leiden, prothrombin gene mutation, and thrombosis risk in patients with antiphospholipid antibodies. J. Rheumatol. 2002, 29, 1683–1688. [Google Scholar]
- Harper, B.E.; Wills, R.; Pierangeli, S.S. Pathophysiological mechanisms in antiphospholipid syndrome. Int. J. Clin. Rheumtol. 2011, 6, 157–171. [Google Scholar] [CrossRef]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investg. 2004, 114, 744–754. [Google Scholar] [CrossRef]
- Silva, J.F.; Serakides, R. Intrauterine trophoblast migration: A comparative view of humans and rodents. Cell Adh. Migr. 2016, 10, 88–110. [Google Scholar] [CrossRef]
- Lawless, L.; Qin, Y.; Xie, L.; Zhang, K. Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications. Nutrients 2023, 15, 3564. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pang, Z.J.; Yu, Y.H. Regulation of trophoblast invasion: The role of matrix metalloproteinases. Rev. Obstet. Gynecol. 2012, 5, e137–e143. [Google Scholar]
- Mastrolia, S.A.; Mazor, M.; Loverro, G.; Klaitman, V.; Erez, O. Placental vascular pathology and increased thrombin generation as mechanisms of disease in obstetrical syndromes. PeerJ 2014, 2, e653. [Google Scholar] [CrossRef]
- Lunghi, L.; Ferretti, M.E.; Medici, S.; Biondi, C.; Vesce, F. Control of human trophoblast function. Reprod. Biol. Endocrinol. 2007, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.J.; Brosens, J.J.; Chamley, L.W.; Giles, I.; Pericleous, C.; Rahman, A.; Joyce, S.K.; Panda, B.; Paidas, M.J.; Abrahams, V.M. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am. J. Reprod. Immunol. 2009, 62, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.B.; Zheng, J. Regulation of placental angiogenesis. Microcirculation 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Zigler, M.; Kamiya, T.; Brantley, E.C.; Villares, G.J.; Bar-Eli, M. PAR-1 and thrombin: The ties that bind the microenvironment to melanoma metastasis. Cancer Res. 2011, 71, 6561–6566. [Google Scholar] [CrossRef]
- Bobircă, A.; Dumitrache, A.; Alexandru, C.; Florescu, A.; Ciobotaru, G.; Bobircă, F.; Sima, R.-M.; Poalelungi, C.; Bojincă, M.; Ancuța, I. Pathophysiology of Placenta in Antiphospholipid Syndrome. Physiologia 2022, 2, 66–79. [Google Scholar] [CrossRef]
- Miller, D.; Motomura, K.; Galaz, J.; Gershater, M.; Lee, E.D.; Romero, R.; Gomez-Lopez, N. Cellular immune responses in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2022, 111, 237–260. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Ernerudh, J.; Buse, E.; Cline, J.M.; Haeger, J.D.; Dixon, D.; Markert, U.R.; Pfarrer, C.; Vos, P.D.; Faas, M.M. The Placenta in Toxicology. Part II: Systemic and Local Immune Adaptations in Pregnancy. Toxicol. Pathol. 2014, 42, 327–338. [Google Scholar] [CrossRef]
- Peach, C.J.; Edgington-Mitchell, L.E.; Bunnett, N.W.; Schmidt, B.L. Protease-activated receptors in health and disease. Physiol. Rev. 2023, 103, 717–785. [Google Scholar] [CrossRef]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- La Farina, F.; Raparelli, V.; Napoleone, L.; Guadagni, F.; Basili, S.; Ferroni, P. Inflammation and Thrombophilia in Pregnancy Complications: Implications for Risk Assessment and Clinical Management. Cardiovasc. Hematol. Disord. Drug Targets 2016, 15, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Krishna, U.; Bhalerao, S. Placental Insufficiency and Fetal Growth Restriction. J. Obstet. Gynaecol. India 2011, 61, 505–511. [Google Scholar] [CrossRef] [PubMed]
- D’Uva, M.; Micco, P.D.; Strina, I.; Placido, G.D. Recurrent Pregnancy Loss and Thrombophilia. J. Clin. Med. Res. 2010, 2, 18–22. [Google Scholar] [CrossRef]
- Simcox, L.E.; Ormesher, L.; Tower, C.; Greer, I.A. Thrombophilia and Pregnancy Complications. Int. J. Mol. Sci. 2015, 16, 28418–28428. [Google Scholar] [CrossRef]
- Chang, K.J.; Seow, K.M.; Chen, K.H. Preeclampsia: Recent Advances in Predicting, Preventing, and Managing the Maternal and Fetal Life-Threatening Condition. Int. J. Environ. Res. Public Health 2023, 20, 2994. [Google Scholar] [CrossRef]
- Bitsadze, V.; Khizroeva, J.; Alexander, M.; Elalamy, I. Venous Thrombosis Risk Factors in Pregnant Women. J. Perinat. Med. 2022, 50, 505–518. [Google Scholar] [CrossRef]
- James, A.H. Pregnancy and Thrombotic Risk. Crit. Care Med. 2010, 38, S57–S63. [Google Scholar] [CrossRef]
- Dado, C.D.; Levinson, A.T.; Bourjeily, G. Pregnancy and Pulmonary Embolism. Clin. Chest Med. 2018, 39, 525–537. [Google Scholar] [CrossRef]
- Gottlieb, J.L.; Blice, J.P.; Mestichelli, B.; Konkle, B.A.; Benson, W.E. Activated Protein C Resistance, Factor V Leiden, and Central Retinal Vein Occlusion in Young Adults. Arch. Ophthalmol. 1998, 116, 577–579. [Google Scholar] [CrossRef]
- Spina, V.; Aleandri, V.; Morini, F. The impact of the factor V Leiden mutation on pregnancy. Hum. Reprod. Update 2000, 6, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A. Prothrombin G20210A polymorphism and thrombophilia. Mayo Clin. Proc. 2000, 75, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.M.; Zhao, Y.; Spong, C.Y.; Sibai, B.; Wendel, G., Jr.; Wenstrom, K.; Samuels, P.; Caritis, S.N.; Sorokin, Y.; Miodovnik, M.; et al. Prothrombin gene G20210A mutation and obstetric complications. Obstet. Gynecol. 2010, 115, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, B.; Garg, R.; Ibrahim, G.; Batra, J. Investigating protein C and S levels in pregnant women with recurrent early pregnancy loss versus normal pregnancy. J. Med. Life 2023, 16, 160–166. [Google Scholar] [CrossRef]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef]
- Turgal, M.; Gumruk, F.; Karaagaoglu, E.; Beksac, M.S. Methylenetetrahydrofolate Reductase Polymorphisms and Pregnancy Outcome. Geburtshilfe Frauenheilkd. 2018, 78, 871–878. [Google Scholar] [CrossRef]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X. A Novel Review of Homocysteine and Pregnancy Complications. Biomed. Res. Int. 2021, 2021, 6652231. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Chamley, L.W.; Salmon, J.E. Emerging Treatment Models in Rheumatology: Antiphospholipid Syndrome and Pregnancy: Pathogenesis to Translation. Arthritis Rheumatol. 2017, 69, 1710–1721. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Anunciación-Llunell, A.; Marques-Soares, J.; Pardos-Gea, J.; Miró-Mur, F. Pathogenesis, Diagnosis and Management of Obstetric Antiphospholipid Syndrome: A Comprehensive Review. J. Clin. Med. 2022, 11, 675. [Google Scholar] [CrossRef]
- Vrzić Petronijević, S.; Vilotić, A.; Bojić-Trbojević, Ž.; Kostić, S.; Petronijević, M.; Vićovac, L.; Jovanović Krivokuća, M. Trophoblast Cell Function in the Antiphospholipid Syndrome. Biomedicines 2023, 11, 2681. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Barbaro, G.; Paciullo, C.; Tersigni, C.; Scambia, G.; Di Simone, N. Antiphospholipid Syndrome in Pregnancy: New and Old Pathogenetic Mechanisms. Int. J. Mol. Sci. 2023, 24, 3195. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, G.; Mertowska, P.; Mertowski, S.; Przysucha, A.; Strużyna, J.; Grywalska, E.; Torres, K. The Crossroads of the Coagulation System and the Immune System: Interactions and Connections. Int. J. Mol. Sci. 2023, 24, 12563. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.L.; Pereira, E.R.; Oliveira, C.S.D.; Ferreira, E.D.S.; Menon, E.T.N.; Diniz, S.N.; Pezuk, J.A. MicroRNAs: Understanding their role in gene expression and cancer. Einstein 2021, 19, eRB5996. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Pavlov, V.; Du, W.; Yang, B. MiRNAs and Their Role in Venous Thromboembolic Complications. Diagnostics 2023, 13, 3383. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Ren, J.; Geng, Q.; Song, J.; Lee, C.; Cao, C.; Zhang, J.; Xu, N. MicroRNA-223 inhibits tissue factor expression in vascular endothelial cells. Atherosclerosis 2014, 237, 514–520. [Google Scholar] [CrossRef]
- Bijak, M.; Dzieciol, M.; Rywaniak, J.; Saluk, J.; Zielinska, M. Platelets miRNA as a Prediction Marker of Thrombotic Episodes. Dis. Markers 2016, 2016, 2872507. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Lu, D. MicroRNA-223-3p Downregulates the Inflammatory Response in Preeclampsia Placenta via Targeting NLRP3. BMC Pregnancy Childbirth 2024, 24, 175. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, O.J.; Kim, S.Y.; Oh, S.H.; Oh, D.; Kim, O.J.; Shin, B.S.; Kim, N.K. Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 420–430. [Google Scholar] [CrossRef]
- Ricke-Hoch, M.; Hoes, M.F.; Pfeffer, T.J.; Schlothauer, S.; Nonhoff, J.; Haidari, S.; Bomer, N.; Scherr, M.; Stapel, B.; Stelling, E.; et al. In peripartum cardiomyopathy plasminogen activator inhibitor-1 is a potential new biomarker with controversial roles. Cardiovasc. Res. 2020, 116, 1875–1886. [Google Scholar] [CrossRef]
- Lopez-Pedrera, C.; Barbarroja, N.; Patiño-Trives, A.M.; Collantes, E.; Aguirre, M.A.; Perez-Sanchez, C. New Biomarkers for Atherothrombosis in Antiphospholipid Syndrome: Genomics and Epigenetics Approaches. Front. Immunol. 2019, 10, 764. [Google Scholar] [CrossRef]
- Jairajpuri, D.S.; Malalla, Z.H.; Sarray, S.; Mahmood, N. Analysis of differential expression of hypoxia-inducible microRNA-210 gene targets in mild and severe preeclamptic patients. Noncoding RNA Res. 2021, 6, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Wang, Y.; Xu, P.; Cao, G.; Zhao, Y.; Shao, X.; Li, Y.X.; Chang, C.; Peng, C.; Wang, Y.L. Hypoxia-inducible miR-210 contributes to preeclampsia via targeting thrombospondin type I domain containing 7A. Sci. Rep. 2016, 6, 19588. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Gu, Y.; Sheng, W.; Sun, J.; Morgan, J.A.; Lewis, D.F.; Cooper, D.B.; McCathran, C.E.; Wang, Y. Downregulation of miR-126-3p expression contributes to increased inflammatory response in placental trophoblasts in preeclampsia. J. Reprod. Immunol. 2021, 144, 103281. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liu, Y.; Cui, K.; Hu, B.; Wang, F.; Zou, L. MicroRNA-126 regulates EPCs function: Implications for a role of miR-126 in preeclampsia. J. Cell Biochem. 2013, 114, 2148–2159. [Google Scholar] [CrossRef]
- Sahu, A.; Jha, P.K.; Prabhakar, A.; Singh, H.D.; Gupta, N.; Chatterjee, T.; Tyagi, T.; Sharma, S.; Kumari, B.; Singh, S.; et al. MicroRNA-145 Impedes Thrombus Formation via Targeting Tissue Factor in Venous Thrombosis. EBioMedicine 2017, 26, 175–186. [Google Scholar] [CrossRef]
- Islam, R.; Lai, C. A Brief Overview of lncRNAs in Endothelial Dysfunction-Associated Diseases: From Discovery to Characterization. Epigenomes 2019, 3, 20. [Google Scholar] [CrossRef]
- Lam, F.; Leisegang, M.S.; Brandes, R.P. LncRNAs Are Key Regulators of Transcription Factor-Mediated Endothelial Stress Responses. Int. J. Mol. Sci. 2024, 25, 9726. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; He, X.; Wang, N.; Sun, R.; Li, X.; Yu, T.; Chu, X.-M. Roles of long non-coding RNAs in angiogenesis-related diseases: Focusing on non-neoplastic aspects. Life Sci. 2023, 330, 122006. [Google Scholar] [CrossRef]
- Chen, H.; Meng, T.; Liu, X.; Sun, M.; Tong, C.; Liu, J.; Wang, H.; Du, J. Long non-coding RNA MALAT-1 is downregulated in preeclampsia and regulates proliferation, apoptosis, migration and invasion of JEG-3 trophoblast cells. Int. J. Clin. Exp. Pathol. 2015, 8, 12718–12727. [Google Scholar]
- Li, Q.; Wang, T.; Huang, S.; Zuo, Q.; Jiang, Z.; Yang, N.; Sun, L. LncRNA MALAT1 affects the migration and invasion of trophoblast cells by regulating FOS expression in early-onset preeclampsia. Pregnancy Hypertens. 2020, 21, 50–57. [Google Scholar] [CrossRef]
- Abdelazim, S.A.; Shaker, O.G.; Aly, Y.A.H.; Senousy, M.A. Uncovering serum placental-related non-coding RNAs as possible biomarkers of preeclampsia risk, onset and severity revealed MALAT-1, miR-363 and miR-17. Sci. Rep. 2022, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, X.; Cheng, J.; Zhang, K.; Ma, C. LncRNA TUG1 regulates proliferation and apoptosis by regulating miR-148b/IGF2 axis in ox-LDL-stimulated VSMC and HUVEC. Life Sci. 2020, 243, 117287. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wang, Y.; Meng, Y.; Hu, J.; Qiao, J.; Zhen, J.; Liang, D.; Fan, M. Hypoxia induced-disruption of lncRNA TUG1/PRC2 interaction impairs human trophoblast invasion through epigenetically activating Nodal/ALK7 signalling. J. Cell. Mol. Med. 2022, 26, 4087–4100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, L.; Wan, F. Molecular mechanisms of TUG1 in the proliferation, apoptosis, migration and invasion of cancer cells. Oncol. Lett. 2019, 18, 4393–4402. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Zhang, Z.; Wang, B.; Wei, R.; Chu, C.; Xu, K.; Li, L.; Liu, Y.; Li, X. Emerging role of miRNAs, lncRNAs, and circRNAs in pregnancy-associated diseases. Chin. Med. J. 2023, 136, 1300–1310. [Google Scholar] [CrossRef]
- Zhao, Q.; Pang, G.; Yang, L.; Chen, S.; Xu, R.; Shao, W. Long Noncoding RNAs Regulate the Inflammatory Responses of Macrophages. Cells 2021, 11, 5. [Google Scholar] [CrossRef]
- Shin, J.J.; Park, J.; Shin, H.S.; Arab, I.; Suk, K.; Lee, W.H. Roles of lncRNAs in NF-κB-Mediated Macrophage Inflammation and Their Implications in the Pathogenesis of Human Diseases. Int. J. Mol. Sci. 2024, 25, 2670. [Google Scholar] [CrossRef]
- Basak, T.; Ain, R. Long non-coding RNAs in placental development and disease. Non-Coding RNA Investig. 2019, 3, 14. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Martellucci, S.; Orefice, N.S.; Angelucci, A.; Luce, A.; Caraglia, M.; Zappavigna, S. Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy? Int. J. Mol. Sci. 2020, 21, 6486. [Google Scholar] [CrossRef]
- He, Y.; Wu, Q. The Effect of Extracellular Vesicles on Thrombosis. J. Cardiovasc. Transl. Res. 2023, 16, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.V.C.; Pantazi, P.; Holder, B. Circulating extracellular vesicles in healthy and pathological pregnancies: A scoping review of methodology, rigour and results. J. Extracell Vesicles 2023, 12, e12377. [Google Scholar] [CrossRef] [PubMed]
- Maligianni, I.; Yapijakis, C.; Nousia, K.; Bacopoulou, F.; Chrousos, G.P. Exosomes and exosomal non-coding RNAs throughout human gestation. Exp. Ther. Med. 2022, 24, 582. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Coenen, C.S.; Hidalgo, T.N.; Lynn, T.; Jones, D.M.; Salmon, J.E.; Chamley, L.W.; Abrahams, V.M. Antiphospholipid-exposed trophoblast-derived extracellular vesicles express elevated levels of TLR7/8-activating microRNAs and induce endometrial endothelial activation, in part, through TLR7. J. Reprod. Immunol. 2024, 164, 104255. [Google Scholar] [CrossRef]
- Tong, M. Antiphospholipid Antibodies Increase the Levels of Mitochondrial DNA in Placental Extracellular Vesicles: Alarmin-g for Preeclampsia. Sci. Rep. 2017, 7, 16556. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Immune Modulation Using Extracellular Vesicles Encapsulated with MicroRNAs as Novel Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 5658. [Google Scholar] [CrossRef]
- Morelli, A.E.; Sadovsky, Y. Extracellular vesicles and immune response during pregnancy: A balancing act. Immunol. Rev. 2022, 308, 105–122. [Google Scholar] [CrossRef]
- Martin, C.; Bergamelli, M.; Malnou, C.E.; D’Angelo, G. Placental extracellular vesicles in maternal-fetal communication during pregnancy. Biochem. Soc. Trans. 2022, 50, 1785–1795. [Google Scholar] [CrossRef]
- Ghosh, S.; Thamotharan, S.; Fong, J.; Lei, M.Y.Y.; Janzen, C.; Devaskar, S.U. Circulating extracellular vesicular microRNA signatures in early gestation show an association with subsequent clinical features of pre-eclampsia. Sci. Rep. 2024, 14, 16770. [Google Scholar] [CrossRef]
- Teruel-Montoya, R.; Rosendaal, F.R.; Martínez, C. MicroRNAs in Hemostasis. J. Thromb. Haemost. 2015, 13, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Olarerin-George, A.O.; Schwartz, N.; Srinivas, S.; Bastek, J.; Hogenesch, J.B.; Elovitz, M.A. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am. J. Pathol. 2013, 183, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Jaszczuk, I.; Koczkodaj, D.; Kondracka, A.; Kwaśniewska, A.; Winkler, I.; Filip, A. The role of miRNA-210 in pre-eclampsia development. Ann. Med. 2022, 54, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Antza, C.; Cifkova, R.; Kotsis, V. Hypertensive Complications of Pregnancy: A Clinical Overview. Metabolism 2018, 86, 102–111. [Google Scholar] [CrossRef]
- López-Pedrera, C.; Cerdó, T.; Jury, E.C.; Muñoz-Barrera, L.; Escudero-Contreras, A.; A Aguirre, M.; Pérez-Sánchez, C. New advances in genomics and epigenetics in antiphospholipid syndrome. Rheumatology 2024, 63, SI14–SI23. [Google Scholar] [CrossRef]
- Pérez-Sánchez, C.; la Rosa, I.A.-D.; Aguirre, M.; Luque-Tévar, M.; Ruiz-Limón, P.; Barbarroja, N.; Jiménez-Gómez, Y.; Ábalos-Aguilera, M.C.; Collantes-Estévez, E.; Segui, P.; et al. Circulating microRNAs as biomarkers of disease and typification of the atherothrombotic status in antiphospholipid syndrome. Haematologica 2018, 103, 908–918. [Google Scholar] [CrossRef]
- Xia, S.; Ye, Y.; Liu, J.; Qiu, H.; Lin, M.; He, Z.; Huang, L.; Wang, M.; Luo, Y. The Role of MALAT1 in Regulating the Proangiogenic Functions, Invasion, and Migration of Trophoblasts in Selective Fetal Growth Restriction. Biomolecules 2024, 14, 988. [Google Scholar] [CrossRef]
- Ogoyama, M.; Takahashi, H.; Suzuki, H.; Ohkuchi, A.; Fujiwara, H.; Takizawa, T. Non-Coding RNAs and Prediction of Preeclampsia in the First Trimester of Pregnancy. Cells 2022, 11, 2428. [Google Scholar] [CrossRef]
- Hell, L.; Ay, C.; Posch, F.; Gebhart, J.; Koder, S.; Mackman, N.; Pabinger, I.; Thaler, J. Low extracellular vesicle-associated tissue factor activity in patients with persistent lupus anticoagulant and a history of thrombosis. Ann. Hematol. 2019, 98, 313–319. [Google Scholar] [CrossRef]
- Zifkos, K.; Dubois, C.; Schäfer, K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 9317. [Google Scholar] [CrossRef]
- Aharon, A.; Rebibo-Sabbah, A.; Ahmad, R.S.; Dangot, A.; Bar-Lev, T.H.; Brenner, B.; Cohen, A.H.; Ben David, C.; Weiner, Z.; Solt, I. Associations of maternal and placental extracellular vesicle miRNA with preeclampsia. Front. Cell Dev. Biol. 2023, 11, 1080419. [Google Scholar] [CrossRef] [PubMed]
- Fusco, P.; Fietta, A.; Esposito, M.R.; Zanella, L.; Micheli, S.; Bastianello, A.; Bova, L.; Borile, G.; Germano, G.; Cimetta, E. miR-210-3p enriched extracellular vesicles from hypoxic neuroblastoma cells stimulate migration and invasion of target cells. Cell Biosci. 2023, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Bozack, A.K.; Merrill, S.M.; Cardenas, A. Epigenetic Biomarkers for Understanding Adverse Experiences and Health. JAMA Netw. Open 2024, 7, e2427070. [Google Scholar] [CrossRef]
- Bates, S.M.; Greer, I.A.; Middeldorp, S.; Veenstra, D.L.; Prabulos, A.M.; Vandvik, P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e691S–e736S. [Google Scholar] [CrossRef] [PubMed]
- Teruel, R.; Pérez-Sánchez, C.; Corral, J.; Herranz, M.T.; Pérez-Andreu, V.; Saiz, E.; García-Barberá, N.; Martínez-Martínez, I.; Roldán, V.; Vicente, V.; et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J. Thromb. Haemost. 2011, 9, 1985–1992. [Google Scholar] [CrossRef]
- Shi, R.; Zhou, X.; Ji, W.-J.; Zhang, Y.-Y.; Ma, Y.-Q.; Zhang, J.-Q.; Li, Y.-M. The Emerging Role of miR-223 in Platelet Reactivity: Implications in Antiplatelet Therapy. Biomed. Res. Int. 2015, 2015, 981841. [Google Scholar] [CrossRef]
- Hromadka, M.; Motovska, Z.; Hlinomaz, O.; Kala, P.; Tousek, F.; Jarkovsky, J.; Beranova, M.; Jansky, P.; Svoboda, M.; Krepelkova, I.; et al. MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. J. Pers. Med. 2021, 11, 508. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, M.; Xue, G.; Zhou, Q.; Jia, Y.; Li, L.; Xin, H.; Sun, S. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: New insights into molecular mechanisms for the disease. J. Cell. Mol. Med. 2012, 16, 249–259. [Google Scholar] [CrossRef]
- Hayder, H.; Shan, Y.; Chen, Y.; O’Brien, J.A.; Peng, C. Role of microRNAs in trophoblast invasion and spiral artery remodeling: Implications for preeclampsia. Front. Cell Dev. Biol. 2022, 10, 995462. [Google Scholar] [CrossRef]
- Sultana, R.; Kamihira, M. Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy. Pharmaceuticals 2024, 17, 1362. [Google Scholar] [CrossRef]
- Riess, H.; Verhamme, P.; Weitz, J.I.; Young, A.; Bauersachs, R.; Beyer-Westendorf, J.; Crowther, M.; Maraveyas, A. Treatment of cancer-associated thrombosis: The evolution of anticoagulant choice and clinical insights into practical management. Crit. Rev. Oncol. Hematol. 2021, 157, 103125. [Google Scholar] [CrossRef] [PubMed]
- Spanos, M.; Gokulnath, P.; Chatterjee, E.; Li, G.; Varrias, D.; Das, S. Expanding the horizon of EV-RNAs: LncRNAs in EVs as biomarkers for disease pathways. Extra Cell. Vesicle 2023, 2, 100025. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Yahya, E.B.; Mohamed, M.M.I.; Abdulsamad, M.A.; Allaq, A.A.; Gielecińska, A.; Kontek, R. Insights into the Role of LncRNAs and miRNAs in Glioma Progression and Their Potential as Novel Therapeutic Targets. Cancers 2023, 15, 3298. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, Y.; Wang, X.; Wang, S. An integrated hypothesis for miR-126 in vascular disease. Med. Res. Arch. 2020, 8, 2133. [Google Scholar] [CrossRef]
- Anton, L.; DeVine, A.; Polyak, E.; Olarerin-George, A.; Brown, A.G.; Falk, M.J.; Elovitz, M.A. HIF-1α Stabilization Increases miR-210 Eliciting First Trimester Extravillous Trophoblast Mitochondrial Dysfunction. Front. Physiol. 2019, 10, 699. [Google Scholar] [CrossRef]
- Salet, D.M.; Bekkering, S.; Middeldorp, S.; van den Hoogen, L.L. Targeting thromboinflammation in antiphospholipid syndrome. J. Thromb. Haemost. 2023, 21, 744–757. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Ding, J.; Cheng, Y.; Diao, L.; Li, L.; Zhang, Y.; Yin, t. Multiomics Studies Investigating Recurrent Pregnancy Loss: An Effective Tool for Mechanism Exploration. Front. Immunol. 2022, 13, 826198. [Google Scholar] [CrossRef]
- Hu, Y.; Lan, W.; Miller, D. Next-Generation Sequencing for MicroRNA Expression Profile. Methods Mol. Biol. 2017, 1617, 169–177. [Google Scholar] [CrossRef]
- Micheel, J.; Safrastyan, A.; Wollny, D. Advances in Non-Coding RNA Sequencing. Non-Coding RNA 2021, 7, 70. [Google Scholar] [CrossRef]
- Peng, L.; Yang, J.; Wang, M.; Zhou, L. Editorial: Machine learning-based methods for RNA data analysis-Volume II. Front. Genet. 2022, 13, 1010089. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shan, D.; Xie, Y.; Luo, X.; Wu, Y.; Chen, Q.; Dong, R.; Hu, Y. Single cell RNA sequencing research in maternal fetal interface. Front. Cell Dev. Biol. 2023, 10, 1079961. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Wang, S.; Deng, Q.; An, Y.; Xing, Y.; Dai, X.; Li, Z.; Ma, Q.; Wang, K.; et al. Single-cell transcriptional profiling reveals cellular and molecular divergence in human maternal-fetal interface. Sci. Rep. 2022, 12, 10892. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wei, L.; Li, S.; Cheng, T.; Zhang, X.; Wang, X. Single-cell Transcriptomes Reveal Characteristics of MicroRNAs in Gene Expression Noise Reduction. Genom. Proteom. Bioinform. 2021, 19, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.; Bhaskar, S.M.M. Evaluating Machine Learning Models for Stroke Prognosis and Prediction in Atrial Fibrillation Patients: A Comprehensive Meta-Analysis. Diagnostics 2024, 14, 2391. [Google Scholar] [CrossRef]
- Ryvkin, P.; Leung, Y.Y.; Ungar, L.H.; Gregory, B.D.; Wang, L.S. Using Machine Learning and High-Throughput RNA Sequencing to Classify the Precursors of Small Non-Coding RNAs. Methods 2014, 67, 28–35. [Google Scholar] [CrossRef]
- Dixon, D.; Sattar, H.; Moros, N.; Kesireddy, S.R.; Ahsan, H.; Lakkimsetti, M.; Fatima, M.; Doshi, D.; Sadhu, K.; Hassan, M.J. Unveiling the Influence of AI Predictive Analytics on Patient Outcomes: A Comprehensive Narrative Review. Cureus 2024, 16, e59954. [Google Scholar] [CrossRef]
- Mishra, A.; Ashraf, M.Z. Using Artificial Intelligence to Manage Thrombosis Research, Diagnosis, and Clinical Management. Semin. Thromb. Hemost. 2020, 46, 410–418. [Google Scholar] [CrossRef]
- Karalis, V.D. The Integration of Artificial Intelligence into Clinical Practice. Appl. Biosci. 2024, 3, 14–44. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Seco-Cervera, M.; Tollefsbol, T.O.; Romá-Mateo, C.; Peiró-Chova, L.; Lapunzina, P.; Pallardó, F.V. Epigenetic Biomarkers: Current Strategies and Future Challenges for Their Use in the Clinical Laboratory. Crit. Rev. Clin. Lab. Sci. 2017, 54, 529–550. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Devonshire, A.S.; Sanders, R.; Wilkes, T.M.; Taylor, M.S.; Foy, C.A.; Huggett, J.F. Application of Next Generation qPCR and Sequencing Platforms to mRNA Biomarker Analysis. Methods 2013, 59, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Hund, M.; Andraczek, T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension 2020, 75, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Olgun, G.; Gopalan, V.; Hannenhalli, S. miRSCAPE—Inferring miRNA expression from scRNA-seq data. iScience 2022, 25, 104962. [Google Scholar] [CrossRef]
- Sanders, R.; Bustin, S.; Huggett, J.; Mason, D. Improving the standardization of mRNA measurement by RT-qPCR. Biomol. Detect. Quantif. 2018, 15, 13–17. [Google Scholar] [CrossRef]
- Bernad, B.C.; Tomescu, M.-C.; Anghel, T.; Lungeanu, D.; Enătescu, V.; Bernad, E.S.; Nicoraș, V.; Arnautu, D.-A.; Hogea, L. Epigenetic and Coping Mechanisms of Stress in Affective Disorders: A Scoping Review. Medicina 2024, 60, 709. [Google Scholar] [CrossRef]
- Knight, A.K.; Smith, A.K. Epigenetic Biomarkers of Preterm Birth and Its Risk Factors. Genes 2016, 7, 15. [Google Scholar] [CrossRef]

| Pregnancy-Induced Normal Adaptations of the Coagulation System | Modifications Induced by Thrombophilias During Pregnancy | |
|---|---|---|
| Adaptations in the coagulation cascade | - The maternal body shifts early during pregnancy to a hypercoagulable state, increasing clotting factors (fibrinogen, Factor VII, Factor VIII) to prevent hemorrhage during childbirth | - Congenital thrombophilias exaggerate this hypercoagulable state, causing excess thrombin production and a high risk of thromboembolic events - Acquired thrombophilias further increase clot formation, leading to placental vascular damage |
| Trophoblast formation and invasion | - Thrombin supports the invasion of the maternal endometrium by activating matrix metalloproteinases (MMPs), which remodel spiral arteries and establish the blood flow to the placenta | - In thrombophilias, excessive thrombin production can lead to premature fibrin deposition around spiral arteries, restricting the placental blood flow - In APS, antiphospholipid antibodies trigger trophoblast apoptosis, resulting in a disrupted blood supply and nutrient exchange |
| Angiogenesis and placental vascularization | - Adequate angiogenesis ensures placental development, supplying the fetus with oxygen and nutrients - Thrombin promotes endothelial cell proliferation and induces pro-angiogenic factors like VEGF for vessel formation | - Excessive thrombin activates anti-angiogenic pathways reducing VEGF expression and impairing vessel formation - Increased fibrin deposition further restricts blood flow and oxygenation |
| Immune regulation at maternal–fetal interface | - Immune tolerance is achieved through a balance between Th1 and Th2 responses, allowing the mother to tolerate the fetus while remaining protected from infections - Thrombin supports this immune modulation through PARs on immune cells | - Thrombophilias shift the immune balance toward a Th1-dominant pro-inflammatory response - Elevated thrombin levels promote pro-inflammatory cytokine release - The inflammatory response also disrupts immune tolerance |
| Nutrient and Oxygen Exchange | - Proper placental structure and blood flow ensure efficient nutrient and oxygen exchange between mother and fetus | - Thrombophilias can lead to excessive fibrin deposition in placental vessels, impairing nutrient and oxygen exchange |
| Epigenetic Component | Description and Mechanism of Action | Role in Pregnancy and Coagulation | Diagnostic Potential | Therapy Assessment and Future Applications |
|---|---|---|---|---|
| miRNAs | Small non-coding RNAs that regulate gene expression by binding to mRNA, inhibiting translation or promoting degradation | - Coagulation regulation: Modulate key coagulation factors influencing thrombin production - Vascular health: Regulate endothelial function and angiogenesis - Immune tolerance: Balance pro- and anti-inflammatory signals | - miR-223: Marker for thrombin generation, assessing thrombotic risk. - miR-210: Hypoxia and placental development - miR-126: Early marker for endothelial dysfunction - miR-145, miR-19b: Markers for immune-mediated thrombosis in APS | - miR-223: Monitoring tool for anticoagulant efficacy, reducing thrombotic events in response to LMWH - miR-210: Evaluation of therapy impact on placental oxygenation - Therapeutic targeting: miRNA mimics/inhibitors could be explored to adjust miRNA levels |
| lncRNAs | Long non-coding RNAs that influence gene transcription, chromatin remodeling, and post-transcriptional regulation | - Endothelial function: Regulate vascular health, influencing VEGF production - Coagulation control: Affect coagulation factors like Factor V and VIII, modulating thrombin production - Immune modulation: Control pro-inflammatory cytokines, balancing immune responses | - HOTAIR: Elevated in APS, linked to immune response and thrombotic risk - MALAT1: Downregulated in pre-eclampsia/IUGR - H19: Low levels indicate placental dysfunction | - HOTAIR: Tracks immune and inflammatory response under therapy - MALAT1: Monitors endothelial health, assessing anti-inflammatory therapy - Therapeutic targeting: Target lncRNAs to reduce immune activation and thrombosis |
| Extracellular Vesicles (EVs) | Membrane-bound particles (exosomes and microvesicles) that transport proteins, miRNAs, and lncRNAs between cells | - Coagulation: Carry tissue factor and other pro-coagulant proteins, promoting the extrinsic pathway, increasing hypercoagulability in thrombophilic pregnancies - Endothelial and immune modulation: Transfer miRNAs/lncRNAs affecting vascular integrity and immune responses at the maternal–fetal interface | - Tissue Factor-Bearing EVs: Elevated levels indicate hypercoagulability, predictive of placental thrombosis and vascular insufficiency - EV with miRNA/lncRNA cargo: Presence of miR-210 (placental hypoxia) or HOTAIR (immune dysregulation) reflects specific pregnancy risks | - EV Levels: Used to assess anticoagulant therapy success - Therapeutic cargo: EVs could be engineered to carry therapeutic miRNAs/lncRNAs to target placental or vascular cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardan, C.R.; Ioniță, I.; Iordache, M.; Călămar-Popovici, D.; Todorescu, V.; Popescu, R.; Bernad, B.C.; Bardan, R.; Bernad, E.S. Epigenetic Biomarkers in Thrombophilia-Related Pregnancy Complications: Mechanisms, Diagnostic Potential, and Therapeutic Implications: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 13634. https://doi.org/10.3390/ijms252413634
Bardan CR, Ioniță I, Iordache M, Călămar-Popovici D, Todorescu V, Popescu R, Bernad BC, Bardan R, Bernad ES. Epigenetic Biomarkers in Thrombophilia-Related Pregnancy Complications: Mechanisms, Diagnostic Potential, and Therapeutic Implications: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(24):13634. https://doi.org/10.3390/ijms252413634
Chicago/Turabian StyleBardan, Claudia Ramona, Ioana Ioniță, Maria Iordache, Despina Călămar-Popovici, Violeta Todorescu, Roxana Popescu, Brenda Cristiana Bernad, Răzvan Bardan, and Elena Silvia Bernad. 2024. "Epigenetic Biomarkers in Thrombophilia-Related Pregnancy Complications: Mechanisms, Diagnostic Potential, and Therapeutic Implications: A Narrative Review" International Journal of Molecular Sciences 25, no. 24: 13634. https://doi.org/10.3390/ijms252413634
APA StyleBardan, C. R., Ioniță, I., Iordache, M., Călămar-Popovici, D., Todorescu, V., Popescu, R., Bernad, B. C., Bardan, R., & Bernad, E. S. (2024). Epigenetic Biomarkers in Thrombophilia-Related Pregnancy Complications: Mechanisms, Diagnostic Potential, and Therapeutic Implications: A Narrative Review. International Journal of Molecular Sciences, 25(24), 13634. https://doi.org/10.3390/ijms252413634








