Maintaining the Cartilage Phenotype of Late-Passage Chondrocytes Using Salidroside, TGF-β, and Sulfated Alginate for Cartilage Tissue Engineering Applications
Abstract
1. Introduction
2. Results
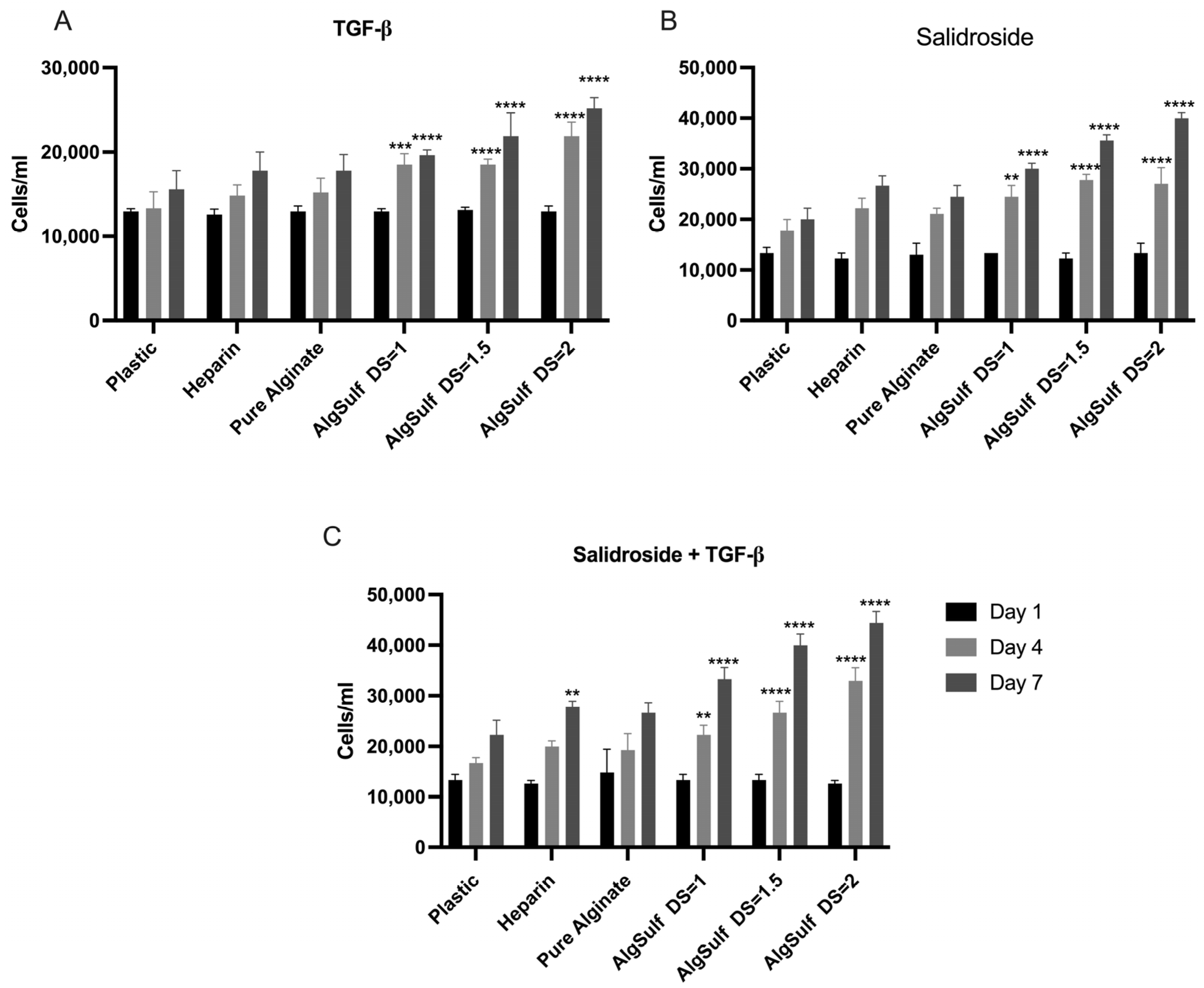
2.1. Proliferation Assay for P2 Chondrocytes
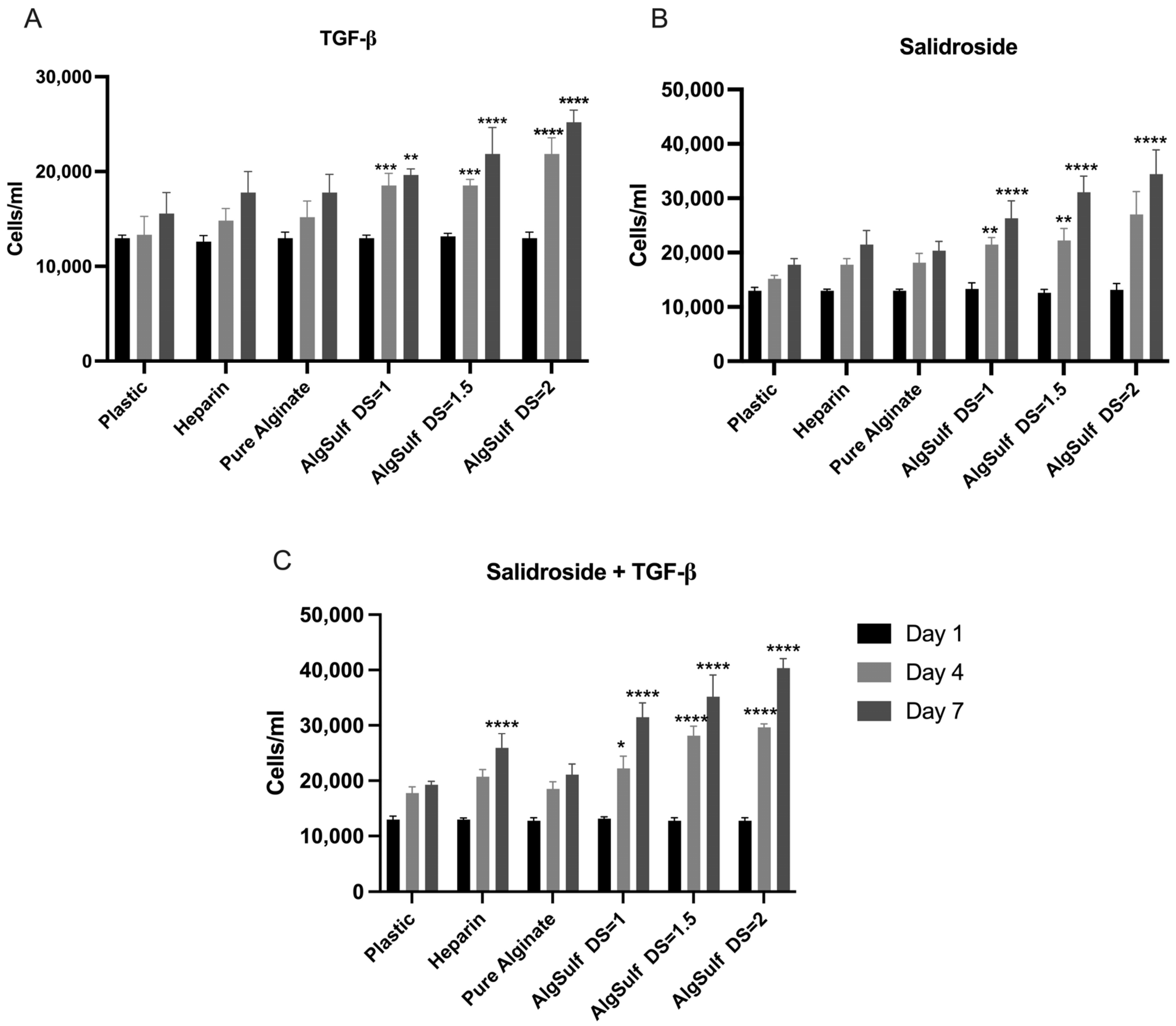
2.2. Proliferation Assay for P4 Chondrocytes
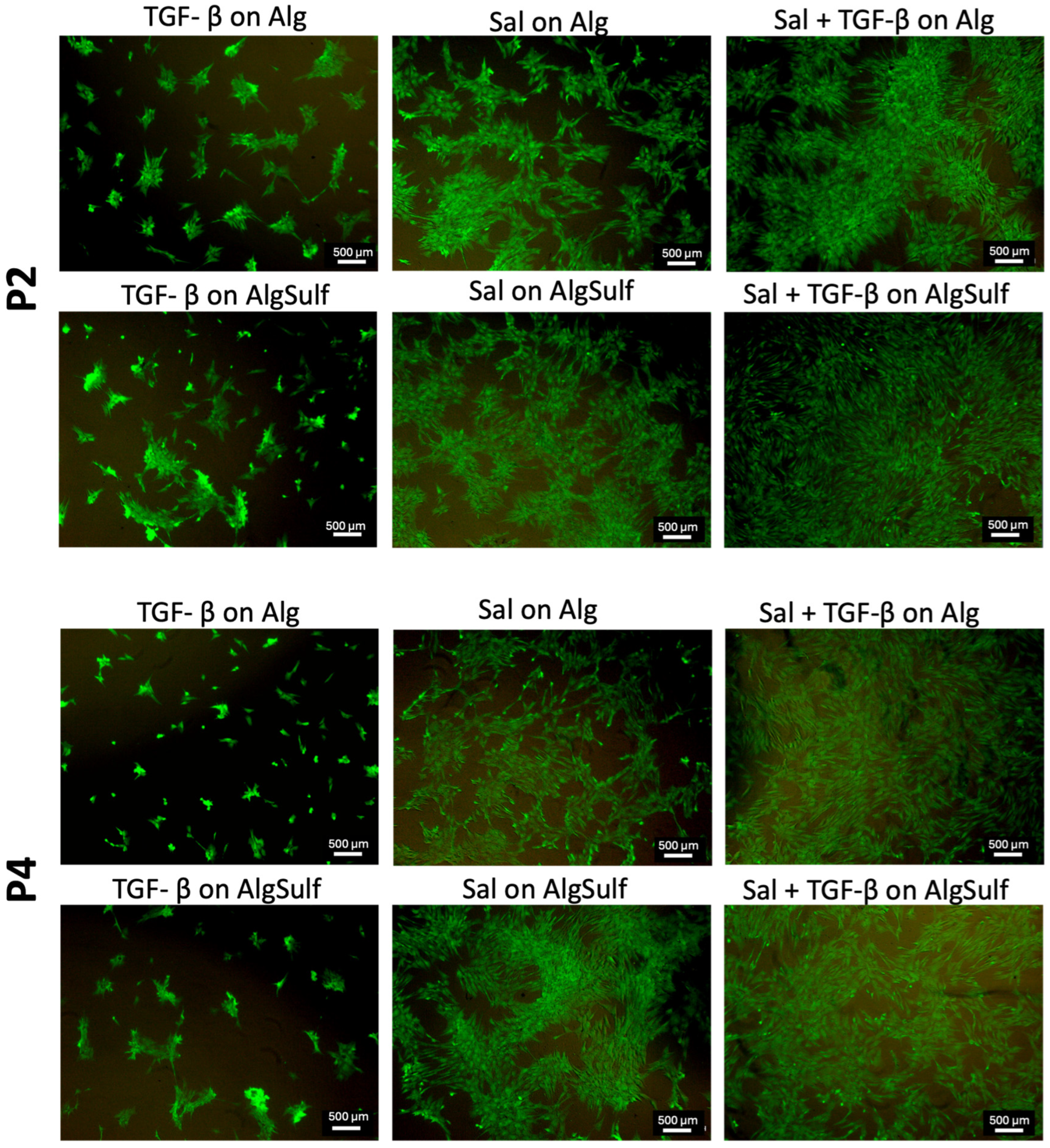
2.3. Cell Viability Findings
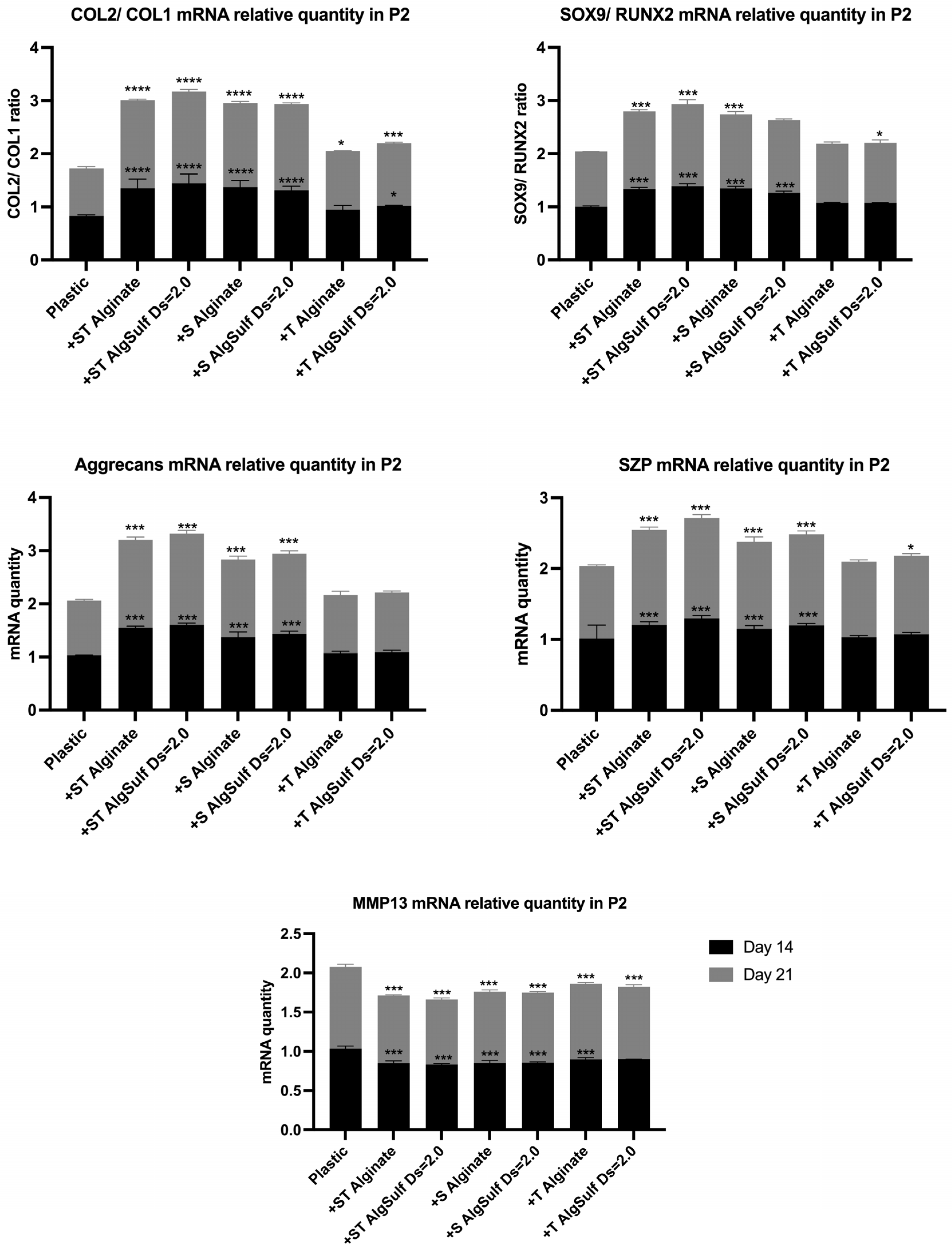
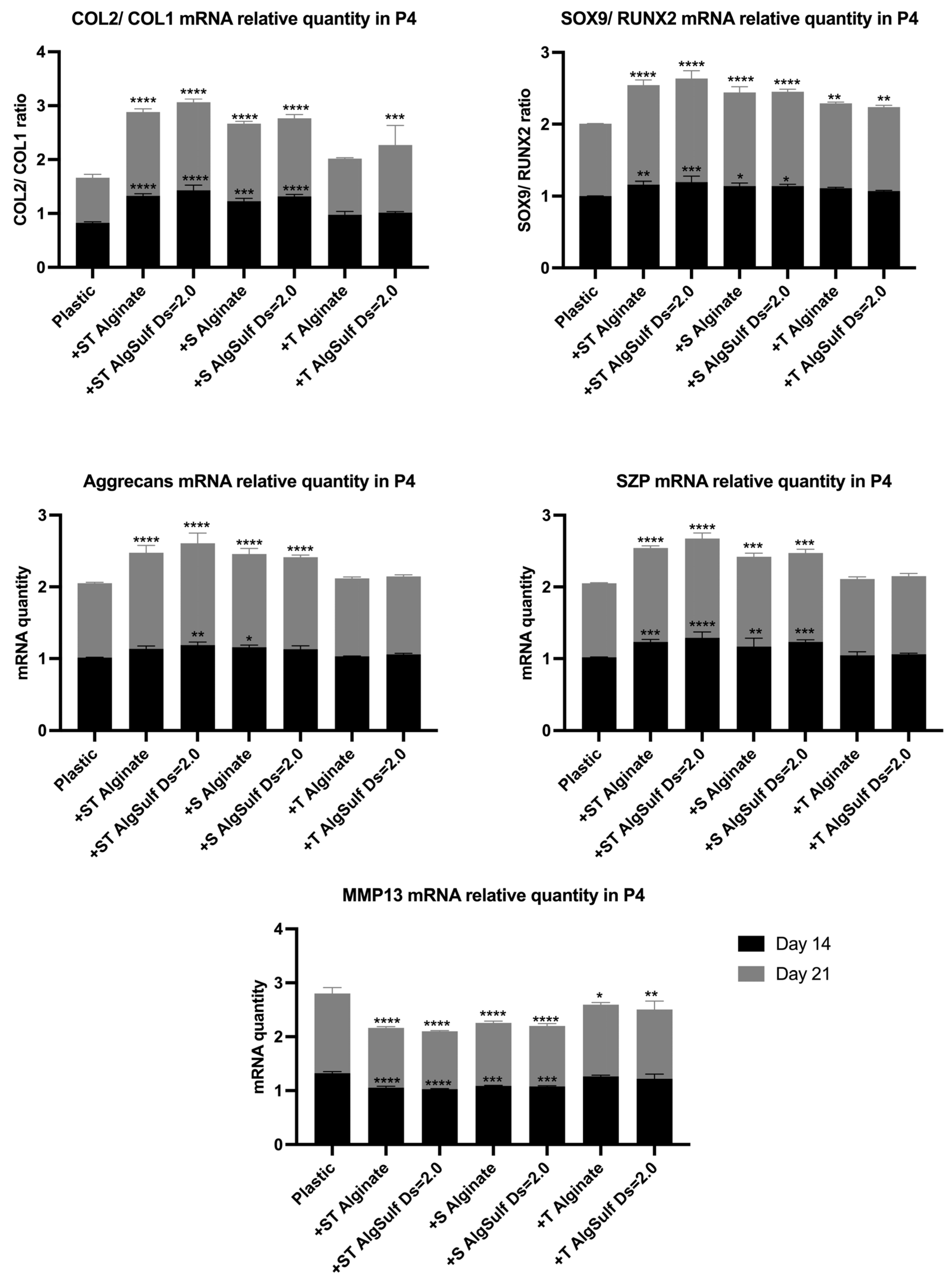
2.4. Gene Expression
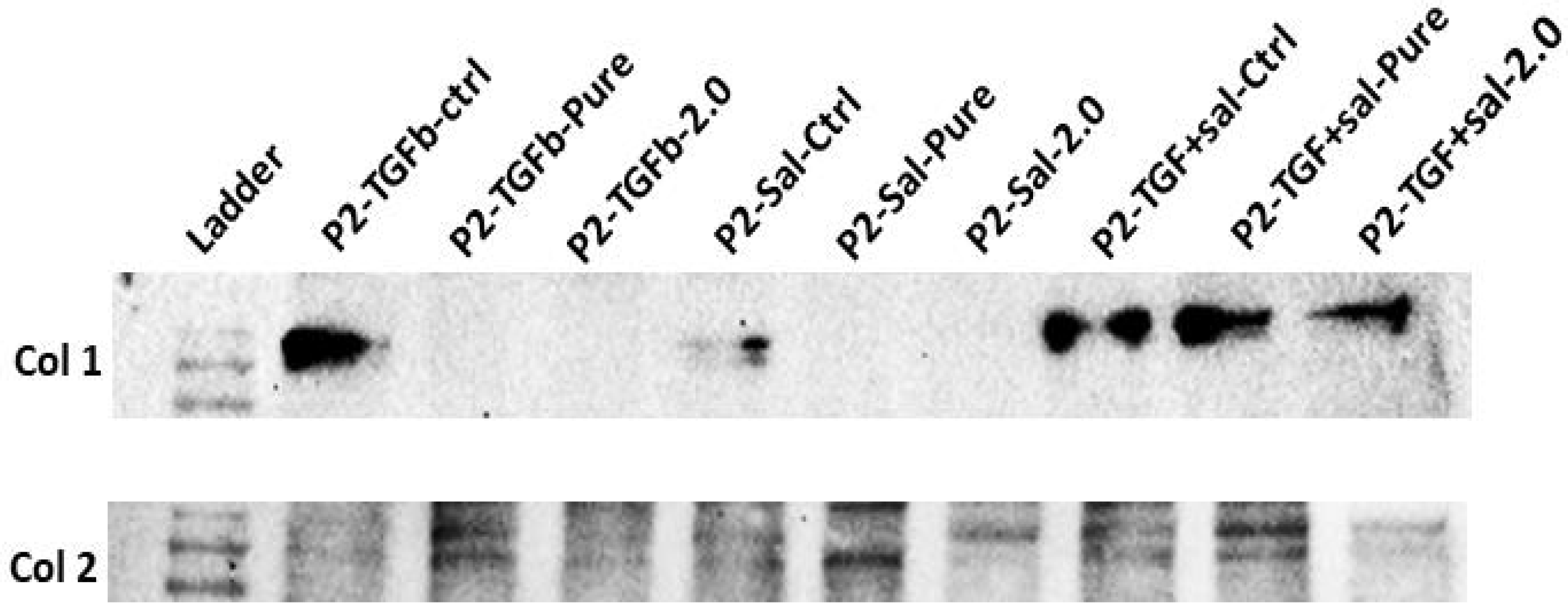
2.5. Protein Expression
3. Discussion
4. Materials and Methods
4.1. Chondrocyte Isolation
4.2. Cell Culture
4.3. Two-Dimensional Film Build-Up and Cell Seeding
4.4. Cell Viability
4.5. qRT-PCR
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-S.; Qu, Z.; Yu, D.-Q.; Wang, W.; Yan, S.; Huang, H.-F. Sex Steroids and Osteoarthritis: A Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 683226. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. Syst. Rev. Clin. Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef]
- Blaker, C.L.; Clarke, E.C.; Little, C.B. Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. J. Orthop. Res. 2017, 35, 424–439. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Franceschi, F.; Longo, U.G.; Ruzzini, L.; Marinozzi, A.; Maffulli, N.; Denaro, V. Simultaneous arthroscopic implantation of autologous chondrocytes and high tibial osteotomy for tibial chondral defects in the varus knee. Knee 2008, 15, 309–313. [Google Scholar] [CrossRef]
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, G.; Lin, Q.; Zhang, W.; Liu, H.; Su, J. Articular cartilage repair biomaterials: Strategies and applications. Mater. Today Bio 2024, 24, 100948. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Zapata-Linares, N.; Eymard, F.; Berenbaum, F.; Houard, X. Role of adipose tissues in osteoarthritis. Curr. Opin. Rheumatol. 2021, 33, 84–93. [Google Scholar] [CrossRef]
- Sun, Y.; Leng, P.; Guo, P.; Gao, H.; Liu, Y.; Li, C.; Li, Z.; Zhang, H. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol. Med. 2021, 27, 96. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Vinther, M.; Maloney, M.D.; Schwarz, E.M.; Rosier, R.; O’Keefe, R.J. Articular cartilage biology. J. Am. Acad. Orthop. Surg. 2003, 11, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.M.-G.; Beier, F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth. Defects Res. C Embryo Today 2014, 102, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Q. Salidroside attenuates interleukin-1β-induced inflammation in human osteoarthritis chondrocytes. J. Cell. Biochem. 2018, 120, 1203–1209. [Google Scholar] [CrossRef]
- Gao, H.; Peng, L.; Li, C.; Ji, Q.; Li, P. Salidroside Alleviates Cartilage Degeneration Through NF-κB Pathway in Osteoarthritis Rats. Drug Des. Dev. Ther. 2020, 14, 1445–1454. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7166061/ (accessed on 4 December 2024). [CrossRef]
- Sun, M.; Lu, Z.; Cai, P.; Zheng, L.; Zhao, J. Salidroside enhances proliferation and maintains phenotype of articular chondrocytes for autologous chondrocyte implantation (ACI) via TGF-β/Smad3 Signal. Biomed. Pharmacother. 2020, 122, 109388. Available online: https://www.sciencedirect.com/science/article/pii/S075333221931577X (accessed on 2 June 2023). [CrossRef]
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef]
- Öztürk, E.; Stauber, T.; Levinson, C.; Cavalli, E.; Arlov, Ø.; Zenobi-Wong, M. Tyrosinase-crosslinked, tissue adhesive and biomimetic alginate sulfate hydrogels for cartilage repair. Biomed. Mater. 2020, 15, 45019. [Google Scholar] [CrossRef]
- Wu, M.; Hu, R.; Wang, J.; An, Y.; Lu, L.; Long, C.; Yan, L. Salidroside Suppresses IL-1β-Induced Apoptosis in Chondrocytes via Phosphatidylinositol 3-Kinases (PI3K)/Akt Signaling Inhibition. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 5833–5840. [Google Scholar] [CrossRef]
- van der Kraan, P.M.; Blaney Davidson, E.N.; Blom, A.; van den Berg, W.B. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: Modulation and integration of signaling pathways through receptor-Smads. Osteoarthr. Cartil. 2009, 17, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Barisón, M.J.; Nogoceke, R.; Josino, R.; Horinouchi, C.D.D.S.; Marcon, B.H.; Correa, A.; Stimamiglio, M.A.; Robert, A.W. Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling. Int. J. Mol. Sci. 2022, 23, 6010. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Xiong, J.; Zhu, W.; Liu, Q.; Wang, D.; Liu, W.; Li, Z.; Wang, D. E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res. Ther. 2016, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Lekerika, P.; Crump, K.B.; Wuertz-Kozak, K.; Le Maitre, C.L.; Gantenbein, B. Sulfated Hydrogels as Primary Intervertebral Disc Cell Culture Systems. Gels 2024, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Kerschenmeyer, A.; Arlov, Ø.; Malheiro, V.; Steinwachs, M.; Rottmar, M.; Maniura-Weber, K.; Palazzolo, G.; Zenobi-Wong, M. Anti-oxidant and immune-modulatory properties of sulfated alginate derivatives on human chondrocytes and macrophages. Biomater. Sci. 2017, 5, 1756–1765. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Li, X.; Zhong, D.; Li, Y.; Li, J.; Jin, R. Strategies to modulate the redifferentiation of chondrocytes. Front. Bioeng. Biotechnol. 2021, 9, 764193. [Google Scholar] [CrossRef]
- Mhanna, R.F.; Vörös, J.; Zenobi-Wong, M. Layer-by-layer films made from extracellular matrix macromolecules on silicone substrates. Biomacromolecules 2011, 12, 609–616. [Google Scholar] [CrossRef]
- Kfoury, G.; El Habbaki, V.; Malaeb, W.; Weaver, S.; Momotenko, D.; Mhanna, R. Alginate Sulfate Substrates Control Growth Factor Binding and Growth of Primary Neurons: Toward Engineered 3D Neural Networks. Adv. Biosyst. 2020, 4, e2000047. [Google Scholar] [CrossRef]
- Malaeb, W.; Bahmad, H.F.; Abou-Kheir, W.; Mhanna, R. The sulfation of biomimetic glycosaminoglycan substrates controls binding of growth factors and subsequent neural and glial cell growth. Biomater. Sci. 2019, 7, 4283–4298. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene Name | Primer Sequence |
|---|---|
| Col1 | F-CGAGGGCAACAGCAGATTCAC TTA R-GCA GGC GAG ATG GCT TGT TTG |
| Col2 | F-GGC CAG CGT CCC CAA GAA R-AGCAGGCGC AGG AAG GTC AT |
| SOX9 | F-ACG CGG CCC CAG GAG AAC R-CGGATG CACACGGGGAACTT |
| RUNX2 | F-TTTTCAGACCCCAGGCAGTT R-TTGGAGAAGCGGCTCTCAGT |
| Aggrecan | F-AGTAGAGGACATCAGCGGGCTT R-CCGCTGATGTCCTCTACTCCAG |
| SZP | F-TTGCGCAATGGGACATTAGTT R-AGCTGGAGATGGTGGACTGAA |
| MMP13 | F-CCTTGATGCCATTACCAGTCTCC R-AAACAGCTCCGCATCAACCTGC |
| IL-1β | F-ACCCCAAAGTCTACCCCAAG R-CCAGTTAGGGTACAGGACAGAC |
| IL-6 | F-GTGCTCCTGGTGATGACTT R-GAAGTAGTCTGCCTGGGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, R.G.; Deeb, G.; Roda, R.; Karam, M.; Faraj, M.; Harajli, M.; Damiati, L.A.; Mhanna, R. Maintaining the Cartilage Phenotype of Late-Passage Chondrocytes Using Salidroside, TGF-β, and Sulfated Alginate for Cartilage Tissue Engineering Applications. Int. J. Mol. Sci. 2024, 25, 13623. https://doi.org/10.3390/ijms252413623
Diab RG, Deeb G, Roda R, Karam M, Faraj M, Harajli M, Damiati LA, Mhanna R. Maintaining the Cartilage Phenotype of Late-Passage Chondrocytes Using Salidroside, TGF-β, and Sulfated Alginate for Cartilage Tissue Engineering Applications. International Journal of Molecular Sciences. 2024; 25(24):13623. https://doi.org/10.3390/ijms252413623
Chicago/Turabian StyleDiab, Rita G., George Deeb, Rena Roda, Mia Karam, Marwa Faraj, Mohamad Harajli, Laila A. Damiati, and Rami Mhanna. 2024. "Maintaining the Cartilage Phenotype of Late-Passage Chondrocytes Using Salidroside, TGF-β, and Sulfated Alginate for Cartilage Tissue Engineering Applications" International Journal of Molecular Sciences 25, no. 24: 13623. https://doi.org/10.3390/ijms252413623
APA StyleDiab, R. G., Deeb, G., Roda, R., Karam, M., Faraj, M., Harajli, M., Damiati, L. A., & Mhanna, R. (2024). Maintaining the Cartilage Phenotype of Late-Passage Chondrocytes Using Salidroside, TGF-β, and Sulfated Alginate for Cartilage Tissue Engineering Applications. International Journal of Molecular Sciences, 25(24), 13623. https://doi.org/10.3390/ijms252413623





