Comparative Physiological, Proteomic, and Metabolomic Insights into a Promising Low-Pruning Mulberry Cultivar for Silkworm Rearing
Abstract
1. Introduction
2. Results
2.1. Low-Pruning Cultivation Mulberry Showed Characteristics of High Yield and Mechanical Harvestability
2.2. Impact of Low-Pruning Mulberry ZJ1 on the Production Performance in the Silkworm Rearing Industry
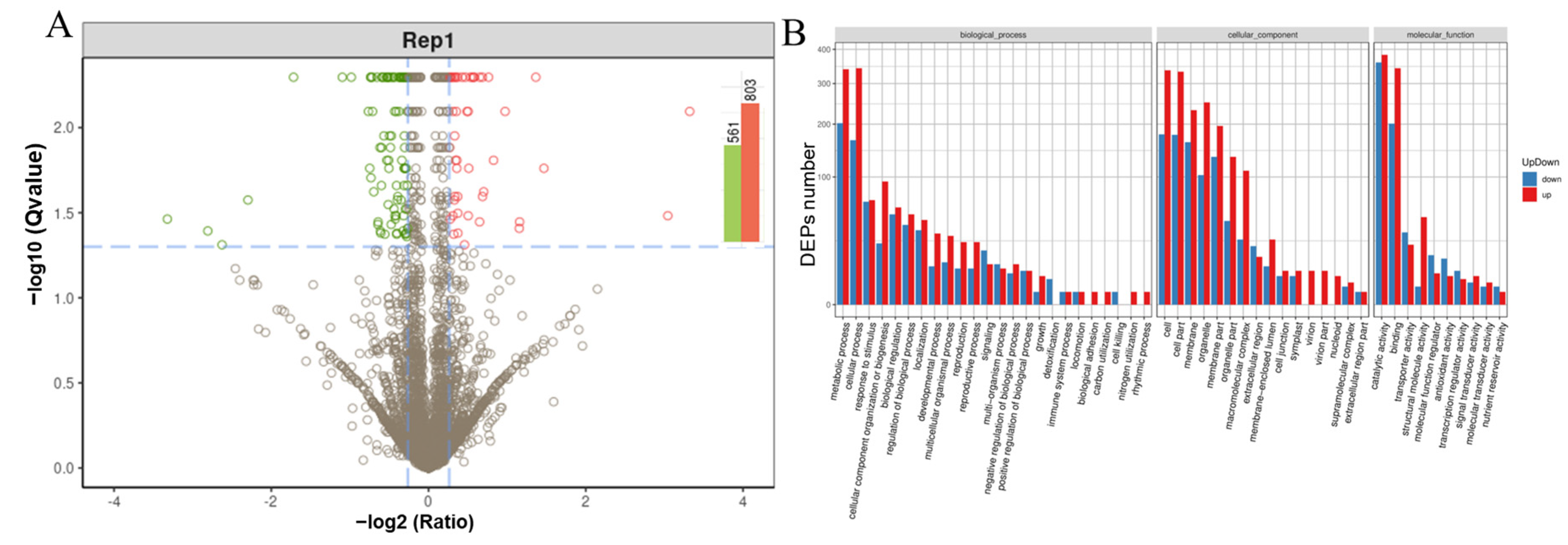
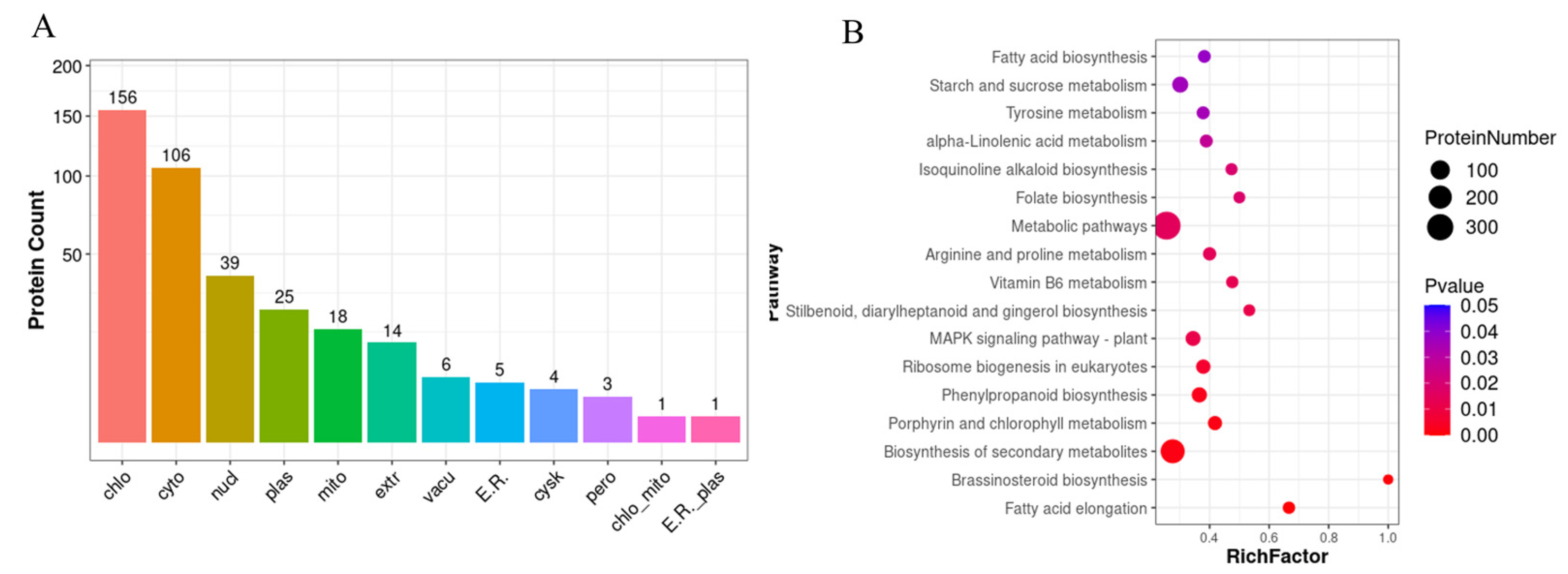
2.3. iTRAQ Comparison of Leaf Protein Profile from the Two Kinds of Cultivated Mulberry Varieties
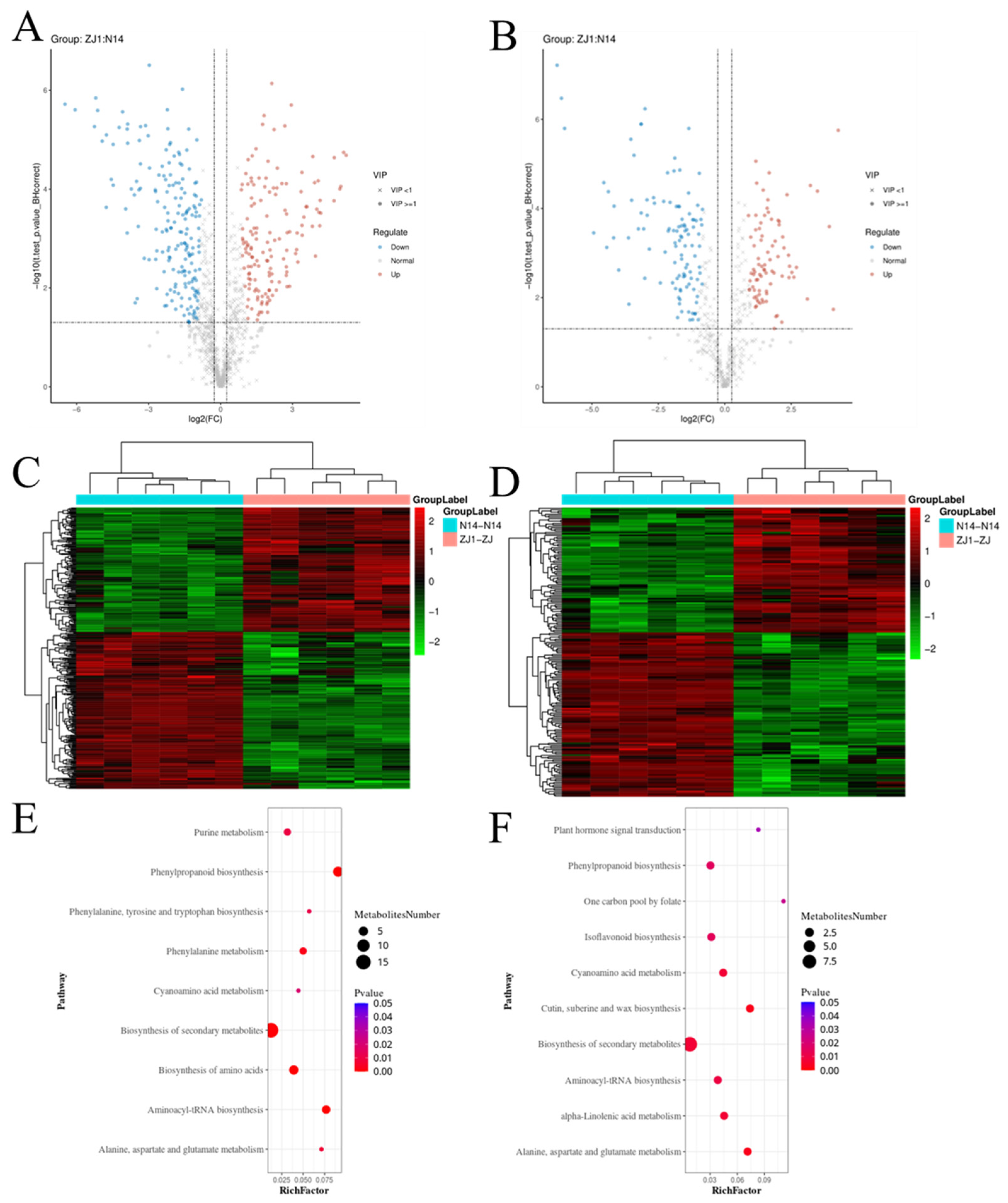
2.4. LC-MS/MS Metabolomics Analysis of the Leaves of Two Mulberry Cultivars
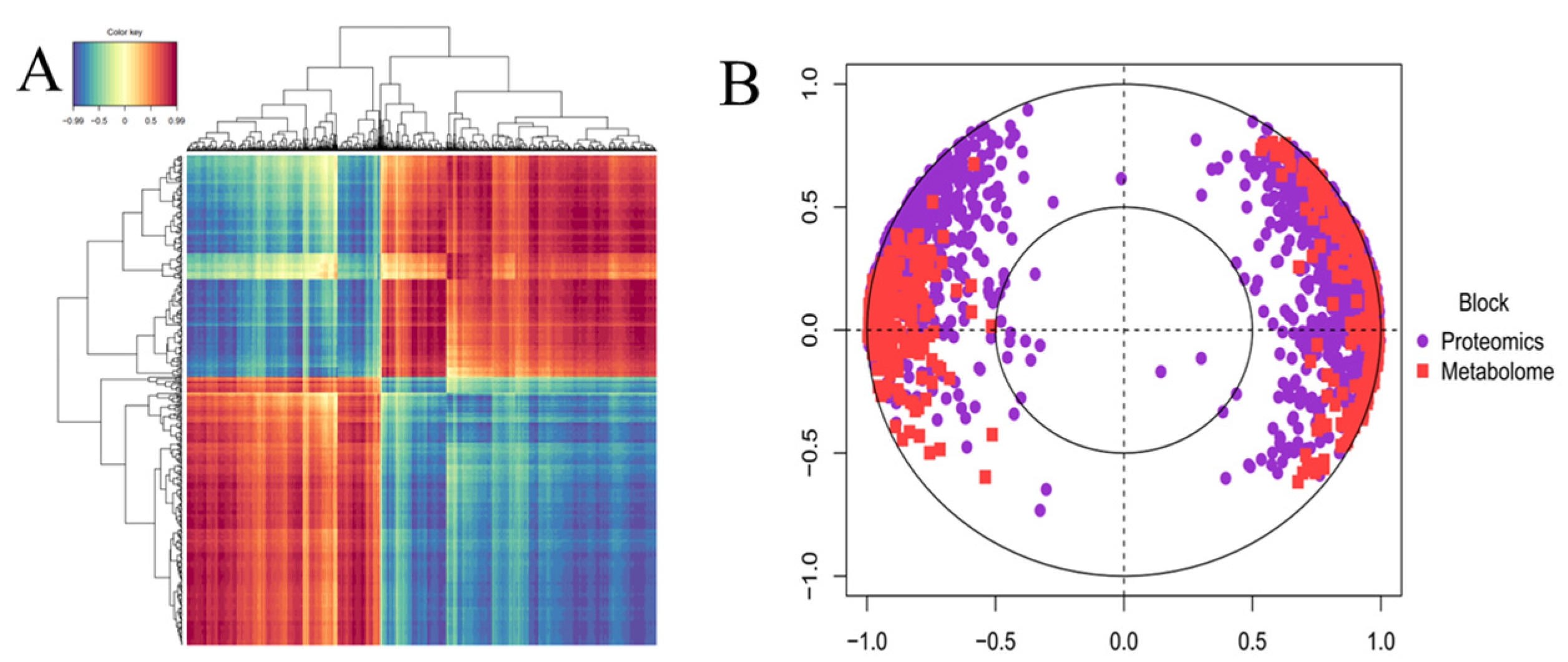
2.5. Integrated Correlation Analyses of Proteomics and Metabolomics Data
3. Discussion
3.1. Low-Pruning Hybrid Variety ZJ1 Exhibited Superior Performance in Mulberry Cultivation and Silkworm Rearing
3.2. Diet Amino Acids Composition Affected the Performance and Yield of Silkworm Silk
3.3. Carbohydrate Metabolism Pathway Functioned Under Different Cultivation Models
4. Materials and Methods
4.1. Experimental Materials
4.2. Physicochemical Index Measurement
4.3. Silkworm Rearing
4.4. Protein Sample Preparation and iTRAQ Labeling
4.5. Peptide Separation and LC-MS/MS Analysis
4.6. Protein Data Processing and Bioinformatics Analysis
4.7. Untargeted Metabolomics Analysis
4.8. Proteome and Metabolic Sequencing Cooperation Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A/N-INV | alkaline/neutral invertase |
| DEP | differentially expressed protein |
| DEM | differentially expressed metabolite |
| FDR | false discovery rate |
| GO | gene ontology |
| iTRAQ | isobaric tags for relative and absolute quantitation |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| rpS14 | 40 S ribosomal protein S14 |
| sPLS-DA | squares-discriminant analysis model |
| SPS | sucrose phosphate synthase |
| SUS | sucrose synthesis |
| UDPG | uridine diphosphate glucose, UDP-glucose |
References
- Rohela, G.K.; Shukla, P.; Muttanna, R.; Kumar, S.R. Chowdhury, Mulberry (Morus spp.): An ideal plant for sustainable development. Trees For. People 2020, 2, 100011. [Google Scholar] [CrossRef]
- Savithri, G.; Sujathamma, P.; Asha, K.V. Silkworm Bombyx mori an economic insect. Int. J. Sci. Res. 2013, 2, 2535–2537. [Google Scholar] [CrossRef]
- Krishna, K.J.; Ishita, B.; Mayookha, V.P. Recent trends in the development and diversification of sericulture natural products for innovative and sustainable applications. Bioresour. Technol. Rep. 2021, 13, 100614. [Google Scholar] [CrossRef]
- Murthy, D.V.N.Y.; Ramesh, H.L.; Munirajappa, D.; Yadav, D.B.R.D. Nutritional quality assessment of ten mulberry (Morus) germplasm varieties through moulting test, silkworm rearing technique and economical characters of bivoltine silkworms (Bombyx mori L.) for commercial exploitation. Int. Res. J. Natural Sci. 2013, 1, 11–22. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Wang, Y.; Li, Q.; Liang, X.; Wang, G.; Li, J.; Peng, R.; Sima, Y.; Xu, S. Ectopic expression of sericin enables efficient production of ancient silk with structural changes in silkworm. Nat. Commun. 2022, 13, 6295. [Google Scholar] [CrossRef]
- Khamenei-Tabrizi, A.S.; Sendi, J.J.; Imaani, S.; Shojaee, M. Can feeding of silkworm on different mulberry variety affect its performance? J. Econ. Entomol. 2020, 113, 281–287. [Google Scholar] [CrossRef]
- Kumar, H.; Priya, Y.S.; Kumar, M.; Elangovan, V. Effect of different mulberry varieties and seasons on growth and economic traits of bivoltine silkworm (Bombyx mori). J. Entomol. 2013, 10, 147–155. [Google Scholar] [CrossRef]
- Burgel, L.; Hartung, J.; Pflugfelder, A.; Graeff-Hönninger, S. Impact of growth stage and biomass fractions on cannabinoid content and yield of different hemp (Cannabis Sativa L.) genotypes. Agronomy 2020, 10, 372. [Google Scholar] [CrossRef]
- Crispim, M.D.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Hönninger, S. Impact of harvest time and pruning technique on total CBD concentration and yield of medicinal cannabis. Plants 2022, 11, 140. [Google Scholar] [CrossRef]
- Mazzoni, L.; Medori, I.; Balducci, F.; Marcellini, M.; Acciarri, P.; Mezzetti, B.; Capocasa, F. Branch numbers and crop load combination effects on production and fruit quality of flat peach cultivars (Prunus persica (L.) Batsch) trained catalonian vase. Plants 2022, 11, 308. [Google Scholar] [CrossRef]
- Herbst, R.H.; Bar-Zvi, D.; Reikhav, S.; Soifer, I.; Breker, M.; Jona, G.; Shimoni, E.; Schuldiner, M.; Levy, A.A.; Barkai, N. Heterosis as a consequence of regulatory incompatibility. BMC Biol. 2017, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, X.; Zhang, Q. Understanding the genetic and molecular constitutions of heterosis for developing hybrid rice. J. Genet. Genom. 2022, 49, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Low, J.E.; Åslund, M.L.; Rutter, A.; Zeeb, B.A. The effects of pruning and nodal adventitious roots on polychlorinated biphenyl uptake by Cucurbita pepo grown in field conditions. Environ. Pollut. 2011, 159, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, U.M. Effects of pruning on radial growth and biomass increment of trees growing in homegardens of Kerala, India. Agroforest Syst. 2007, 69, 231–237. [Google Scholar] [CrossRef]
- Nadella, K.D.; Marla, S.S.; Kumar, P.A. Metabolomics in agriculture. OMICS 2012, 16, 149–159. [Google Scholar] [CrossRef]
- Pedras, M.S.; Zheng, Q.A. Metabolic responses of Thellungiella halophila/salsuginea to biotic and abiotic stresses: Metabolite profiles and quantitative analyses. Phytochemistry 2010, 71, 581–589. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.; Mu, Q.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.; Yu, X. Differences in total phenolics, antioxidant activity and metabolic characteristics in peach fruits at different stages of ripening. LWT-Food Sci. Technol. 2023, 178, 114586. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Liu, Z.; Zhao, Z.; Zhao, J.; Wang, Z.; Zhou, G.; Liu, P.; Liu, M. Transcriptome and metabolome profiling unveil the mechanisms of Ziziphus jujuba Mill. peel coloration. Food Chem. 2020, 312, 125903. [Google Scholar] [CrossRef]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Li, S.; Yu, J.; Yang, L.; Hong, L. Widely targeted metabolomic analysis provides new insights into the effect of rootstocks on citrus fruit quality. Metabolites 2024, 14, 242. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Ji, C.; Wei, L.; Zhao, J.; Yu, J. An integrated proteomic and metabolomic study on the chronic effects of mercury in Suaeda salsa under an environmentally relevant salinity. PLoS ONE 2013, 8, e64041. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Qi, X. Mining plant metabolomes: Methods, applications, and perspectives. Plant Commun. 2021, 2, 100238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, L.; Fang, X.; Shi, Z.; Zhang, Y.; Chen, H.; Yan, X.; Dai, J. Biological responses to perfluorododecanoic acid exposure in rat kidneys as determined by integrated proteomic and metabonomic studies. PLoS ONE 2011, 6, e20862. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Ji, X.; Gao, H.; Zhang, S.; Xie, Q. High yield performance and sericulture effect of Nongsang 14 grafted mulberry herb cultivation. China Seric. 2020, 41, 6–10. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Feng, K.; Lin, T.; Liang, D.; Liu, P.; Liu, Y.; Wei, J.; Lv, Z. The results and analysis of 4 new hybrid mulberry varieties. Bull. Seric. 2022, 53, 20–23. (In Chinese) [Google Scholar]
- Qian, W.; Lin, T.; Pan, M.; Lv, Z.; Wu, H.; Shi, X.; Liu, L. Key technology of mechanical cultivation and harvest in hybrid herbaceous mulberry. Bull. Seric. 2021, 52, 48–49. (In Chinese) [Google Scholar]
- Hou, T.; Li, X.; Liu, S.; Zhou, J.; Bian, Y.; Zhou, L.; Sun, M.; Zhou, W.; Yang, B. High-performance artificially reeled silkworm silk via a multi-task and high-efficiency centrifugal reeling technique and its application in soft actuators. Mater. Horiz. 2023, 10, 2854–2867. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, C.; Huang, T.; Yu, L.; Sun, Y.; Tao, Y.; Cao, Y.; Du, R.; Lin, W.; Zeng, J. Silkworm cocoon: Dual functions as a traditional Chinese medicine and the raw material of promising biocompatible carriers. Pharmaceuticals 2024, 17, 817. [Google Scholar] [CrossRef]
- Ruth, L.; Ghatak, S.; Subbarayan, S.; Choudhury, B.N.; Gurusubramanian, G.; Kumar, N.S.; Bin, T. Influence of micronutrients on the food consumption rate and silk production of Bombyx mori (Lepidoptera: Bombycidae) reared on mulberry plants grown in a mountainous agro-ecological condition. Front. Physiol. 2019, 10, 878. [Google Scholar] [CrossRef]
- Saad, M.; El-Samad, L.M.; Gomaa, R.A.; Augustyniak, M.; Hassan, M.A. A comprehensive review of recent advances in silk sericin: Extraction approaches, structure, biochemical characterization, and biomedical applications. Int. J. Biol. Macromol. 2023, 250, 126067. [Google Scholar] [CrossRef] [PubMed]
- Muzamil, A.; Tahir, H.M.; Ali, A.; Bhatti, M.F.; Munir, F.; Ijaz, F.; Adnan, M.; Khan, H.A.; Abdul, Q.K. Effect of amino acid fortified mulberry leaves on economic and biological traits of Bombyx mori L. Heliyon. 2023, 9, e21053. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ye, X.; Wang, X.; Zhao, S.; Wu, M.; Ruan, J.; Zhong, B. High mechanical property silk produced by transgenic silkworms expressing the spidroins PySp1 and ASG1. Sci. Rep. 2021, 1, 20980. [Google Scholar] [CrossRef] [PubMed]
- Murugesh, K.A.; Chozhan, K.; Aruna, R. Enhancement of larval and cocoon traits of silkworm, Bombyx mori L. through the application of amino acids. J. Entomol. Zool. Stud. 2021, 9, 2198–2203. [Google Scholar]
- Mahanta, D.K.; Komal, J.; Samal, I.; Bhoi, T.K.; Dubey, V.K.; Pradhan, K.; Nekkanti, A.; Gouda, M.N.R.; Saini, V.; Negi, N.; et al. Nutritional aspects and dietary benefits of “Silkworms”: Current scenario and future outlook. Front. Nutr. 2023, 10, 1121508. [Google Scholar] [CrossRef]
- Jin, X.; Ackah, M.; Acheampong, A.; Zhang, Q.; Wang, L.; Lin, Q.; Qiu, C.; Zhao, W. Genome-wide identification of candidate genes associated with heat stress in mulberry (Morus alba L.). Curr. Issues Mol. Biol. 2023, 45, 4151–4167. [Google Scholar] [CrossRef]
- Nelson, V.L.; Eadie, A.L.; Perez, L.; Madhu, M.; Platt, M.; Mercer, A.; Pulinilkunnil, T.; Kienesberger, P.; Simpson, J.A.; Brunt, K.R. Yap is a nutrient sensor sensitive to the amino acid L-isoleucine and regulates the expression of Ctgf in cardiomyocytes. Biomolecules 2024, 14, 1299. [Google Scholar] [CrossRef]
- Jakovljevic, J.; de Mayolo, P.A.; Miles, T.D.; Nguyen, T.M.L.; Léger-Silvestre, I.; Gas, N.; Woolford, J.L., Jr. Woolford, The carboxy-terminal extension of yeast ribosomal protein S14 is necessary for maturation of 43S preribosomes. Mol. Cell. 2004, 14, 331–342. [Google Scholar] [CrossRef]
- Cashikar, A.G.; Duennwald, M.; Lindquistc, S.L. A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J. Biol. Chem. 2005, 280, 23869–23875. [Google Scholar] [CrossRef]
- Yan, Y.; Duan, F.; Li, X.; Zhao, R.; Hou, P.; Zhao, M.; Li, S.; Wang, Y.; Dai, T.; Zhou, W. Photosynthetic capacity and assimilate transport of the lower canopy influence maize yield under high planting density. Plant Physiol. 2024, 195, 2652–2667. [Google Scholar] [CrossRef]
- Li, S.; Yin, Y.; Chen, J.; Cui, X.; Fu, J. H2O2 promotes trimming-induced tillering by regulating energy supply and redox status in bermudagrass. PeerJ 2024, 12, e16985. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wei, Z.; Zhang, J.; Zheng, C.; Zhu, H.; Zhai, H.; He, S.; Gao, S.; Zhao, N.; Zhang, H.; et al. Source-sink synergy is the key unlocking sweet potato starch yield potential. Nat. Commun. 2024, 15, 7260. [Google Scholar] [CrossRef] [PubMed]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport and metabolism control carbon partitioning between stem and grain in rice. J. Exp. Bot. 2021, 72, 4355–4372. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Y.; Pan, M.; Li, X.; Hebelstrup, K.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Transport and spatio-temporal conversion of sugar facilitate the formation of spatial gradients of starch in wheat caryopses. Commun. Biol. 2024, 7, 928. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Wang, M.; Yu, D.; Wei, X. Elevated CO2 concentration enhance carbon and nitrogen metabolism and biomass accumulation of Ormosiahosiei. Plant Physiol. Biochem. 2024, 212, 108725. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhen, X.H.; Zhou, Y.J.; Wang, Y.L.; Hou, J.Y.; Wang, X.; Li, R.M.; Liu, J.; Hu, X.W.; Geng, M.T.; et al. MeNINV1: An alkaline/neutral invertase gene of Manihot esculenta, enhanced sucrose catabolism and promoted plant vegetative growth in transgenic Arabidopsis. Plants 2022, 11, 946. [Google Scholar] [CrossRef]
- Weerasooriya, H.N.; Longstreth, D.J.; DiMario, R.J.; Rosati, V.C.; Cassel, B.A.; Moroney, J.V. Carbonic anhydrases in the cell wall and plasma membrane of Arabidopsis thaliana are required for optimal plant growth on low CO2. Front. Mol. Biosci. 2024, 11, 1267046. [Google Scholar] [CrossRef]
- Merino, V.M.; Aguilar, R.I.; Rivero, M.J.; Ordóñez, I.P.; Piña, L.F.; López-Belchí, M.D.; Schoebitz, M.I.; Noriega, F.A.; Pérez, C.I.; Cooke, A.S.; et al. Distribution of non-Structural carbohydrates and root structure of Plantago lanceolata L. under different defoliationfFrequencies and intensities. Plants 2024, 13, 2773. [Google Scholar] [CrossRef]
- Weng, J.; Rehman, A.; Li, P.; Chang, L.; Zhang, Y.; Niu, Q. Physiological and transcriptomic analysis reveals the responses and difference to high temperature and humidity stress in two melon genotypes. Int. J. Mol. Sci. 2022, 23, 734. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, M.; Ruan, J.; Peng, Y.; Ma, C.; Wu, W.; Gao, A.; Weng, W.; Cheng, J. Physiological and biochemical regulation mechanism of exogenous hydrogen peroxide in alleviating NaCl stress toxicity in tartary buckwheat (Fagopyrum tataricum (L.) Gaertn). Int. J. Mol. Sci. 2022, 23, 10698. [Google Scholar] [CrossRef]
- Kambhampati, S.; Li, J.; Evans, B.S.; Allen, D.K. Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry. Plant Methods 2019, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Fang, Y.; Li, D.C.; Chen, D.S.; Wu, F. Effect of lactic acid supplementation on the growth and reproduction of Bombyx mori (Lepidopteria: Bombycidae). J. Insect Sci. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Peng, L.L.; Cao, Y.Y.; Thakur, K.; Hu, F.; Tang, S.M.; Wei, Z.J. Effect of dietary selenium supplementation on growth and reproduction of silkworm Bombyx mori L. Biol. Trace Elem. Res. 2020, 193, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and Proteomic Responses of Mulberry Trees (Morus alba L.) Comb. Salt Drought Stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef]
- Zi, J.; Zhang, J.; Wang, Q.; Zhou, B.; Zhong, J.; Zhang, C.; Qiu, X.; Wen, B.; Zhang, S.; Fu, X.; et al. Stress responsive proteins are actively regulated during rice (Oryza sativa) embryogenesis as indicated by quantitative proteomics analysis. PLoS ONE 2013, 8, e74229. [Google Scholar] [CrossRef]
- Zhao, F.; Wei, Z.; Bai, Y.; Li, C.; Zhou, G.; Kristiansen, K.; Wang, C. Proteomics and metabolomics profiling of pork exudate reveals meat spoilage during storage. Metabolites 2022, 12, 570. [Google Scholar] [CrossRef]
- Zhang, H.; Gou, X.; Ma, L.; Zhang, X.; Qu, J.; Wang, X.; Huang, W.; Yan, S.; Zhang, X.; Xue, J.; et al. Reveal the kernel dehydration mechanisms in maize based on proteomic and metabolomic analysis. BMC Plant Biol. 2024, 24, 15. [Google Scholar] [CrossRef]
- Lê Cao, K.A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef]


| Varieties | 10 May (Kg/Acre) | 1 July (Kg/Acre) | 30 August (Kg/Acre) | 10 October (Kg/Acre) | Annual Leaf Yield (Kg/Acre) |
|---|---|---|---|---|---|
| ZJ1 | 923.93a | 904.94b | 960.82b | 600.17b | 3389.87b |
| N14 | 1295.01b | 226.88a | 320.64a | 512.48a | 2355.01a |
| Varieties | N14 | ZJ1 | p-Value |
|---|---|---|---|
| Water content (%) | 73.61 ± 0.35 | 73.37 ± 1.18 | 0.24 |
| Chlorophyll a content (mg.g−1) | 8.08 ± 0.01 | 7.84 ± 0.01 | 0.02 |
| Chlorophyll b content (mg.g−1) | 2.81 ± 0.01 | 2.65 ± 0.01 | 0.05 |
| Total protein content (mg.g−1) | 22.76 ± 3.67 | 24.18 ± 2.86 | 0.34 |
| Dietary fiber content (mg.g−1) | 420.07 ± 6.12 | 413.65 ± 5.57 | 0.29 |
| Ash content (mg.g−1) | 129.30 ± 0.24 | 124.03 ± 0.85 | 0.05 |
| Amino acid content (mg.g−1) | |||
| Aspartic acid | 19.32 ± 0.02 | 21.46 ± 0.09 | 0.06 |
| Threonine | 9.14 ± 0.07 | 9.78 ± 0.05 | 0.06 |
| Serine | 8.60 ± 0.08 | 9.63 ± 0.01 | 0.04 |
| Glutamic acid | 22.26 ± 0.12 | 26.04 ± 0.11 | 0.06 |
| Proline | 9.56 ± 0.07 | 10.67 ± 0.14 | 0.14 |
| Glycine | 11.43 ± 0.05 | 13.11 ± 0.05 | 0.09 |
| Alanine | 13.16 ± 0.02 | 14.43 ± 0.06 | 0.10 |
| Valine | 12.72 ± 0.13 | 13.90 ± 0.03 | 0.22 |
| Methionine | 1.45 ± 0.01 | 1.72 ± 0.01 | 0.13 |
| Isoleucine | 9.70 ± 0.11 | 11.84 ± 0.12 | 0.04 |
| Leucine | 18.53 ± 0.04 | 21.06 ± 0.06 | 0.10 |
| Tyrosine | 4.91 ± 0.01 | 6.33 ± 0.07 | 0.09 |
| Phenylalanine | 11.54 ± 0.03 | 13.34 ± 0.05 | 0.10 |
| Histidine | 4.63± 0.03 | 5.52 ± 0.04 | 0.25 |
| Lysine | 9.54 ± 0.12 | 10.56 ± 0.07 | 0.32 |
| Arginine | 9.40 ± 0.03 | 11.97 ± 0.22 | 0.22 |
| Groups | Stage | Larvae Number | Final Cocoon Number | Weight of 50 Whole Cocoons (g) | Weight of 50 Cocoon Shells (g) | Average Cocoon Shell Ratio (%) | Cocoon Shell Weight of Per 10,000 Larvae (g) |
|---|---|---|---|---|---|---|---|
| ZJ1 | Spring | 400 | 378.67 ± 8.74a | 109.13 ± 0.92b | 22.72 ± 0.36b | 20.72 ± 0.19b | 4261.45 ± 43.65b |
| Autumn | 400 | 371.33 ± 11.59a | 94.67 ± 0.06a | 19.43 ± 0.34a | 20.53 ± 0.01a | 3672.39 ± 77.89a | |
| N14 | Spring | 400 | 381.33 ± 9.61a | 110.42 ± 0.43c | 22.54 ± 0.09b | 20.41 ± 0.07a | 4295.70 ± 10.34b |
| Autumn | 400 | 374.33 ± 4.73a | 93.08 ± 0.91a | 18.86 ± 0.38a | 20.26 ± 0.02a | 3555.25 ± 85.31a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lv, Z.; Wei, J.; Liu, P.; Pan, M.; Ma, H.; Lin, T. Comparative Physiological, Proteomic, and Metabolomic Insights into a Promising Low-Pruning Mulberry Cultivar for Silkworm Rearing. Int. J. Mol. Sci. 2024, 25, 13483. https://doi.org/10.3390/ijms252413483
Liu Y, Lv Z, Wei J, Liu P, Pan M, Ma H, Lin T. Comparative Physiological, Proteomic, and Metabolomic Insights into a Promising Low-Pruning Mulberry Cultivar for Silkworm Rearing. International Journal of Molecular Sciences. 2024; 25(24):13483. https://doi.org/10.3390/ijms252413483
Chicago/Turabian StyleLiu, Yan, Zhiqiang Lv, Jia Wei, Peigang Liu, Meiliang Pan, Huanyan Ma, and Tianbao Lin. 2024. "Comparative Physiological, Proteomic, and Metabolomic Insights into a Promising Low-Pruning Mulberry Cultivar for Silkworm Rearing" International Journal of Molecular Sciences 25, no. 24: 13483. https://doi.org/10.3390/ijms252413483
APA StyleLiu, Y., Lv, Z., Wei, J., Liu, P., Pan, M., Ma, H., & Lin, T. (2024). Comparative Physiological, Proteomic, and Metabolomic Insights into a Promising Low-Pruning Mulberry Cultivar for Silkworm Rearing. International Journal of Molecular Sciences, 25(24), 13483. https://doi.org/10.3390/ijms252413483







