In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers
Abstract
1. Introduction
1.1. Acute Myeloid Leukemia
1.2. Dendritic Cell (DC)-Based Immunotherapy
2. Results
2.1. Animals
2.1.1. Ex Vivo Priming of Rat T Cells with Kit M-Treated (DC/DCleu-Containing) Rat WB Results in Improved Antileukemic Reactivity
2.1.2. In Vivo Treatment of Healthy Rats with PGE1 Is Safe
2.1.3. In Vivo Treatment of BNML Rats with Kit M Is Safe and Leads to Antileukemic Immune Reactions
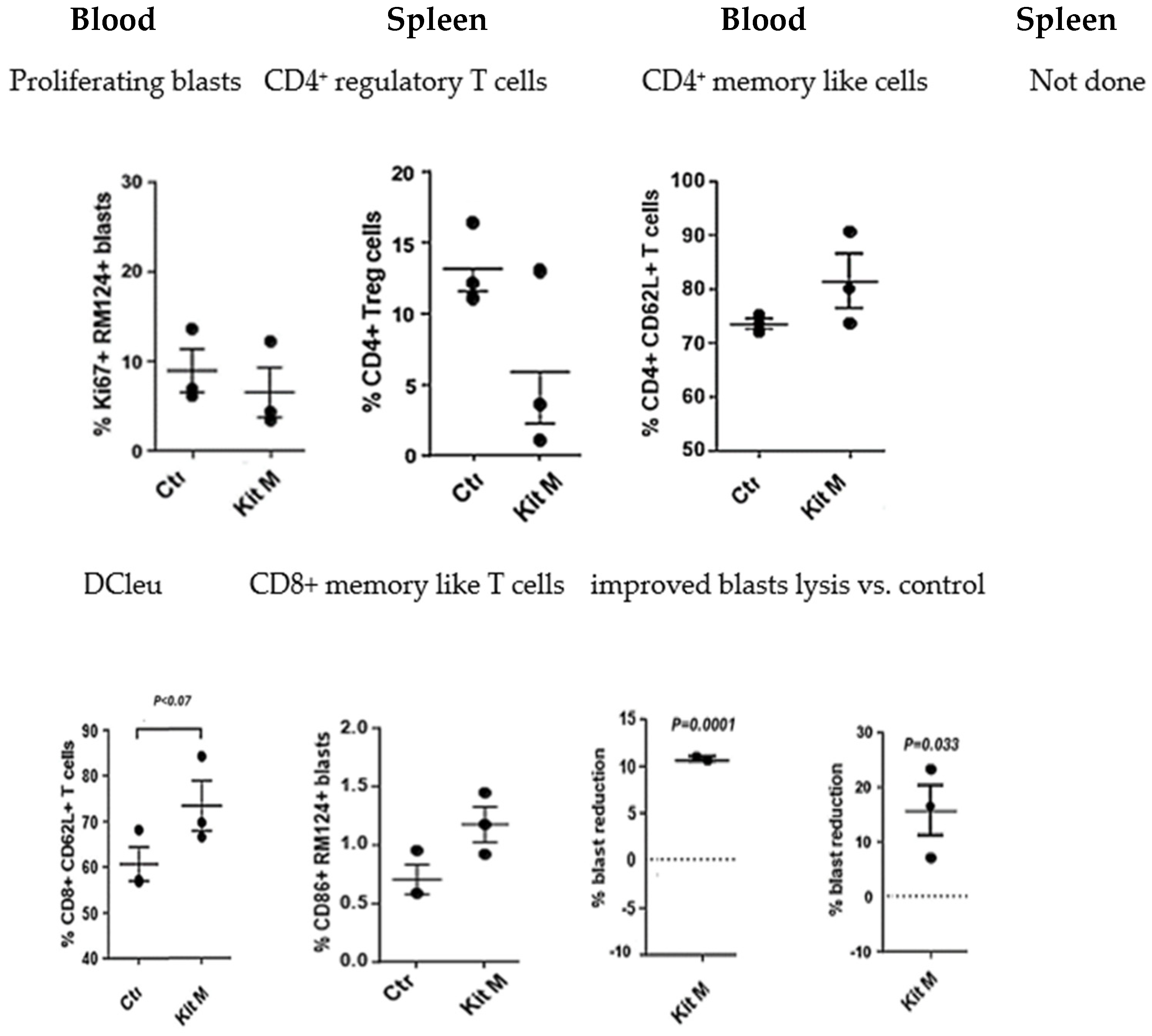
2.2. Patients
2.2.1. Before Clinical Treatment: Successful Ex Vivo Generation of DC/DCleu from Patients’ Blasts with Kit M
2.2.2. Clinical Courses and Immune Monitoring
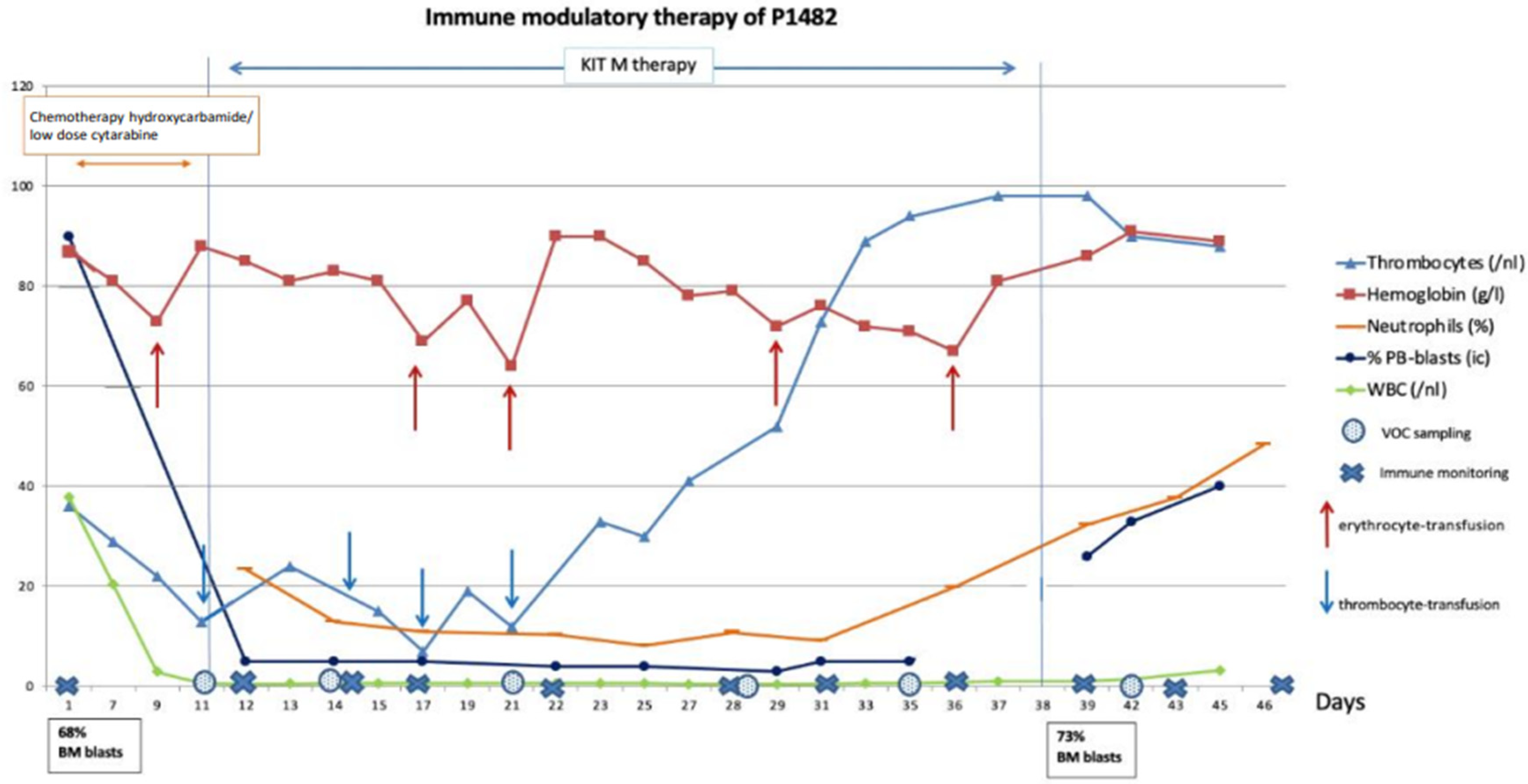
2.2.3. Patient 1482: Clinical Response, Improvement in Blood Counts, and Immunological Effects
2.2.4. Patient 1601: Induced Immunological Effects and Transient Reduction in Peripheral Blasts
 pleural punctures.
pleural punctures.
 pleural punctures.
pleural punctures.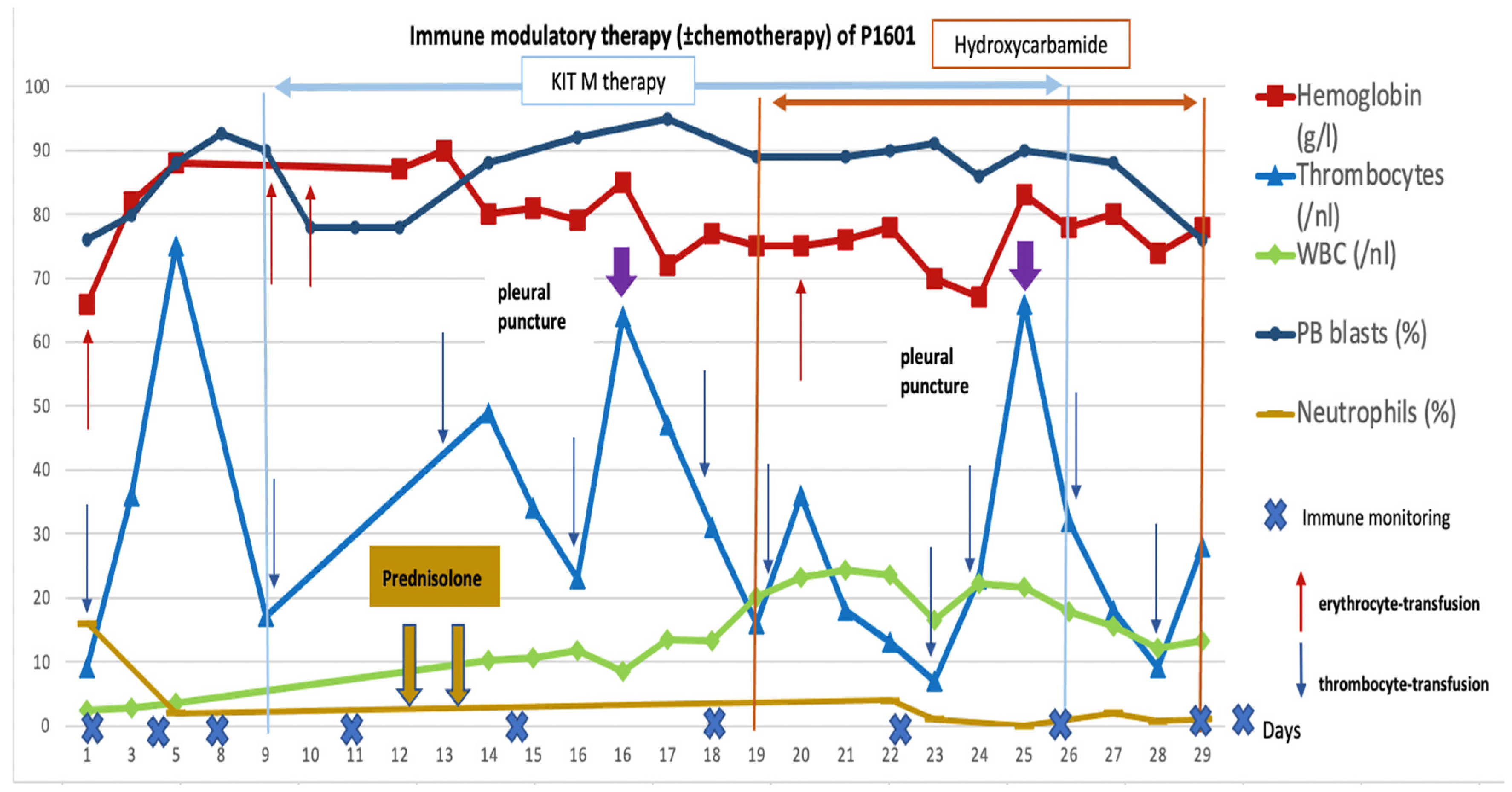
2.2.5. Control Patient 1511: No Immunological or Clinical Response to Treatment

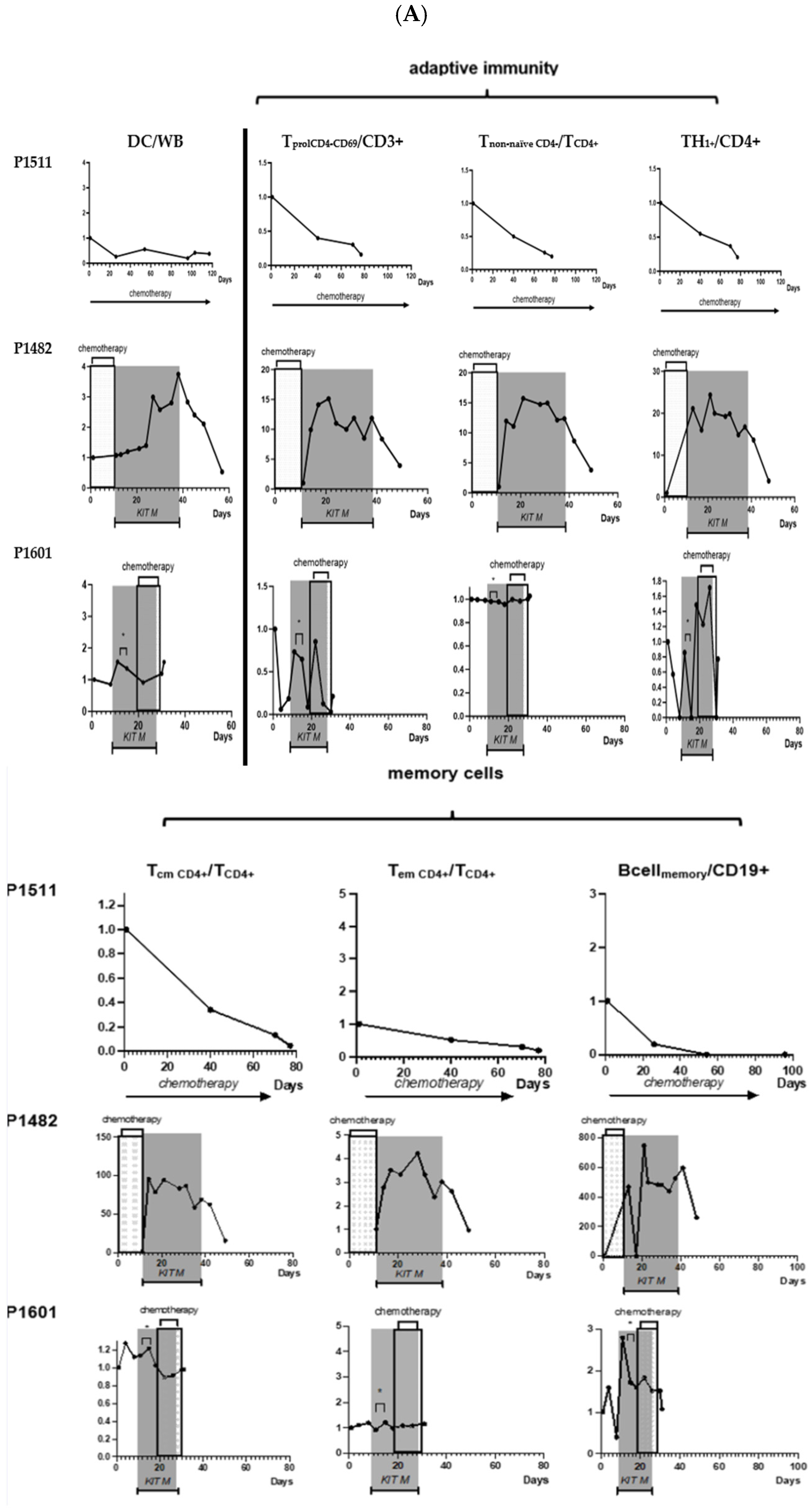

3. Discussion
3.1. Treatment of Leukemia-Diseased Rats with Kit M Was Well Tolerated, Reduced Regulatory T Cells, and Induced Antileukemic Responses
3.2. Treatment of Refractory AML Patients with Blast Modulatory Kit M Is Safe, May Induce Platelet Regeneration, and Improve the Composition of (Antileukemic) Immunoreactive Cells
4. Material and Methods
4.1. Animal Experiments
4.1.1. Animal Model
4.1.2. Application of Single Response Modifiers to Healthy Rats (Safety Analysis)
4.1.3. Kit M Treatment of Rats Diseased with Leukemia
4.1.4. Flow Cytometric Analyses on Rat Cells
4.2. Individualized Clinical Treatments in Two Patients with Refractory AML
4.2.1. Flow Cytometrc Analyses on Human Cells
4.2.2. Detection of Antigen-Specific Cells (Interferon Gamma (IFNy)-Cytokine Secretion Assay (CSA), Intracellular IFNy Secretion Assay (InCyt), IFNy-ELISPOT)
4.3. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamamyan, G.; Tervonen, H.; Mendoza, L.; Batmunkh, T.; Yap, M.L.; Rodin, D.; Moraes, F.Y. Future of Global Cancer From the Perspective of Young Oncology Leaders. JGO 2018, 4, 74s. [Google Scholar] [CrossRef]
- O’Donnell, M.R.; Tallman, M.S.; Abboud, C.N.; Altman, J.K.; Appelbaum, F.R.; Arber, D.A.; Bhatt, V.; Bixby, D.; Blum, W.; Coutre, S.E.; et al. Acute Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 926–957. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Burnett, A.K.; Hills, R.K.; Nielsen, O.J.; Freeman, S.; Ali, A.; Cahalin, P.; Hunter, A.; Thomas, I.F.; Russell, N.H. A comparison of FLAG-Ida and daunorubicin combined with clofarabine in high-risk acute myeloid leukaemia: Data from the UK NCRI AML17 Trial. Leukemia 2018, 32, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Döhner, H.; Ganser, A. How I treat refractory and relapsed acute myeloid leukemia. Blood 2024, 143, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, F.S.; Krupka, C.; Haubner, S.; Köhnke, T.; Subklewe, M. Recent developments in immunotherapy of acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Ansprenger, C.; Amberger, D.C.; Schmetzer, H.M. Potential of immunotherapies in the mediation of antileukemic responses for patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS)—With a focus on Dendritic cells of leukemic origin (DCleu). Clin. Immunol. 2020, 217, 108467. [Google Scholar] [CrossRef]
- Wan, H.; Dupasquier, M. Dendritic cells in vivo and in vitro. Cell. Mol. Immunol. 2005, 2, 28–35. [Google Scholar] [PubMed]
- Palucka, K.; Banchereau, J. Dendritic cells: A link between innate and adaptive immunity. J. Clin. Immunol. 1999, 19, 12–25. [Google Scholar] [CrossRef]
- Unterfrauner, M.; Rejeski, H.A.; Hartz, A.; Bohlscheid, S.; Baudrexler, T.; Feng, X.; Rackl, E.; Li, L.; Rank, A.; Filippini Velázquez, G.; et al. Granulocyte-Macrophage-Colony-Stimulating-Factor Combined with Prostaglandin E1 Create Dendritic Cells of Leukemic Origin from AML Patients’ Whole Blood and Whole Bone Marrow That Mediate Antileukemic Processes after Mixed Lymphocyte Culture. Int. J. Mol. Sci. 2023, 24, 17436. [Google Scholar] [CrossRef] [PubMed]
- Plett, C.; Klauer, L.K.; Amberger, D.C.; Ugur, S.; Rabe, A.; Fischer, Z.; Deen, D.; Hirn-Lopez, A.; Gunsilius, C.; Werner, J.-O.; et al. Immunomodulatory kits generating leukaemia derived dendritic cells do not induce blast proliferation ex vivo: IPO-38 as a novel marker to quantify proliferating blasts in acute myeloid leukaemia. Clin. Immunol. 2022, 242, 109083. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Sato, M. Toll-like receptor signaling in anti-cancer immunity. J. Med. Investig. 2003, 50, 9–24. [Google Scholar]
- Conejo-Garcia, J.R.; Rutkowski, M.R.; Cubillos-Ruiz, J.R. State-of-the-art of regulatory dendritic cells in cancer. Pharmacol. Ther. 2016, 164, 97–104. [Google Scholar] [CrossRef]
- Amberger, D.C.; Doraneh-Gard, F.; Gunsilius, C.; Weinmann, M.; Möbius, S.; Kugler, C.; Rogers, N.; Böck, C.; Ködel, U.; Werner, J.-O.; et al. PGE1-Containing Protocols Generate Mature (Leukemia-Derived) Dendritic Cells Directly from Leukemic Whole Blood. Int. J. Mol. Sci. 2019, 20, 4590. [Google Scholar] [CrossRef]
- Kremser, A.; Dressig, J.; Grabrucker, C.; Liepert, A.; Kroell, T.; Scholl, N.; Schmid, C.; Tischer, J.; Kufner, S.; Salih, H.; et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: An evaluation of different methods. J. Immunother. 2010, 33, 185–199. [Google Scholar] [CrossRef]
- Hirn Lopez, A.; Deen, D.; Fischer, Z.; Rabe, A.; Ansprenger, C.; Stein, K.; Vogt, V.; Schick, J.; Kroell, T.; Kraemer, D.; et al. Role of Interferon (IFN)α in “Cocktails” for the Generation of (Leukemia-derived) Dendritic Cells (DCleu) From Blasts in Blood From Patients (pts) With Acute Myeloid Leukemia (AML) and the Induction of Antileukemic Reactions. J. Immunother. 2019, 42, 143–161. [Google Scholar] [CrossRef]
- van Acker, H.H.; Versteven, M.; Lichtenegger, F.S.; Roex, G.; Campillo-Davo, D.; Lion, E.; Subklewe, M.; van Tendeloo, V.F.; Berneman, Z.N.; Anguille, S. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J. Clin. Med. 2019, 8, 579. [Google Scholar] [CrossRef]
- Schwepcke, C.; Klauer, L.K.; Deen, D.; Amberger, D.C.; Fischer, Z.; Doraneh-Gard, F.; Gunsilius, C.; Hirn-Lopez, A.; Kroell, T.; Tischer, J.; et al. Generation of Leukaemia-Derived Dendritic Cells (DCleu) to Improve Anti-Leukaemic Activity in AML: Selection of the Most Efficient Response Modifier Combinations. Int. J. Mol. Sci. 2022, 23, 8333. [Google Scholar] [CrossRef]
- Kim, G.G.; Donnenberg, V.S.; Donnenberg, A.D.; Gooding, W.; Whiteside, T.L. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: Comparisons to a 4 h 51Cr-release assay. J. Immunol. Methods 2007, 325, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Nestvold, J.M.; Omdal, B.K.; Dai, K.-Z.; Martens, A.; Benestad, H.B.; Vaage, J.T.; Rolstad, B. A second prophylactic MHC-mismatched bone marrow transplantation protects against rat acute myeloid leukemia (BNML) without lethal graft-versus-host disease. Transplantation 2008, 85, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Amberger, D.C.; Schmetzer, H.M. Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treatment Tools for Patients with Myeloid Leukemia. Transfus. Med. Hemother. 2020, 47, 432–443. [Google Scholar] [CrossRef]
- Schutti, O.; Klauer, L.; Baudrexler, T.; Burkert, F.; Schmohl, J.; Hentrich, M.; Bojko, P.; Kraemer, D.; Rank, A.; Schmid, C.; et al. Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients’ Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays. Int. J. Mol. Sci. 2024, 25, 6983. [Google Scholar] [CrossRef] [PubMed]
- Stahl, C.P.; Winton, E.F.; Monroe, M.C.; Haff, E.; Holman, R.C.; Myers, L.; Liehl, E.; Evatt, B.L. Differential effects of sequential, simultaneous, and single agent interleukin-3 and granulocyte-macrophage colony-stimulating factor on megakaryocyte maturation and platelet response in primates. Blood 1992, 80, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, K.; Roesler, J.; Emmendoerffer, A.; Elsner, J.; Welte, K. Functional features of neutrophils induced by G-CSF and GM-CSF treatment: Differential effects and clinical implications. Leukemia 1997, 11, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, M.; Ye, J.; Ma, H. Targeting PRAME for acute myeloid leukemia therapy. Front. Immunol. 2024, 15, 1378277. [Google Scholar] [CrossRef]
- van Tendeloo, V.F.; van de Velde, A.; van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA 2010, 107, 13824–13829. [Google Scholar] [CrossRef]
- Steger, B.; Milosevic, S.; Doessinger, G.; Reuther, S.; Liepert, A.; Braeu, M.; Schick, J.; Vogt, V.; Schuster, F.; Kroell, T.; et al. CD4(+)and CD8(+)T-cell reactions against leukemia-associated- or minor-histocompatibility-antigens in AML-patients after allogeneic SCT. Immunobiology 2014, 219, 247–260. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Stone, R.M.; Uhl, L.; Neuberg, D.; Joyce, R.; Levine, J.D.; Arnason, J.; McMasters, M.; Luptakova, K.; Jain, S.; et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016, 8, 368ra171. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.C.; van Bekkum, D.W.; Hagenbeek, A. The BN acute myelocytic leukemia (BNML) (a rat model for studying human acute myelocytic leukemia (AML)). Leukemia 1990, 4, 241–257. [Google Scholar]
- Waidhauser, J.; Nerlinger, P.; Arndt, T.T.; Schiele, S.; Sommer, F.; Wolf, S.; Löhr, P.; Eser, S.; Müller, G.; Claus, R.; et al. Alterations of circulating lymphocyte subsets in patients with colorectal carcinoma. Cancer Immunol. Immunother. 2022, 71, 1937–1947. [Google Scholar] [CrossRef]
- Klauer, L.K.; Schutti, O.; Ugur, S.; Doraneh-Gard, F.; Amberger, D.C.; Rogers, N.; Krämer, D.; Rank, A.; Schmid, C.; Eiz-Vesper, B.; et al. Interferon Gamma Secretion of Adaptive and Innate Immune Cells as a Parameter to Describe Leukaemia-Derived Dendritic-Cell-Mediated Immune Responses in Acute Myeloid Leukaemia in vitro. Transfus. Med. Hemother. 2022, 49, 44–61. [Google Scholar] [CrossRef]
- Schillingmann, D.A.; Riese, S.B.; Vijayan, V.; Tischer-Zimmermann, S.; Schmetzer, H.; Maecker-Kolhoff, B.; Blasczyk, R.; Immenschuh, S.; Eiz-Vesper, B. Inhibition of Heme Oxygenase-1 Activity Enhances Wilms Tumor-1-Specific T-Cell Responses in Cancer Immunotherapy. Int. J. Mol. Sci. 2019, 20, 482. [Google Scholar] [CrossRef] [PubMed]
| Patient ID | Diagnosis | Stage | Age at Diagnosis (Years) | Sex | ELN Risk Stratification at Diagnosis | First-Line Treatment | Further Treatments | Blast Immune Phenotype (CD-Positivity) | BM/PB Blasts (%) Before Kit M Treatment | Conducted Cell Biological Experiments |
|---|---|---|---|---|---|---|---|---|---|---|
| P1511 | AML | Refractory Relapse | 76 | m | Intermediate | Decitabine (22 cycles) | Cytarabine + Midostaurin | 13, 33, 34, 117 | 54/40 | DC, MLC, CTX, CSA |
| P1482 | AML | Refractory Relapse | 74 | m | Unfavorable | 5-Azacytidin (8 cycles) | Second line: Daunorubicin/AraC (2 cycles), followed by HD AraC (three cycles) | 13, 15, 33, 34, 64, 117 | <5/68 | DC, MLC, CTX, CSA |
| Third line: Decitabine (two cycles) | ||||||||||
| Fourth line: Hydroxyurea/ Cytarabine | ||||||||||
| P1601 | AML | Refractory Relapse | 74 | f | Unfavorable | 5-Azacytidin (four cycles) and Venetoclax (one cycle) | Hydroxyurea | 13, 33, 34, 117 | 90/68 | DC, MLC, CTX, InCyt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzler, M.; Baudrexler, T.; Amberger, D.C.; Rogers, N.; Rabe, A.; Schmohl, J.; Wang, R.; Rank, A.; Schutti, O.; Hirschbühl, K.; et al. In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers. Int. J. Mol. Sci. 2024, 25, 13469. https://doi.org/10.3390/ijms252413469
Atzler M, Baudrexler T, Amberger DC, Rogers N, Rabe A, Schmohl J, Wang R, Rank A, Schutti O, Hirschbühl K, et al. In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers. International Journal of Molecular Sciences. 2024; 25(24):13469. https://doi.org/10.3390/ijms252413469
Chicago/Turabian StyleAtzler, Michael, Tobias Baudrexler, Daniel Christoph Amberger, Nicole Rogers, Alexander Rabe, Joerg Schmohl, Ruixiao Wang, Andreas Rank, Olga Schutti, Klaus Hirschbühl, and et al. 2024. "In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers" International Journal of Molecular Sciences 25, no. 24: 13469. https://doi.org/10.3390/ijms252413469
APA StyleAtzler, M., Baudrexler, T., Amberger, D. C., Rogers, N., Rabe, A., Schmohl, J., Wang, R., Rank, A., Schutti, O., Hirschbühl, K., Inngjerdingen, M., Deen, D., Eiz-Vesper, B., Schmid, C., & Schmetzer, H. M. (2024). In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers. International Journal of Molecular Sciences, 25(24), 13469. https://doi.org/10.3390/ijms252413469






